Abstract
The aim of the research has been to assay the contents of Zn, Cu, Mn, Fe, and Pb in soil and in the organs of wild Thymus serpyllum L. acquired from three natural habitats from the Kuyavia-Pomerania Province (Poland) not exposed to contamination with metals. As compared with the contents of the geochemical background of the soils in the region and the value of enrichment factor (EF), there was noted a considerable content of Pb in the soil from two locations. The Pb content in plant tissues in one of the three locations was higher than the admissible content specified by the World Health Organization WHO (above 10 mg kg−1 d.w.). As for the Zn content, only the plants from the locations with the relatively youngest phytocenosis met the conditions for herbs to be used for phytotherapy. The Zn content in the dry weight of the plants collected from the other two locations exceeded 50 mg kg−1. The risk of an elevated content of Zn and Pb in the plants makes it necessary to monitor the soil environment and to limit the collection of the plants from natural habitats, as well as to encourage the collection from controlled habitats.
1. Introduction
For sustainable global development, food safety is a high-priority issue. In recent decades, adverse effects of toxic substances on crop quality have threatened both food safety and human health [1]. Heavy metals and metalloids (e.g., As, Cd, Pb, and Hg) are classified as non-essential for metabolic and other biological functions as they can disturb human metabolism, contributing to a higher morbidity [2]. Trace elements and other contaminants are released into the environment by both natural and anthropogenic sources. Highly reactive, and often toxic even at low concentrations, they may enter soils and bioaccumulate in food webs. Certain heavy metals, such as Cu, Zn, and Fe, are essential components of metabolic processes in herbs, including enzymes, linked to the metabolic functioning of biota [3].
Heavy metals also may persist in the environment for years, posing long-term risks to human health [4]. For that reason, some studies have focused on understanding how to reduce these toxic elements from natural environments with an eco-friendly approach, highlighting an interesting correspondence between plant communities and heavy metal concentration in soil [5]. Recent decades have witnessed an increase in alternative medical approaches, including the use of herbal supplements, natural products, and complementary medicines. The therapeutic activity of herbs is associated with the content of bio-active elements and organic compounds, such as vitamins, alkaloids and tannins. Herbs and spices constitute an important role in the transfer of essential metals from soil to human body [6,7]. Many trace metals are essential for the formation of constituents responsible for curative properties [8]. However, there is an increasing concern about the safety and health benefits of these traditional medicines as one of the main hazards with the use of herbs is the presence of trace elements. Many medicinal plants have been shown to bioaccumulate various heavy metals when grown near industrial areas [9]. Thus, soil-plant systems provide an example of abiotic-biotic interactions in the environment. With that in mind, this article focuses on and describes heavy metal contamination in soil–herb crop subsystems with respect to human health risks.
The pharmaceutical herbal material legislature, concerning the content of heavy metals, in the European Union (EU) countries and globally, follow the requirements of the European Pharmacopoeia [10] and the requirements of the World Health Organization [11]. The contents of trace elements, especially the contents of Fe, Mn, Zn and Cu in medicinal plant substances, are not regulated by law. In the light of the current regulations and the WHO guidelines, the admissible lead content in medicinal plants is Pb 10 mg kg−1 d.w. According to the Polish Pharmacopoeia, the regulations are more restrictive and they specify the limit values of Pb concentrations below 5.0 mg kg−1 d.w. in herbs [12].
One of the plants used in herbal medicine is T. serpyllum. This plant is a frost-hardy plant of the family Lamiaceae, a chamephyte with lignified creeping stems, reaching up to 30–40 cm in length. It creates low sods with a compact habit. The flowers are tiny, pink-violet in colour, with dense clusters on the top of the stems. The species occurs in the temperate climate zone; in Europe and in Asia. It is also found in India, as well as on the Kola Peninsula and in Iceland. It also occurs in North America. In Poland, it is common and it represents a native flora [13,14,15,16,17]. In the natural state, T. serpyllum plants grow in very sunny spots, in sandy permeable soil with a slightly acid reaction. It is very common in dry pine forests, well-lit shrubs, on slopes and roadsides. The species is common for the Koelerio-Corynephoretea class [18]. In Poland, in natural stands, one can find 12 species of the genus Thymus, however, only two occur frequently: T. pulegioides and T. serpyllum [17,19].
The herbal material of T. serpyllum is mostly collected from natural habitats [20]. The material acquisition method is part of the sustainable development of rural areas, which involves a rational and environment-friendly use of natural resources [21,22]. A rational acquisition of material from natural habitats helps limiting a negative effect of the crop protection chemicals applied throughout the cultivation on the natural environment. The herbal material collected from the areas with no agrotechnical, and phytosanitary treatments applied shows a high quality and medicinal properties. In response to a growing social demand for natural treatment methods, including herbal medicine, the number of pharmaceutical and cosmetic enterprises interested in purchasing the herbal material increases [23]. A collection from undeveloped areas, with respect to the natural resources, including woodland, supervised by qualified advisors, enhances the job market and the income of rural areas residents [24].
T. serpyllum plant has a characteristic and slightly spicy fragrance, and it has been, for a long time, used as seasoning in herbal medicine [20,25,26]. It contains luteolin, apigenin, glycosides, scutellareins, triterpenes, organic acids, mineral salts and bitter serpyllin. Thymi oleum essential oil contains more than 200 chemical compounds, and the main group are terpenes, especially thymol and carvacrol [10]. Their content in plants varies and it depends on the herbal material origin [27,28,29]. Thanks to the presence of active compounds, it has relaxant, expectorant, mucoactive, disinfecting, anti-inflammatory, and diuretic properties. Thyme essential oil shows antibacterial, antifungal, antiviral [30,31], and antineoplastic effects [27,32]. T. serpyllum preparations can be also used to treat rheumatic disorders and herbal infusion can prevent hair loss [33,34,35,36,37]. Moreover, the thyme oil shows insecticidal properties, and it can be used as an insect repellent [36,38].
The macro- and microelements found in plant tissues are indispensable for an adequate plant growth and development as well as for the health and life of the people and animals consuming the plants. The group includes Zn, Cu, Mn, and Fe, while other metals, especially As, Cd, and Pb, have a negative effect on the course of metabolic processes [1]. The impact of heavy metals on plants is very complex. One of the effects is a decreased content of active medicinal compounds, which deteriorates the herbal material quality [39,40]. The amount of microelements and essential oils in wild herbal material depends on growth habitat [41] and on the development stage that is species-specific [42]. The uptake of trace elements by plants is complex and it is conditioned by the interaction of environmental factors. Wild T. serpyllum shows a specific capacity for trace elements phytoaccumulation [43]. There are three stages of the process of metal hyperaccumulation in plants: the uptake with soil solution by the root system, the transport of the ions uptaken by vascular bundles, and the compartmentation and detoxication of metals in the stem [44]. The translocation of metals from the roots to the stem decreases their availability in root cells [45]. It is related to the specific features of root cell tonoplast and to the increased capacity for accumulating metals in leaf cell vacuoles [46,47]. However, an excessive lead content has a negative impact on many metabolic processes in plants. The Pb ions decrease the accumulation of potassium, calcium, magnesium, manganese, zinc and iron cations in plant cells. Lead in mostly accumulated in roots. However, it can be also transported to aboveground parts [48,49]. The lead availability and uptake from soil is affected by pH, the root system area and by the chemicals released by roots [40].
The factors determining the occurrence of many plant species in soils with a high content of heavy metals also include a capacity for forming the arbuscular mycorrhiza with fungi representing the assemblage Glomeromycota [50,51]. That type of mycorrhiza is found in most herbal plants from the ecosystems of the temperate and semidesert zones [52]. The presence of mycorrhizal fungi affects not only the root system of the host but also the functioning of the stem by modifying the hormonal status of the plant and the effect of stomata [53,54]. Abscisic acid can block transpiration, which results in the accumulation of elements in the aboveground plant organs [55].
The plants of the family Lamiaceae, represented by T. serpyllum, cover the species known as heavy metal hyperaccumulators [56] which, in the aboveground parts, can accumulate from 10 to 500 times more metal than the representatives of the same species growing in the uncontaminated stands. The group includes also the plants for which the ratio of metal content in the aboveground parts to the content in roots and in soil is higher than 1 [57].
The aim of the research has been to assay the content of Zn, Cu, Mn, Fe, and Pb in soil and in the organs of T. serpyllum acquired from three natural habitats not exposed to contamination with metals. There was determined the content of metals in soil and in plant tissues, as compared to their biologically permissible contents. The possibilities of using the herbal material derived from rural stands were evaluated as well as a possibility of using herbal material from rural areas from undeveloped agricultural stands, with protection and sustainable use of biological diversity in mind. Assaying the content of metals indispensable to plants, including Zn, Cu, Mn, and Fe, in soil facilitates the evaluation of the soil environment and estimating a potential threat of pollution with those metals. For that reason, the contents of the forms of those metals available to plants were assayed. There is a lack of Cd, Hg, or As pollution emitters in the neighbourhood of the sampling locations; hence no assays of the content of those metals. The reports by other authors did not point to the presence of those metals in the parent material of the soils in the region. There was assayed a total Pb content due to the earlier results of herbal plants [23,58,59], pointing to a threat of the accumulation of that metal in plant material despite a relatively low total content in soil.
2. Materials and Methods
The total content of Zn, Cu, Mn, Fe and Pb and their forms available to plants were assayed in the soil samples and their content in T. serpyllum organs. Soil and plants were sampled in July 2018 in three locations from the Kuyavia-Pomerania Province (north-central Poland). They were as follows: A–Łosiny (53°37′22″ N 17°59′08″ E), B–Okole (53°17′52″ N 17°56′37″ E) and C–Łochowo (53°07′19″ N 17°50′19″ E) (Figure 1). For the purpose of representing the geographic locations, the WGS-84 reference system was used. The distance from the source of pollution was considered when selecting the study area. Sampling locations B and C were chosen as the nearest, and A as the most distant from the urban agglomeration. Soil and plant were sampled from the edge of the pine forest. The locations were most representative in terms of the plant habitat.
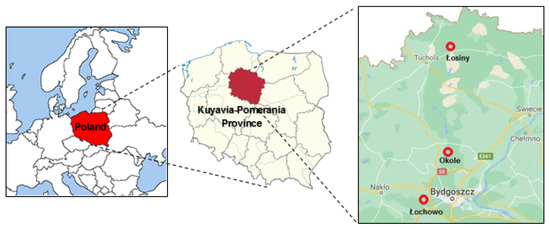
Figure 1.
Localization study area.
Soil samples were taken from two depths of soil profiles using a spade. The soil was sampled from the plant root zone (0–30 cm). The average soil samples were made from three individual subsamples. Some soil was also sampled from a depth of 120–150 cm to estimate the metal content of the local geochemical background. Soil samples were air-dried and screened through a 2-mm sieve for physical and chemical analysis (three replications). Soil pH was measured using a glass electrode in 1M KCl solution (1:2.5 soil-solution ratio) [60]. The total organic carbon (TOC) content was assayed using analyser vario Max CN Elementar provided by Analysensysteme GmbH (Hanau, Germany). The soil texture was measured applying the laser diffraction method with Mastersizer 2000 (Malvern Instrument, Malvern, UK). The total metal contents were determined applying digestion with HF and HClO4 acid solutions, according to the Crock and Severson (1980) [61] method. The certified reference materials (TILL–3, the Canadian Certified Reference Materials) were used to verify the accuracy of the results. The recovery rates for the elements were as follows: 98%, 99%, 103%, 97%, and 99%, for Zn, Cu, Pb, Mn, and Fe, respectively. The contents of metals available to plant forms were determined with 1 M HCl solution (Rinkis method) [62]. To evaluate the bioavailability of metals in soil samples, there was applied a test with the solution of 1 M HCl, which is a strong extractant to facilitate assaying metals both exchangeable forms, bonded with carbonates, Fe-Mn oxides, as well as with organic matter. The content of Zn, Cu, Mn, Fe forms susceptible to exctraction with 1M HCl was compared with the so-called limit numbers developed by the State Research Institute–Institute of Soil Science and Plant Cultivation (IUNG) in Puławy, Poland [63]. The method is commonly used in the research of soils in Poland to evaluate the content of the metal forms potentially available to plants [64,65,66]. The metal contents in soil samples and plant material were assayed applying the atomic absorption spectrometry method (AAS), with the SOLAAR S4 AA spectrometer (ThermoElemental, Cambridge, UK).
For the purpose of research, T. serpyllum plants were collected at the flowering stage. The aboveground plant parts, namely leaves, stems and inflorescences, were separated from the roots, and then rinsed in distilled water and dried (at 40 °C) to reach the air-dry state. Then the plant material was crushed in the agate mortar. The homogenized material (300 mg) was microwave-digested in the Speedwave Two mineraliser (Berghof, Germany) with the wet mineralization method (5 mL 65% HNO3, 1 mL 30% H2O2).
The total contents of Zn, Cu, Mn, Fe, and Pb in inflorescences, leaves and stems following mineralization were determined using the atomic absorption spectrophotometry, with a SOLAAR S4 AA spectrometer (ThermoElemental, Cambridge, UK).
Statistical analyses were performed applying Statistica 12.0 software (StatSoft Inc., Tulsa, OK, USA) [67]. The contents of heavy metals in soil and in plant parts are expressed as mean ± standard deviation (Sd). The relationship between the soil properties selected and the content of metals of T. serpyllum was also calculated (correlation coefficient R, coefficient of determination R2). Hierarchical cluster analysis (CA) using Ward’s method (1963) [68] was used to identify the similarity groups across samples.
The coefficient of variation in the metal content in the plant was calculated as follows:
where: CV is the coefficient of variation (%), Sd is the standard deviation, and X–arithmetic mean. The values, 0–15%, 16–35% and >36%, indicate low, moderate, or high variation, respectively [69].
CV = (Sd/X) × 100%
The human impact on soils was estimated with enrichment factor (EF), based on the normalization of the metal measured against a reference metal. Generally, Al and Fe are used as reference elements. The values of enrichment factor (EF) facilitate evaluating the risk of contamination with heavy metals. The values of enrichment factor (EF) were calculated according to the Equation (1):
where Cn—total content of metal in soil sample; CnFe—total content of Fe in soil sample; Bn—content of metal for the geochemical background; BnFe—content of Fe for the geochemical background as the reference element [70]. For the calculations, there was assumed the content of metals in parent material at the depth of 120–150 cm. Based on the EF value, the categories were determined as: <2—deficient to minimal enrichment, 2–5 moderate; 5–20—considerable; 20–40 very high; >40—extremely high enrichment [71].
EF = [Cn/CnFe]/[Bn/BnFe]
3. Results
3.1. Physical and Chemical Properties
With the percentage content of respective grain size fractions, the soil was classified as sand for A and loamy sand for B, C [72]. The results of grain size composition and selected parameters of soil samples (0–30 cm) are presented in Table 1. They include from 77.9–87.4% of sand fraction (2.0–0.05 mm), from 11.7–20.7% of silt fraction (0.05–0.002 mm) and from 0.9–1.4% of clay fraction (<0.002 mm). The soil reaction ranged from very acid for location A to slightly acid for location C. The soils demonstrated a varied content of organic carbon. The highest value was found in the soil from sampling location B (13.8 g kg−1).

Table 1.
Selected properties of soil samples (0–30 cm).
The soil and T. serpyllum plant sampling locations are the stands with sandy texture (Table 1). The soil sample from location A was most acid and it recorded the lowest content of clay fraction. However, the soil reaction in location C was slightly acid (pH in 1 M KCl) and the soil revealed the highest content of clay fraction, thus creating better plant development conditions. The soil sampled from location B recorded the highest total organic carbon (13.8 g kg−1).
3.2. Heavy Metal Content in Soil Samples
The soil from locations A and C recorded a similar Znt content, while the soil from location B contained over two-fold more of that metal. The content of Cut in the soil samples differed and it ranged from 4.03 mg kg−1 in location A, to 9.1 mg kg−1 in location B (Table 2), whereas the total lead content ranged from 45.0–54.1 mg kg−1. The content of manganese in soils varied a lot, which is evident from a high value of the coefficient of variation (CV). The soil sampled from location A contained 87.6 mg kg−1 of Mn and a three-fold higher total content was noted in the soil sampled from location B, and more than six-fold higher–from location C. The least varied were the total contents of lead and iron in soil for which the CV values were 9.27% and 14.74%, respectively.

Table 2.
Total content of Zn, Cu, Mn, Pb and Fe in topsoil.
The average total content of metals in soil at a depth of 120–150 cm (Table 3) was similar to the geochemical background values. Soil sampled from location B had the highest total content of Zn, Cu, Pb, and Fe content of the three habitats studied. There was also assayed the content of metal forms available to plants (Table 4). The content of Zna, Cua, Mna and Fea varied a lot across the locations. All the soil samples recorded a high content (according to the Polish norms) [63] of available-to-plants zinc forms; Zna ranging from 6.7 mg kg−1 (C) to 14.2 mg kg−1 (B), while the content of copper (Cua) available to plants in the soil sampled from location A and C was average and high in the soil from location B. In all the soil sampling locations, the content of Mna was average.

Table 3.
Total content of metals in parent material (120–150 cm).

Table 4.
Content of metals available to plants (extracted with 1M HCl).
The value of enrichment factor (EF) amounted to, respectively: Zn from 1.20–2.78; Cu from 1.25–2.19; Mn from 0.92–1.75; Pb from 4.17–5.98 (Table 5).

Table 5.
Values of enrichment factor EF in topsoil.
3.3. Content of Heavy Metal in T. serpyllum
Depending on the sampling location, there was recorded a varied content of metals in plant organs. There was no accumulation in the respective plant parts studied (Table 6). The highest Zn content was reported in the plants from location A (Table 6), collected from the edge of the pine forest, about 50 years of age, from 68.55 to 116.9 mg kg−1. However, in stems and leaves, the content was, on average, 62% higher than in roots. In location B, where the secondary succession process started in the 1990s, the plants contained, on average, 2.4% zinc less, and its highest content was recorded in plant roots (41% higher than in inflorescences and 26% higher than in stems with leaves). T. serpyllum plants from location C, from a relatively younger phytocenosis, contained, on average, 46.92 mg kg−1 of zinc, which is twice less than the plants from locations A and B. There was found a highly significantly negative correlation between the content of Zn L+S and pH KCl of soil; r = −0.998 (Table 7).

Table 6.
Content of Zn, Cu, Pb, Mn, Fe mg kg−1 in the dry weight of T. serpyllum plants.

Table 7.
Correlation (R), determination coefficient (R2) and linear regression models for selected properties of soils and T. serpyllum plants.
In the plants growing in all the habitats under study, the lowest contents of Cu were noted in stems with leaves from sampling locations A, B, C–7.8, 11.35, 16.2 mg kg−1, respectively (Table 6). In the other organs from sampling locations A, B, and C, the content of that element was, on average, 3.3- and 1.9- as well as 1.7-fold higher, respectively. At the same time in the plants from locations A and B there was recorded a slightly higher copper content in plant roots than in inflorescences. However, in the T. serpyllum plants from location C the opposite tendency was identified.
The herb samples showed a high variation in the lead content, depending on the sampling location and on the plant organ. The highest Pb content was noted in the roots of T. serpyllum plants from location B (Table 6) 20.8 mg kg−1. However, the lowest lead content was found in the inflorescences of the plants collected from location C–4.1 mg kg−1. An equally low content of the element was reported in stems with leaves from the plants from location B and the roots of the plants from location A. The herbal material acquired from the stands in locations A and C, where the average lead content, 8.85 and 9.9 mg kg−1, respectively, meets the conditions provided for in the WHO norm for herbs: Pb 10 mg kg−1. The lead content in the leaves and stems in T. serpyllum plants (Pb L+S) was significantly negatively correlated (r = −0.999) with soil reaction (pH KCl) (Table 7).
The content of manganese in leaves also varied a lot and it depended on the sampling location and on the organ. There was noted a highly significantly positive correlation (r = 0.999) between the content of manganese in leaves and in stems (Mn L+S) and the content of that element in inflorescences (Mn I) in T. serpyllum (Table 7). The highest Mn content was noted in the inflorescences of T. serpyllum plants collected from location A (Table 6) (338.6 mg kg−1). On average, in the aboveground parts of the plants from the same stand, 313.85 mg kg−1 of manganese was recorded. However, the content of that element in the roots of T. serpyllum plants was 55.8% lower (138.65 mg kg−1). In the other stands, in the stems and leaves of plants, a similar content was noted. However, in the roots of T. serpyllum plants from location B, the content of manganese was more than two-fold higher than in the aboveground stems.
The highest mean content of iron was identified in the plants from location B (Table 6). In the other cases, the mean content of Fe in tissues was definitely lower: 52.5% lower in the plants from location A and 62.1% lower in the plants from location C. The plants collected from location B accumulated similar amounts of iron in the aboveground and underground parts, while T. serpyllum plants from location A contained most of that element in inflorescences, and in the organs of T. serpyllum plants sampled from location C the iron content was similar.
The results of cluster analysis indicate that the soil demonstrated a similar total metal content in locations A and B in both the surface layer and the parent material, in comparison to location C (Figure 2a,b). The inflorescences of T. serpyllum collected from habitats A and B showed similar concentrations of metals, as compared with the content of metals in the inflorescences collected from location C (Figure 3a), whereas a similar concentration of metals in stems and leaves were found in plants collected from habitats A and C (Figure 3b). The cluster analysis showed differences in the content of metals in respective organs of the herbs across the sampling locations. The plants collected from location B showed a slightly different habit, they were higher than the plants sampled from the other two locations, which could be due to slightly better soil conditions. Soil in location B showed a considerably higher content of organic matter (Table 1) and, as for that content, it demonstrated slightly more favorable water holding capacity, as compared with locations A and C, which could be the reason for such variation, despite a similar content of metals in soil.
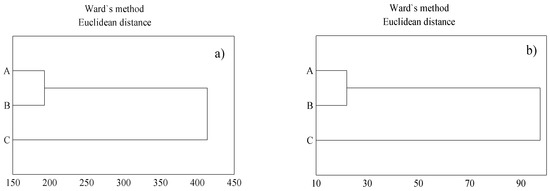
Figure 2.
Total content of metals in topsoil (a); total content of metal in parent material (b). A, B, C—location vicinity: explanations as in Table 1.
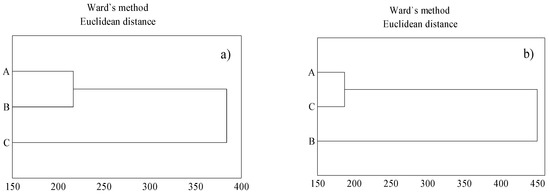
Figure 3.
Concentration of metal in inflorescences (a); concentration of metals in stems and leaves (b). A, B, C—location vicinity: explanations as in Table 1.
4. Discussion
The soils were not contaminated with heavy metals [73] and their content in the parent material was typical for soils (north-central Poland) developed from Baltic glacial sediments [74]. However, as compared with the contents of the geochemical background of the soils in the region and the value of enrichment factor (EF), there was noted an average content of lead in the soil from location C, an average content of manganese in the soil from location B, and a considerable content of lead in the soil from two locations A and B (Table 5). Enrichment factor is a relatively simple and easy tool to assess the enrichment of elements in soils [58,75,76]. The content of Zna, Cua, Mna and Fea was location-specific. As reported by Mercik 2004 and Ociepa 2011 [77,78], the availability of heavy metals to plants is affected by the parent material, the soil reaction and by the content of organic substance, clay minerals and the interaction with other elements. The total content of respective metals in the soil samples was found in a decreasing series: Fet >> Mnt > Pbt > Znt > Cut.
For the purpose of research, T. serpyllum plants were collected at the flowering stage. The concentration of heavy metals in the plant varies throughout the phenological stages and it is different in different parts: root, leaves, stems and flowers [23,59,79]. In their roots, stems, leaves, and inflorescences, T. serpyllum plants accumulated relatively high contents of Zn and Pb, which is species-specific. T. serpyllum is a species with a natural tolerance to high contents of heavy metals in the environment and it is considered an metallophyte [80]. T. serpyllum is a plant which can grow in soils with a very high content of metals [81,82]. The soil sampling locations were classified as dry psammophytic grasses with sandy soils, easily drying, with an acid reaction [18]. The differences were recorded for the secondary succession period in those stands. With the age of the stands neighbouring with location A and own observations, that phytocenosis was considered the oldest one, with more than 50 years of age. The other two phytocenoses were estimated to be around 30 years of age. It could have determined the richness of the soil in mycorrhizal mycelia and, at the same time, it could have affected the accumulation of metals in plant tissues. Importantly, the village of Łosiny is found on the border of the Tuchola Landscape Park and it is adjacent to the “Jelenia Wyspa” reserve and so it is not a neighbour of any pollution emitters. Nevertheless, in the T. serpyllum plants there was found a relatively high content of Pb and Zn. In the present studies, the Zn content in the cells of the aboveground part of the plants from locations A and C was higher than in the roots.
The average content of zinc in crops ranges from 10–100 mg kg−1 d.w. [83]. However, to meet the physiological requirements of most plants, the content of zinc in stems ranging from 15–30 mg kg−1 is enough [48]. It means that in the T. serpyllum plants acquired from natural locations A and B, the zinc content was higher than average. In all the T. serpyllum plants, the zinc content exceeded their average physiological requirements. Only the herbal material acquired from location C satisfied the conditions provided for in the WHO norm for the herbs applied in herbal medicine according to which the Zn content cannot exceed 50 mg kg−1.
Copper is a metal which is considered indispensable for the plant life and functioning, however, its excess is toxic. It shows a low mobility in the plant and it is mostly accumulated in roots [40]. The content of copper in plants is usually below 4–5 mg kg−1, and its mean content in the aboveground parts of plants ranges from 5 to 20 mg kg−1 [84]. In the T. serpyllum plants under study, a definitely higher Cu content was noted in the roots and the inflorescences.
The content of Zn, Cu, Mn, and Fe in T. serpyllum plants can determine their applicability for herbal medicine. T. serpyllum plants collected as herbal material from location B (considering the average Pb value in the entire plant) are not suitable for herbal medicine applications. The risk of an increased lead content in plants (above 10 mg kg−1 d.w.) makes soil environment monitoring necessary. The collection of that plant from natural stands must be limited and the collection from controlled plantations–increased.
5. Conclusions
The soils studied from the Kuyavia-Pomerania Province (Poland) were not contaminated with heavy metals and their content in the parent material was typical for soils (north-central Poland) developed from Baltic glacial sediments. However, as compared with the contents of the geochemical background of the soils in the region and the value of enrichment factor (EF), there was noted a considerable content of lead in the soil from two locations A (Łosiny) and B (Okole). The content of Zn, Cu, Mn, and Fe in T. serpyllum plants can determine their applicability for herbal medicine. T. serpyllum plants collected as herbal material from location B, when considering the average Pb value in the entire plant, are not suitable for herbal medicine applications. The risk of an increased Pb content in plants (above 10 mg kg−1 d.w.) makes soil environment monitoring necessary. The collection of that plant from natural stands must be limited and the collection from controlled plantations–increased. In their roots, stems, leaves, and inflorescences, the T. serpyllum plants accumulated relatively high contents of Zn and Pb, which is species-specific. A relatively high lead content in the T. serpyllum cells makes it impossible for plants to be used in herbal medicine. The Pb content above 10 mg kg−1 d.w. suggests abandoning the acquisition of herbal material from natural stands. As for the Zn content, only the T. serpyllum plants from the habitat of the youngest phytocenosis from the vicinity of Łochowo (C) satisfied the conditions for herbs allocated to phytotherapy. Special control measures are required for the materials from natural stands and if the permissible heavy metal content norms are exceeded, the collection of the plant must be limited and replaced with a collection from controlled plantations. Thus, we will be working to facilitate combining the effect of enhancing the material quality and the incomes generated from growing herbs with a rational use of natural resources, with the sustainable development of rural areas in mind. The results are preliminary and so further research is required.
Author Contributions
Conceptualization, A.K.S., A.F., M.T.-S., M.K., and K.K.; methodology, A.F., M.T.-S., M.K., and A.K.S.; software, A.F., M.T.-S., and M.K.; validation, A.F., M.K., A.K.S., and M.T.-S.; formal analysis, M.T.-S., A.F., M.K., and K.K.; investigation A.K.S., A.F., M.K., and M.T.-S.; resources, A.K.S., A.F., M.K., and M.T.-S.; data curation, M.T.-S. and A.F.; writing—original draft preparation, A.K.S., A.F., M.T.-S., and M.K.; writing—review and editing A.F., M.T.-S., A.K.S., M.K., and K.K.; visualization, A.F., M.T.-S., M.K., and K.K.; supervision, A.F.; project administration, A.F. and A.K.S.; funding acquisition, M.T.-S., A.K.S., and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsangd, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Muchuweti, M.; Birkett, J.W.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J.N. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef]
- Perrino, E.V.; Brunetti, G.; Farrag, K. Plant communities of multi-metal contaminated soils: A case study in the National Park of Alta Murgia (Apulia Region - southern Italy). J. Phytoremediat. 2014, 16, 871–888. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kita, A.; Janoska, P.; Połowniak, M.; Kozik, V. Multi-element analysis of mineral and trace elements in medicinal herbs and their infusions. Food Chem. 2012, 135, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Arceusz, A.; Wesolowski, M. Essential metals and phenolic acids in commercial herbs and spices. Multivariate analysis of correlations among them. Open Chem. 2015, 13, 1196–1208. [Google Scholar] [CrossRef]
- Ducat, G.; Torres, R.Y.; Santa, H.S.D.; Kaminski, C.I.; Kleinubing, S.A.; Stock, D.; Tussolini, L.; Justo, T.H.; Quinaia, S.P. Correlation among metallic ions, phenolic compounds and antimicrobial action in medicinal plants extracts. J. Food Qual. 2011, 34, 306–314. [Google Scholar] [CrossRef]
- Bolan, S.; Kunhikrishnan, A.; Seshadri, B.; Choppala, G.; Naidu, R.; Bolan, N.S.; Ok, Y.S.; Zhang, M.; Li, C.G.; Li, F.; et al. Sources, distribution, bioavailability, toxicity, and risk assessment of heavy metal(loid)s in complementary medicines. Environ. Int. 2017, 108, 103–118. [Google Scholar] [CrossRef]
- Farmakopea Europejska/European Pharmacopoeia 10.0; Komisja Farmakopei Europejskiej: Strasburg, France, 2020; Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 1 January 2020).
- WHO—World Health Organization. Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO Press, World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Farmakopea Polska/Polish Pharmacopoeia; X; Urząd Rejestracji Produktów Leczniczych: Warszawa, Poland, 2014; ISBN 978-83-63724-47-4.
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Rośliny Polskie; PWN: Warszawa, Poland, 1953. [Google Scholar]
- Jalas, J. Thymus L. In Flora Europaea 3; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, S.M., Walters, A.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; pp. 172–182. [Google Scholar]
- Henslowa, M. Z badań nad wiedzą ludową o roślinach. II. Thymus serpyllum L.—macierzanka piaskowa. III. Ruta graveolens L.—Ruta zwyczajna. Slavia Antiq. 1977, 24, 163–212. [Google Scholar]
- Fiedoruk, Ł.; Mazik, M.; Pastwa, M. Encyklopedia Ziół; Wydawnictwo Dragon: Bielsko-Biała, Poland, 2012; pp. 124–125, 180–181. [Google Scholar]
- Rutkowski, L. Klucz Do Oznaczania Roślin Naczyniowych Polski Niżowej; PWN: Warszawa, Poland, 2019; pp. 397–398. [Google Scholar]
- Matuszkiewicz, W. Przewodnik Do Oznaczania Zbiorowisk Roślinnych Polski; PWN: Warszawa, Poland, 2011. [Google Scholar]
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Rośliny Polskie. Opisy i Klucze Do Oznaczania Wszystkich Gatunków Roślin Naczyniowych Rosnących w Polsce Bądź Dziko, Bądź Też Zdziczałych Lub Częściej Hodowanych; PWN: Warszawa, Poland, 1969. [Google Scholar]
- Gortat, M. Macierzanka piaskowa (Thymus serpyllum L.) i tymianek pospolity (Thymus vulgaris L.)—źródło surowca dla przemysłu zielarskiego i właściwości lecznicze. Cz. I. In Nauka Dla Rozwoju Rolnictwa, Ekologii i Medycyny w Świetle Współczesnych Osiągnięć; Gortat, M., Ed.; Stowarzyszenie Studentów Nauk Przyrodniczych: Lublin, Poland, 2015; pp. 31–44. [Google Scholar]
- Prus, P. Sustainable Farming Production and its Impact on the Natural Environment—Case Study Based on a Selected Group of Farmers. In Proceedings of the 8th International Scientific Conference Rural Development, Aleksandras Stulginskis University, Lithuania, 23–24 November 2017; pp. 1280–1285. [Google Scholar] [CrossRef]
- Murawska, A.; Prus, P. The Progress of Sustainable Management of Ammonia Emissions from Agriculture in European Union States Including Poland—Variation, Trends, and Economic Conditions. Sustainability 2021, 13, 1035. [Google Scholar] [CrossRef]
- Tomaszewska-Sowa, M.; Kobierski, M.; Sawilska, A.K.; Figas, A. Assessment of phytoaccumulation of trace elements in medicinal plants from natural habitats. Herba Pol. 2018, 64, 11–19. [Google Scholar] [CrossRef]
- Bączek, K. Ekologiczny Zbiór Dziko Rosnących Roślin Leczniczych i ich Obróbka Pozbiorcza-Materiały Szkoleniowe; Instytut Nauk Ogrodniczych, SGGW: Warszawa, Poland, 2020. [Google Scholar]
- Łuczaj, Ł. Dzikie Rośliny Jadalne Polski—Przewodnik Survivalowy/Wild Edible Plants of Poland; Wydawnicwo Chemigrafia: Krosno, Poland, 2004. [Google Scholar]
- Łuczaj, Ł. Dziko rosnące rośliny jadalne użytkowane w Polsce od połowy XIX w. do czasów współczesnych. Pol. Ethnobiol. 2011, 1, 57–125. [Google Scholar]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss and Reut and Thymus vulgaris L. essential oils. Ind. Crop. Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Kirillov, V.; Stikhareva, T.; Mukanov, B.; Chebotko, N.; Ryazantsev, O.; Atazhanova, G.; Adekenov, S. Composition of the essential oil of Thymus serpyllum L. from Northern Kazakhstan. J. Essent. Oil Bear. Plants 2016, 19, 212–222. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regionsof Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sokovic, M.; Marin, P.D.; Brkic, D.; van Griensven, L.J.L.D. Chemical composition and antibacterial activity of essential oils against human pathogenic bacteria. Food 2008, 1, 220–226. [Google Scholar]
- Sokovic, M.D.; Vukojevic, J.; Marin, P.D.; Brkic, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical composition of essential oils of Thymus and Mentha speciesand their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Salehi-Fathabadi, Z.; Maghsoudlou, Y.; Akhavan, H.; Moayedi, A.; Khorasani, S. The assessment of the effect of Aloe vera gel coating containing salicylic acid and thyme extract on the shelf life of fresh pistachios during storage. FSCT 2019, 16, 297–312. [Google Scholar]
- Jadczak, D.; Grzeszczuk, M. Tymianek. Panacea 2006, 3, 30–31. [Google Scholar]
- Aziz, S.; Rehman, H. Studies on the chemical constituents of Thymus serpyllum. Turk. J. Chem. 2008, 32, 605–614. [Google Scholar]
- Treben, M. Apteka Pana Boga; Wydawnictwo Ex Libris: Warszawa, Poland, 2013; p. 120. [Google Scholar]
- Gortat, M. Macierzanka piaskowa (Thymus serpyllum L.) i tymianek pospolity (Thymus vulgaris L.)—źródło surowca dla przemysłu zielarskiego i właściwości lecznicze. Cz. II. In Nauka Dla Rozwoju Rolnictwa, Ekologii i Medycyny w Świetle Współczesnych Osiągnięć; Gortat, M., Ed.; Stowarzyszenie Studentów Nauk Przyrodniczych: Lublin, Poland, 2015; pp. 45–54. [Google Scholar]
- Wesołowska, A.; Grzeszczuk, M.; Jadczak, D.; Nawrotek, P.; Struk, M. Comparison of the chemical composition and antimicrobial activity of Thymus serpyllum essential oils. Not. Bot. Horti Agrobot. 2015, 43, 432–438. [Google Scholar] [CrossRef]
- Rehman, A.; Mannan, A.; Inayatullah, S.; Akhtar, M.Z.; Qayyum, M.; Mirza, B. Biological evaluation of wild thyme (Thymus serpyllum). Pharm. Biol. 2009, 47, 628–633. [Google Scholar] [CrossRef]
- Djukic-Cosic, D.; Curcic, M.; Cmiljanovic, M.; Vasovic, I.; Matovic, V. Heavy metal contents in samples of Hypericum and Thymus spec. collected from different mountain areas in Serbia. Planta Med. 2007, 587. [Google Scholar] [CrossRef]
- Cegielska, W.; Michalska-Kacymirow, M.; Wierzbicka, M. Metale ciężkie w środowisku. In Ekotoksykologia. Rośliny, Gleby, Metale; Wierzbicka, M., Ed.; Wydawnictwa UW: Warszawa, Poland, 2015; pp. 22–51. [Google Scholar]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and Plant Community Implication on Essential Oils Composition in Useful Wild Officinal Species: A Pilot Case Study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, Z.; Ebrahimi, M.; Farajpour, M.; Mirza, M.; Ramshini, H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Crop Prod. 2014, 61, 233–239. [Google Scholar] [CrossRef]
- Musielińska, R.; Kowol, J.; Kwapuliński, J.; Rochel, R. Antagonism between lead and zinc ions in plants. Arch. Environ. Prot. 2016, 42, 78–91. [Google Scholar] [CrossRef]
- Visioli, G.; Marmiroli, N. The proteomics of heavy metal hyperaccumulation by plants. J. Proteom. 2013, 79, 133–145. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ociepa-Kubicka, A.; Ociepa, E. Toksyczne oddziaływanie metali ciężkich na rośliny, zwierzęta i ludzi. Inżynieria Ochr. Śr. 2012, 15, 169–180. [Google Scholar]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Sawilska, A.K.; Jendrzejczak, E.; Kieliszewska-Rokicka, B. Influence of mycorrhiza on the growth and flowering in cultivated plants of Helichrysum arenarium (L.) Moench (Asteraceae). Pol. J. Ecol. 2010, 58, 767–774. [Google Scholar]
- Turnau, K.; Ryszka, P.; Wojtczak, G. Metal tolerant mycorrhizal plants: A review from the perspective on industrial waste in temperate region. In Arbuscular Mycorrhizas: Physiology And Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 257–276. [Google Scholar]
- Blackwell, M. Terrestrial life: Fungal from the start? Science 2000, 289, 1884–1885. [Google Scholar] [CrossRef] [PubMed]
- Koide, R. The Effect of VA mycorrhizal infection and phosphorus status on sunflower hydraulic and stomatal properties. J. Exp. Bot. 1985, 36, 1087–1098. [Google Scholar] [CrossRef]
- Fitter, A.H. Water relations of red clover Trifolium pratense L. as affected by VA mycorrhizal infection and phosphorus supply before and during drought. J. Exp. Bot. 1988, 39, 595–603. [Google Scholar] [CrossRef]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Przedpełska-Wąsowicz, E.M. Hiperakumulatory metali ciężkich. In Ecotoxicology: Plants, Soils, Metals; Wierzbicka, M., Ed.; Wydawnictwa UW: Warszawa, Poland, 2015; pp. 428–449. [Google Scholar]
- Branquinho, C.; Serrano, H.C.; Pinto, M.J.; Martins-Loução, M.A. Revisiting the plant hyperaccumulation criteria to rare plants and earth abundant elements. Environ. Pollut. 2007, 146, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Kobierski, M.; Tomaszewska-Sowa, M.; Figas, A.; Gatz, A.; Sawilska, A.K. Bioaccumulation of heavy metals in herbal plants from areas not exposed to heavy anthropopressure. Pol. J. Soil Sci. 2017, 50, 41–53. [Google Scholar] [CrossRef]
- Figas, A.; Tomaszewska-Sowa, M.; Sawilska, A.K.; Kobierski, M. Assessment the Phytoaccumulation of Trace Elements in Plants of Evening Primrose Oenothera biennis L. from Kuyavia-Pomerania Provinces (Poland). In Infrastructure and Environment; Springer: Cham, UK, 2019; pp. 252–259. [Google Scholar] [CrossRef]
- ISO 10390:2005. Soil Quality–Determination of pH. International Organization for Standardization; Geneva, Switzerland. 2005. Available online: https://www.iso.org/standard/40879.html (accessed on 1 February 2005).
- Crock, J.G.; Severson, R. Four reference soil and rock samples for measuring element availability in the western energy regions. U.S. Geol. Surv. Circ. 1980, 841, 16. [Google Scholar]
- Gediga, K.; Spiak, Z.; Piszcz, U.; Bielecki, K. Suitability of different soil extractants for determination of available Cu and Mn contents in Polish soils. Commun. Soil Sci. Plant Anal. 2015, 46, 81–93. [Google Scholar] [CrossRef]
- Gąsior, J.; Kaniuczak, J.; Hajduk, E.; Właśniewski, S.; Nazarkiewicz, M. Analytical methods for physico-chemical soil properties. Acta Carpathica 2014, 14, 1–51. [Google Scholar]
- Smoleń, S.; Sady, W.; Ledwożyw-Smoleń, I. Quantitative relations between the content of selected trace elements in soil extracted with 0.03 M CH3COOH or 1 M HCl and its total concentration in lettuce and spinach. Acta. Sci. Hortorum Cultus 2010, 9, 13–23. [Google Scholar]
- Korzeniowska, J.; Stanisławska-Glubiak, E. Comparison of 1 M HCl and Mehlich 3 for assessment of the micronutrient status of polish soils in the context of winter wheat nutritional demands. Commun. Soil Sci. Plant Anal. 2015, 46, 1263–1277. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanisławska-Glubiak, E.; Lipiński, W. New limit values of micronutrient deficiency in soil determined using 1 M HCl extractant for wheat and rapeseed. Soil Sci. Annual. 2020, 71, 205–214. [Google Scholar] [CrossRef]
- StatSoft Inc. OK, USA, STATISTICA, Version 12.0. Data Analysis Software System. 2012. Available online: https://statisticasoftware.wordpress.com/2013/05/15/statsoft-releases-version-12-of-statistica-software (accessed on 7 May 2013).
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Wilding, L.P. Spatial variability: Its documentation, accommodation and implication to soil surveys. In Soil Spatial Variability; Nielsen, D.R., Bouma, J., Eds.; Pudoc: Wageningen, The Netherlands, 1985; pp. 166–194. [Google Scholar]
- Martin, J.M.; Meybeck, M. Elemental mass-balance of material carried by major world rivers. Mar. Chem. 1979, 7, 173–206. [Google Scholar] [CrossRef]
- Sutherland, R.A.; Tolosa, C.A.; Tack, F.M.G.; Verloo, M.G. Characterization of Selected Element Concentration and Enrichment Ratios in Background and Anthropogenically Impacted Roadside Areas. AECT 2000, 38, 428–438. [Google Scholar] [CrossRef]
- USDA (United States Department of Agriculture). Soil Mechanics Level I-Module 3: USDA Textural Classification Study Guide; National Employee Development Staff; Soil Conservation Service: Washington, DC, USA, 1987. [Google Scholar]
- Journal of Laws, Item 1395. Regulation of the Minister of the Environment of 1 September 2016 on the Method for Assessment of Land Surface Contamination. 2016. Available online: www.gdos.gov.pl (accessed on 5 September 2016).
- Kobierski, M.; Dąbkowska-Naskręt, H. Local background concentration of heavy metals in various soil types formed from glacial till of the Inowrocławska Plain. J. Elem. 2012, 17, 559–585. [Google Scholar] [CrossRef]
- Vega, F.A.; Covelo, E.F.; Cerqueira, B.; Andrade, M.L. Enrichment of marsh soils with heavy metals by effect of anthropic pollution. J. Hazard. Mater. 2009, 170, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 237. [Google Scholar] [CrossRef]
- Mercik, S. Chemia Rolna; Wydawnictwo SGGW: Warszawa, Poland, 2004. [Google Scholar]
- Ociepa, E. The effect of fertilization on yielding and heavy metals uptake by maize and virgina fanpetals (Sida Hermaphrodita). Arch. Environ. Prot. 2011, 37, 123–129. [Google Scholar]
- Ali, A.; Badshah, L.; Hussain, F. Screening of five plant species for macro/micro nutrients and heavy metals at various phenological stages. Pak. J. Bot. 2018, 50, 1941–1949. [Google Scholar]
- Rostański, A.; Nowak, T.; Jędrzejczyk-Korycińska, M. Metalolubne gatunki roślin naczyniowych we florze Polski. In Ecotoxicology: Plants, Soils, Metals; Wierzbicka, M., Ed.; Wydawnictwa UW: Warszawa, Poland, 2015; pp. 297–322. [Google Scholar]
- Lucassen, E.C.H.E.T.; Eygensteyn, J.; Bobbink, R.; van de Riet, B.P.; Smolders, A.J.P.; Kuijpers, D.J.C.; Roelofs, J.G.M. The decline of metallophyte vegetation in floodplain grasslands: Implications for conservation and restoration. Appl. Veget. Sci. 2009, 12, 69–80. [Google Scholar] [CrossRef]
- Lucassen, E.C.H.E.T.; van Kempen, M.M.L.; Roelofs, J.G.M.; van der Velde, G. Decline in metallophytes in tertiary polluted floodplain grasslands in the Netherlands: Experimental evidence for metal and nutritional changes in soil as driver factors. Chem. Ecol. 2010, 26, 273–287. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Zinc. Environmental Health Criteria; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemia Pierwiastków Śladowych; PWN: Warszawa, Poland, 1999; pp. 329–336. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).