Vegetation Response to Removal of Plant Groups and Grass Seeding in a Microphyllous Desert Shrubland: A 4-Year Field Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Species
2.3. Experimental Design
2.4. Field Evaluation
2.5. Statistical Analysis
3. Results
3.1. Plant Density and Cover
3.2. Species Richness, Diversity, Evenness, and Aboveground Forage
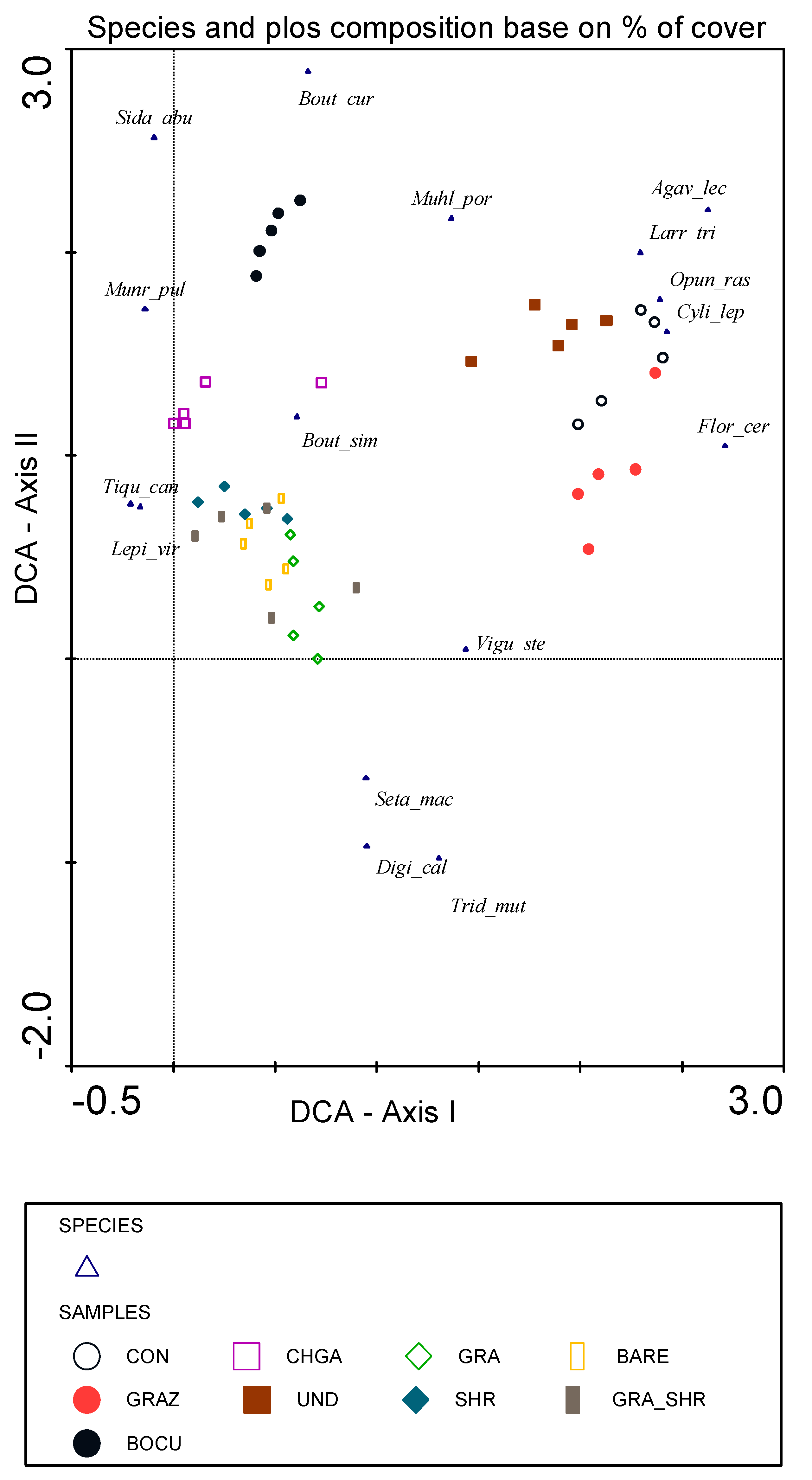
3.3. Species Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Species | Growth Form | Life Cycle | Palatability |
|---|---|---|---|---|
| Asteraceae | Flourensia cernua | Shrub | Perennial | Unpalatable |
| Zygophyllaceae | Larrea tridentata | Shrub | Perennial | Unpalatable |
| Asparagaceae | Agave lechuguilla | Shrub | Perennial | Unpalatable |
| Cactaceae | Cylindropuntia leptocaulis | Shrub | Perennial | Unpalatable |
| Asteraceae | Viguiera stenoloba | Shrub | Perennial | Unpalatable |
| Cactaceae | Opuntia rastrera | Shrub | Perennial | Palatable |
| Malvaceae | Sida abutifolia | Forb | Perennial | Palatable |
| Brassicaceae | Lepidium virginicum | Forb | Annual | Unpalatable |
| Boraginaceae | Tiquilia canescens | Forb | Perennial | Unpalatable |
| Poaceae | Chloris gayana | Graminoid | Perennial | Palatable |
| Poaceae | Muhlenbergia porteri | Graminoid | Perennial | Palatable |
| Poaceae | Digitaria californica | Graminoid | Perennial | Palatable |
| Poaceae | Setaria macrostachya | Graminoid | Perennial | Palatable |
| Poaceae | Tridens muticus | Graminoid | Perennial | Palatable |
| Poaceae | Bouteloua simplex | Graminoid | Annual | Palatable |
| Poaceae | Munroa pulchella | Graminoid | Perennial | Palatable |
| Poaceae | Bouteloua curtipendula | Graminoid | Perennial | Palatable |
References
- Svejcar, T.; Boyd, C.; Davies, K.; Hamerlynck, E.; Svejcar, L. Challenges and limitations to native species restoration in the Great Basin, USA. Plant Ecol. 2017, 218, 81–94. [Google Scholar] [CrossRef]
- Jakoby, O.; Quaas, M.F.; Baumgärtner, S.; Frank, K. Adapting livestock management to spatio-temporal heterogeneity in semi-arid rangelands. J. Environ. Manag. 2015, 162, 179–189. [Google Scholar] [CrossRef]
- Goheen, J.R.; Palmer, T.sM.; Keesing, F.; Riginos, C.; Young, T.P. Large herbivores facilitate savanna tree establishment via diverse and indirect pathways. J. Anim. Ecol. 2010, 79, 372–382. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Fynn, R.W.S.; McGranahan, D.A.; Twidwell, D. Heterogeneity as the basis for rangeland management. In Rangeland Systems; Briske, D.D., Ed.; Springer Series on Environmental Management; Springer: Cham, Switzerland, 2017; pp. 169–196. [Google Scholar]
- Enloe, S.F.; DiTomaso, J.M.; Orloff, S.B.; Drake, D.J. Soil water dynamics differ among rangeland plant communities dominated by yellow starthistle (Centaurea solstitialis), annual grasses, or perennial grasses. Weed Sci. 2004, 52, 929–935. [Google Scholar] [CrossRef]
- Jankju, M. Role of nurse shrubs in restoration of an arid rangeland: Effects of microclimate on grass establishment. J. Arid Environ. 2013, 89, 103–109. [Google Scholar] [CrossRef]
- Munson, S.M.; Lauenroth, W.K. Plant population and community responses to removal of dominant species in the shortgrass steppe. J. Veg. Sci. 2009, 20, 224–232. [Google Scholar] [CrossRef]
- Sala, O.E.; Golluscio, R.A.; Lauenroth, W.K.; Soriano, A. Resource partitioning between shrubs and grasses in the Patagonian steppe. Oecologia 1989, 81, 501–505. [Google Scholar] [CrossRef]
- Aguiar, M.R.; Sala, O.E. Competition, facilitation, seed distribution and the origin of patches in a Patagonian steppe. Oikos 1994, 70, 26–34. [Google Scholar] [CrossRef]
- Fredrickson, E.L.; Estell, R.E.; Laliberte, A.; Anderson, D.M. Mesquite recruitment in the Chihuahuan Desert: Historic and prehistoric patterns with long-term impacts. J. Arid Environ. 2006, 65, 285–295. [Google Scholar] [CrossRef]
- Ndhlovu, T.; Milton-Dean, S.J.; Esler, K.J. Impact of Prosopis (mesquite) invasion and clearing on the grazing capacity of semiarid Nama Karoo rangeland, South Africa. Afr. J. Rangel. Forage Sci. 2011, 28, 129–137. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, G.; Zhuang, Q.; Shimizu, H. Long-Term elimination of grazing reverses the effects of shrub encroachment on soil and vegetation on the Ordos Plateau. J. Geophys Res. Biogeosci. 2020, 125, e2019JG005439. [Google Scholar] [CrossRef]
- Norbury, G.; Byrom, A.; Pech, R.; Smith, J.; Clarke, D.; Anderson, D.; Forrester, G. Invasive mammals and habitat modification interact to generate unforeseen outcomes for indigenous fauna. Ecol. Appl. 2013, 23, 1707–1721. [Google Scholar] [CrossRef]
- Sánchez-Arroyo, J.F.; Wehenkel, C.; Carrete-Carreón, F.; Murillo-Ortiz, M.; Herrera-Torres, E.; Quero-Carrillo, A.R. Establishment attributes of Bouteloua curtipendula (Michx.) Torr. populations native to Mexico. Rev. Fitotec. Mex. 2018, 41, 237–243. [Google Scholar] [CrossRef]
- Herbel, C.H.; Abernathy, G.H.; Yarbrough, C.C.; Gardner, D.K. Rootplowing and seeding arid rangelands in the southwest. J. Rangel. Manag. 1973, 26, 193–197. [Google Scholar] [CrossRef]
- Cruz Campa, J.A.D.L.; Barragán, Z. Campo Experimental Forestal de Zonas Áridas “La Sauceda”, Ramos Arizpe, Coah: Líneas de Investigación y Resultados; Secretaría de Agricultura y Ganadería, Subsecretaría Forestal y de la Fauna, Instituto Nacional de Investigaciones Forestales: Jiutepec, Mexico, 1974; p. 75. [Google Scholar]
- Rodríguez-Arvizu, M.; Rojas-Montes, C.; Esquivel-Romo, A.; Moreno-Jalpa, H.; Abdiel Soto-Pérez, A.N.D. Sistema de Monitoreo de Agostaderos y Pastizales. Available online: https://sites.google.com/a/sima-coahuila.com/agostaderos-de-coahuila/2--coahuila/capacidad-de-carga/mapa (accessed on 12 November 2020).
- Brower, J.E.; Zar, J.H.; von Ende, C.N. Field and Laboratory Methods for General Ecology, 4th ed.; WCB Inc. McGraw-Hill: Boston, MA, USA, 1998. [Google Scholar]
- Smith, B.; Bastow, W. A consumer’s guide to evenness indices. Oikos 1996, 76, 70–82. [Google Scholar] [CrossRef]
- Gauch, H.G., Jr. Multivariate Analysis in Community Ecology; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows, Software for Canonical Community Ordination (Version 4); Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Yurkonis, K.A. Can we reconstruct grasslands to better resist invasion? Ecol. Restor. 2013, 31, 120–123. [Google Scholar] [CrossRef]
- Lair, K.; Redente, E.F. Influence of auxin and sulfonylurea herbicides on seeded native communities. J. Rangel. Manag. 2004, 57, 211–218. [Google Scholar] [CrossRef]
- Seahra, S.E.; Yurkonis, K.A.; Newman, J.A. Species patch size at seeding affects diversity and productivity responses in establishing grasslands. J. Ecol. 2016, 104, 479–486. [Google Scholar] [CrossRef]
- Seahra, S.; Yurkonis, K.A.; Newman, J.A. Structured perennial grassland seeding promotes species establishment and invasion resistance. Restor. Ecol. 2019, 27, 82–91. [Google Scholar] [CrossRef]
- Morales-Nieto, C.R.; Álvarez-Holguín, A.; Santellano-Estrada, E.; Villarreal-Guerrero, F.; Corrales-Lerma, R. Reducing Eragrostis lehmanniana population by preparing seedbeds with unconventional tillage implements and seeding in a semiarid grassland. Invasive Plant Sci. Manag. 2020, 13, 266–275. [Google Scholar] [CrossRef]
- Quero-Carrillo, A.R.; Hernández-Guzmán, F.J.; Velázquez-Martínez, M.; Gámez-Vázquez, H.G.; Landa-Salgado, P.; Aguilar-López, P. Methods for pasture establishment in arid zones of Mexico using crude seeds or caryopses. Trop. Grassl. 2016, 4, 29–37. [Google Scholar] [CrossRef][Green Version]
- Young, S.L.; Barney, J.N.; Kyser, G.B.; Jones, T.S.; DiTomaso, J.M. Functionally similar species confer greater resistance to invasion: Implications for grassland restoration. Restor. Ecol. 2009, 17, 884–892. [Google Scholar] [CrossRef]
- Oakley, C.A.; Knoz, J.S. Plant species richness increases resistance to invasion by non-resident plant species during grassland restoration. Appl. Veg. Sci. 2013, 16, 21–28. [Google Scholar] [CrossRef]
- Bartomeus, I.; Sol, D.; Pino, J.; Vicente, P.; Font, X. Deconstructing the native-exotic richness relationship in plants. Glob. Ecol. Biogeogr. 2012, 21, 524–533. [Google Scholar] [CrossRef]
- Nemec, K.T.; Allen, C.R.; Helzer, C.J.; Wedin, D.A. Influence of richness and seeding density on invasion resistance in experimental tallgrass prairie restorations. Ecol. Restor. 2013, 31, 168–185. [Google Scholar] [CrossRef]
- Staub, J.; Chatterton, J.; Bushman, S.; Johnson, D.; Jones, T.; Larson, S.; Robins, J.; Monaco, T. A history of plant improvement by the usda-ars forage and range research laboratory for rehabilitation of degraded Western U.S. rangelands. Rangelands 2016, 38, 233–240. [Google Scholar] [CrossRef]
- Hardegree, S.P.; Jones, T.A.; Roundy, B.A.; Shaw, N.L. Assessment of range planting as a conservation practice. In Conservation Benefits of Rangeland Practices Assessment, Recommendations, and Knowledge Gaps; Briske, D.D., Ed.; United States Department of Agriculture, Natural Resources Conservation Service: Lawrence, KS, USA, 2016. [Google Scholar]
- James, J.J.; Davies, K.W.; Sheley, R.L.; Aanderud, Z.T. Linking nitrogen partitioning and species abundance to invasion resistance in the Great Basin. Oecologia 2008, 156, 637–648. [Google Scholar] [CrossRef]
- Stonecipher, C.A.; Panter, K.E.; Jensen, K.B.; Rigby, C.W.; Villalba, J.J. Revegetation of medusahead-invaded rangelands in the Channeled Scablands of Eastern Washington. Rangel. Ecol. Manag. 2017, 70, 388–395. [Google Scholar] [CrossRef]
- Snyman, H.A. Revegetation of bare patches in a semi-arid rangeland of South Africa: An evaluation of various techniques. J. Arid Environ. 2003, 55, 417–432. [Google Scholar] [CrossRef]
- Ruthven, D.C.; Fulbright, T.E.; Beasom, S.L.; Hellgren, E.C. Long-term effects of root plowing on vegetation in the eastern south Texas plains. J. Rangel. Manag. 1993, 461, 351–354. [Google Scholar] [CrossRef][Green Version]
- Mellado, M.; Foote, H.; Rodriguez, A.; Zarate, P. Botanical composition and nutrient content of diets selected by goats grazing on desert grassland in northern Mexico. Small Rumin. Res. 1991, 6, 141–150. [Google Scholar] [CrossRef]
- Morton, H.L.; Ibarra, F.A.; Martin, M.H.; Cox, J.R. Creosotebush control and forage production in the Chihuahuan and Sonoran Deserts. J. Rangel. Manag. 1990, 43, 43–48. [Google Scholar] [CrossRef]
- Mata-González, R.; Figueroa-Sandoval, B.; Clemente, F.; Manzano, M. Vegetation changes after livestock grazing exclusion and shrub control in the southern Chihuahuan Desert. West. North Am. Nat. 2007, 67, 63–70. [Google Scholar] [CrossRef]
- Brock, J.; Brandau, B.; Arthun, D.; Humphrey, A.L.; Domínguez, G.; Jacobs, A. Long-term results of tebuthiuron herbicide treatment on creosote bush (Larrea tridentata) in southeast Arizona, USA. J. Arid Environ. 2014, 110, 44–46. [Google Scholar] [CrossRef]
- Valone, T.J.; Sauter, P. Effects of long-term cattle exclosure on vegetation and rodents at a desertified aridgrassland site. J. Arid Environ. 2005, 61, 161–170. [Google Scholar] [CrossRef]
- Ansley, R.J.; Pinchak, W.E.; Teague, W.R.; Kramp, B.A.; Jones, D.L.; Jacoby, P.W. Long-term grass yields following chemical control of honey mesquite. J. Rangel. Manag. 2004, 57, 49–57. [Google Scholar] [CrossRef]
- Whitford, W.G.; Nielson, R.; De Soyza, A. Establishment and effects of establishment of creosotebush, Larrea tridentata, on a Chihuahuan Desert watershed. J. Arid Environ. 2001, 47, 1–10. [Google Scholar] [CrossRef]
- Bilotta, G.S.; Brazier, R.E.; Haygarth, P.M. The impacts of grazing animals on the quality of soils, vegetation, and surface waters in intensively managed grasslands. Adv. Agronom. 2007, 94, 237–280. [Google Scholar]
- Tessema, Z.K.; de Boer, W.F.; Baars, R.M.T.; Prins, H.H.T. Changes in soil nutrients, vegetation structure and herbaceous biomass in response to grazing in a semi-arid savanna of Ethiopia. J. Arid Environ. 2011, 75, 662–670. [Google Scholar] [CrossRef]
- Xu, L.; Freitas, S.M.A.; Yu, F.H.; Dong, M.; Anten, N.P.R.; Werger, M.J.A. Effects of trampling on morphological and mechanical traits of dryland shrub species do not depend on water availability. PLoS ONE 2013, 8, e53021. [Google Scholar] [CrossRef]
- Ondier, J.O.; Okach, D.O.; Onyango, J.C.; Otieno, D.O. Interactive influence of rainfall manipulation and livestock grazing on species diversity of the herbaceous layer community in a humid savannah in Kenya. Plant Divers. 2019, 41, 198–205. [Google Scholar] [CrossRef]
- Gibbens, R.P.; Lenz, J.M. Root systems of some Chihuahuan Desert plants. J. Arid Environ. 2001, 49, 221–263. [Google Scholar] [CrossRef]
- Defalco, L.A.; Esque, T.C.; Nicklas, M.B.; Kane, J.M. Supplementing seed banks to rehabilitate disturbed Mojave Desert shrublands: Where do all the seeds go? Restor. Ecol. 2012, 20, 85–94. [Google Scholar] [CrossRef]
- Kramer, D.W.; Sorensen, G.E.; Taylor, C.A.; Cox, R.D.; Gipson, P.S.; Cain, J.W. Ungulate exclusion, conifer thinning and mule deer forage in northeastern New Mexico. J. Arid Environ. 2015, 113, 29–34. [Google Scholar] [CrossRef]
- Watson, C.; Reid, N. Herbage response to thinning of eucalypt regrowth. Nat. Res. Manag. 2001, 4, 16–21. [Google Scholar]
- Milchunas, D.G.; Lauenroth, W.K. Quantitative effects of grazing on vegetation and soils over a global range of environments: Ecological Archives M063-001. Ecol. Monogr. 1993, 63, 327–366. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Schell, L.D.; Vanden Heuvel, B. How grazing and soil quality affect native and exotic plant diversity in Rocky Mountain grasslands. Ecol. Appl. 1999, 9, 45–64. [Google Scholar] [CrossRef]
- Pykala, J. Cattle grazing increases plant species density of most species trait groups in mesic seminatural grasslands. Plant Ecol. 2004, 175, 217–226. [Google Scholar] [CrossRef]
- Flores, J.; Jurado, E. Are nurse-protégé interactions more common among plants from arid environments? J. Veg. Sci. 2003, 14, 911–916. [Google Scholar] [CrossRef]
- Howard, K.S.C.; Eldridge, D.J.; Soliveres, S. Positive effects of shrubs on plant species diversity do not change along a gradient in grazing pressure in an arid shrubland. Basic Appl. Ecol. 2012, 13, 159–168. [Google Scholar] [CrossRef]

| Species | Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | BOCU | CHGA | GRAS | GRA-SHR | GRAZ | SHR | BARE | UND | |
| Flourensia cernua | 15.2 ± 5.9 b | 0.3 ± 0.2 a | 0.2 ± 0.2 a | 0.7 ± 0.3 a | 1.0 ± 0.8 a | 15.2 ± 5.2 b | 0.7 ± 0.5 a | 0.8 ± 0.4 a | 7.6 ± 3.1 c |
| Larrea tridentata | 8.7 ± 4.2 b | 0.7 ± 0.4 a | 1.4 ± 1.2 a | 0.6 ± 0.2 a | 0.6 ± 0.4 a | 1.9 ± 2.8 a | 0.3 ± 0.2 a | 0.7 ± 0.3 a | 5.2 ± 4.3 c |
| Agave lechuguilla | 7.3 ± 4.9 b | 0.8 ± 0.3 a | 0.04 ± 0.0 a | 0.2 ± 0.2 a | 0.5 ± 0.4 a | 2.5 ± 2.5 a | 0.2 ± 0.2 a | 0.1 ± 0.1 a | 10.5 ± 5.1 c |
| Cylindropuntia leptocaulis | 4.4 ± 1.7 d | 0.3 ± 0.2 ab | 0.1 ± 0.0 a | 0.5 ± 0.5 ab | 0.5 ± 0.3 ab | 2.4 ± 2.1 bc | 0.7 ± 0.1 ab | 0.1 ± 0.1 a | 3.7 ± 3.2 cd |
| Viguiera stenoloba | 0.01± 0.0 a | 0.1 ± 0.0 ab | 0.1 ± 0.0 ab | 0.7 ± 0.3 cd | 0.4 ± 0.4 ab | 0.7 ± 0.5 cd | 0.3 ± 0.3 ab | 0.5 ± 0.4 bcd | 1.2 ± 1.0 d |
| Opuntia rastrera | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.1 a | 1.2 ± 1.6 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.1 a |
| Sida abutifolia | 0.2 ± 0.1 b | 3.5 ± 2.0 a | 1.0 ± 0.7 b | 0.5 ± 0.2 b | 0.3 ± 0.3 b | 0.1 ± 0.0 b | 0.5 ± 0.3 b | 0.5 ± 0.3 b | 0.3 ± 0.3 b |
| Lepidium virginicum | 1.4 ± 1.2 cd | 4.5 ± 1.0 bc | 4.4 ± 1.8 bc | 3.8 ± 1.3 bc | 9.8 ± 5.2 a | 0.3 ± 0.2 d | 5.7 ± 4.0 b | 4.7 ± 1.1 bc | 1.4 ± 1.0 cd |
| Tiquilia canescens | 1.5 ± 1.1 cd | 5.2 ± 1.6 bc | 5.2 ± 2.4 bc | 4.2 ± 1.3 bc | 12.2 ± 6.1 a | 0.6 ± 0.3 d | 6.4 ± 3.5 b | 4.9 ± 1.4 cb | 1.8 ± 0.8 cd |
| Muhlenbergia porteri | 1.3 ± 0.9 a | 0.8 ± 1.1 ab | 0.5 ± 0.2 ab | 0.2 ± 0.3 b | 1.3 ± 0.7 a | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.3 ± 0.2 b | 0.8 ± 0.6 ab |
| Digitaria californica | 0.6 ± 0.5 cb | 0.1 ± 0.1 c | 0.1 ± 0.0 c | 2.2 ± 0.8 a | 2.3 ± 1.2 a | 1.0 ± 0.5 b | 1.0 ± 0.0 c | 0.2 ± 0.2 c | 0.1 ± 0.0 c |
| Setaria macrostachya | 1.3 ± 0.9 c | 0.7 ± 0.6 c | 0.3 ± 0.1 c | 8.3 ± 4.0 a | 5.4 ± 3.6 ab | 2.4 ± 0.7 bc | 1.4 ± 0.8 c | 5.1 ± 3.1 b | 4.8 ± 1.7 b |
| Tridens muticus | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.9 ± 0.7 | 1.5 ± 3.0 | 0.7 ± 0.3 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| Bouteoloua simplex | 0.1 ± 0.0 | 0.2 ± 0.2 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.5 ± 0.6 | 0.1 ± 0.0 | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.4 ± 0.3 |
| Munroa pulchella | 0.3 ± 0.3 a | 0.6 ± 0.8 a | 5.2 ± 2.3 b | 1.1 ± 0.3 a | 0.2 ± 0.2 a | 0.6 ± 0.3 a | 0.9 ± 0.8 a | 0.9 ± 0.5 a | 0.3 ± 0.5 a |
| Bouteloua curtipendula | 0.1 ± 0.0 c | 19.2 ± 6.1 a | 0.1 ± 0.1 c | 0.1 ± 0.1 c | 0.1 ± 0.1 c | 0.1 ± 0.1 c | 0.0 ± 0.0 c | 0.1 ± 0.0 c | 7.4 ± 4.9 b |
| Others | 0.7 ± 0.5 | 1.3 ± 0.9 | 0.9 ± 0.6 | 1.1 ± 0.6 | 0.8 ± 0.3 | 0.5 ± 0.3 | 0.9 ± 0.7 | 0.6 ± 0.2 | 0.6 ± 0.4 |
| Total | 43.1 ± 7.3 ab | 38.5 ± 3.8 b | 19.8 ± 4.8 d | 24.7 ± 6.1 de | 36.9 ± 4.1 bc | 31.2 ± 6.4 cd | 20.2 ± 4.2 d | 20.0 ± 4.3 d | 46.6 ± 5.8 a |
| Species | Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CON * | BOCU | CHGA | GRAS | GRA-SHR | GRAZ | SHR | BARE | UND | |
| Flourensia cernua | 1.3 ± 0.6 a | 0.3 ± 0.2 b | 1.0 ± 0.7 ab | 1.8 ± 0.7 a | 1.2 ± 0.5 a | 1.4 ± 0.8 a | 1.7 ± 0.5 a | 1.5 ± 0.7 a | 1.2 ± 0.6 a |
| Larrea tridentata | 0.2 ± 0.1 bc | 0.0 ± 0.0 c | 1.0 ± 0.2 a | 0.3 ± 0.1 bc | 0.2 ± 0.1 bc | 0.3 ± 0.1 bc | 0.5 ± 0.3 b | 1.0 ± 0.2 a | 0.5 ± 0.3 b |
| Agave lechuguilla | 0.4 ± 0.3 b | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.2 ± 0.2 bc | 0.0 ± 0.0 c | 0.9 ± 0.2 a | 0.0 ± 0.0 c | 0.1 ± 0.1 c | 0.3 ± 0.2 b |
| Cylindropuntia leptocaulis | 0.4 ± 0.1 cd | 0.7 ± 0.3 bc | 0.1 ± 0.1 d | 1.0 ± 0.4 ab | 0.0 ± 0.0 d | 0.3 ± 0.1 d | 0.4 ± 0.1 cd | 0.8 ± 0.3 ab | 1.1 ± 0.5 a |
| Viguiera stenoloba | 0.1 ± 0.1 a | 0.0 ± 0.0 a | 0.9 ± 0.3 b | 1.0 ± 0.6 b | 0.2 ± 0.2 a | 1.0 ± 0.6 b | 0.2 ± 0.2 a | 0.4 ± 0.1 a | 0.4 ± 0.1 a |
| Opuntia rastrera | 0.1 ± 0.1 bc | 0.1 ± 0.1 b | 0.0 ± 0.0 c | 0.1 ± 0.1 bc | 0.3 ± 0.1 a | 0.3 ± 0.2 a | 0.1 ± 0.1 bc | 0.1 ± 0.1 bc | 0.2 ± 0.2 ab |
| Sida abutifolia | 0.0 ± 0.0 d | 1.0 ± 0.6 a | 0.5 ± 0.1 b | 0.1 ± 0.1 cd | 0.2 ± 0.2 bcd | 0.0 ± 0.0 d | 0.4 ± 0.2 bc | 0.2 ± 0.2 bcd | 0.2 ± 0.2 bcd |
| Lepidium virginicum | 7.0 ± 3.7 cd | 26.0 ± 7.0 a | 17.4 ± 4.3 b | 19.4 ± 5.1 ab | 18.0 ± 4.1 b | 3.2 ± 1.4 d | 21.6 ± 6.3 ab | 25.4 ± 7.4 a | 10.8 ± 1.8 c |
| Tiquilia canescens | 7.8 ± 3.3 c | 23.4 ± 5.7 a | 17.8 ± 7.4 ab | 21.0 ± 9.9 a | 19.2 ± 10.0 ab | 3.4 ± 2.3 c | 19.0 ± 10.3 ab | 24.6 ± 6.3 a | 9.8 ± 3.4 bc |
| Muhlenbergia porteri | 0.1 ± 0.1 a | 0.2 ± 0.2 a | 0.2 ± 0.2 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.2 ± 0.2 a | 1.1 ± 0.7 b | 0.0 ± 0.0 a | 0.3 ± 0.2 a |
| Digitaria californica | 0.1 ± 0.1 d | 0.6 ± 0.5 bc | 0.5 ± 0.4 a | 0.7 ± 0.4 b | 1.6 ± 0.5 a | 0.1 ± 0.1 d | 0.2 ± 0.1 cd | 0.3 ± 0.2 bcd | 0.1 ± 0.1 d |
| Setaria macrostachya | 0.4 ± 0.3 c | 0.8 ± 0.4 c | 0.7 ± 0.4 c | 4.9 ± 2.4 b | 5.9 ± 2.5 ab | 1.4 ± 0.5 c | 7.6 ± 2.4 a | 0.3 ± 0.2c | 1.3 ± 0.4 c |
| Tridens muticus | 0.1 ± 0.1 bc | 0.0 ± 0.0c | 0.1 ± 0.1 bc | 0.1 ± 0.1 bc | 0.4 ± 0.3 bc | 1.0 ± 0.5 a | 0.2 ± 0.2 bc | 1.0 ± 0.5 a | 0.5 ± 0.3 b |
| Bouteloua simplex | 6 ± 2.6 c | 21± 8.4 ab | 26 ± 6.6 a | 25 ± 7.7 a | 27 ± 7.6 a | 24 ± 7.9 a | 25 ± 8.7 a | 27 ± 6.5 a | 14 ± 4.6 bc |
| Munroa pulchella | 3.0 ± 1.4 bc | 1.3 ± 0.3 bc | 5.8 ± 4.7 b | 14.8 ± 7.1 a | 0.8 ± 0.9 bc | 0.1± 0.1 c | 14.4 ± 7.4 a | 2.5 ± 1.6 bc | 3.6 ± 0.9 bc |
| Bouteloua curtipendula | 0.0 ± 0.0b | 16.8 ± 7.2 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 0.1 ± 0.1 b | 0.1 ± 0.1 b | 0.1 ± 0.1 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| Others | 2 ± 0.6 | 2 ± 0.7 | 4 ± 1.3 | 3 ± 1.1 | 2 ± 0.9 | 2 ± 0.8 | 3 ± 1.0 | 1 ± 0.4 | 2 ± 0.8 |
| Total | 92 ± 9 a | 94 ± 11 a | 76 ± 12 b | 88 ± 7 a | 80 ± 17b | 94 ± 8 a | 76 ± 12 b | 86 ± 19 a | 48 ± 14 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellado, M.; Encina-Domínguez, J.A.; García, J.E.; Estrada-Castillón, E.; Arévalo, J.R. Vegetation Response to Removal of Plant Groups and Grass Seeding in a Microphyllous Desert Shrubland: A 4-Year Field Experiment. Agriculture 2021, 11, 322. https://doi.org/10.3390/agriculture11040322
Mellado M, Encina-Domínguez JA, García JE, Estrada-Castillón E, Arévalo JR. Vegetation Response to Removal of Plant Groups and Grass Seeding in a Microphyllous Desert Shrubland: A 4-Year Field Experiment. Agriculture. 2021; 11(4):322. https://doi.org/10.3390/agriculture11040322
Chicago/Turabian StyleMellado, Miguel, Juan A. Encina-Domínguez, José E. García, Eduardo Estrada-Castillón, and José R. Arévalo. 2021. "Vegetation Response to Removal of Plant Groups and Grass Seeding in a Microphyllous Desert Shrubland: A 4-Year Field Experiment" Agriculture 11, no. 4: 322. https://doi.org/10.3390/agriculture11040322
APA StyleMellado, M., Encina-Domínguez, J. A., García, J. E., Estrada-Castillón, E., & Arévalo, J. R. (2021). Vegetation Response to Removal of Plant Groups and Grass Seeding in a Microphyllous Desert Shrubland: A 4-Year Field Experiment. Agriculture, 11(4), 322. https://doi.org/10.3390/agriculture11040322








