A Spectroscopic Approach to Evaluate the Effects of Different Soil Tillage Methods and Nitrogen Fertilization Levels on the Biochemical Composition of Durum Wheat (Triticum turgidum subsp. durum) Leaves and Caryopses
Abstract
1. Introduction
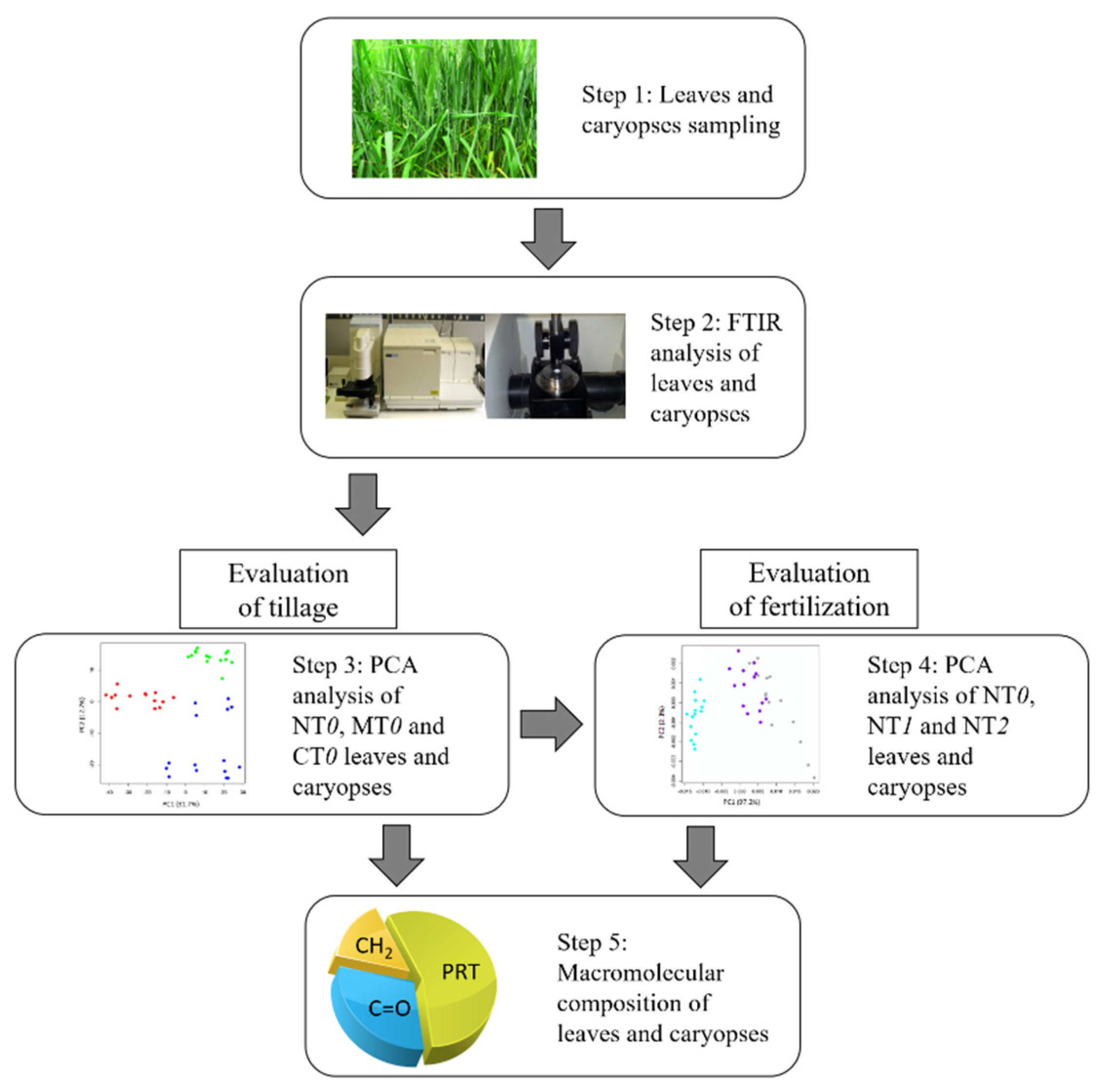
2. Materials and Methods
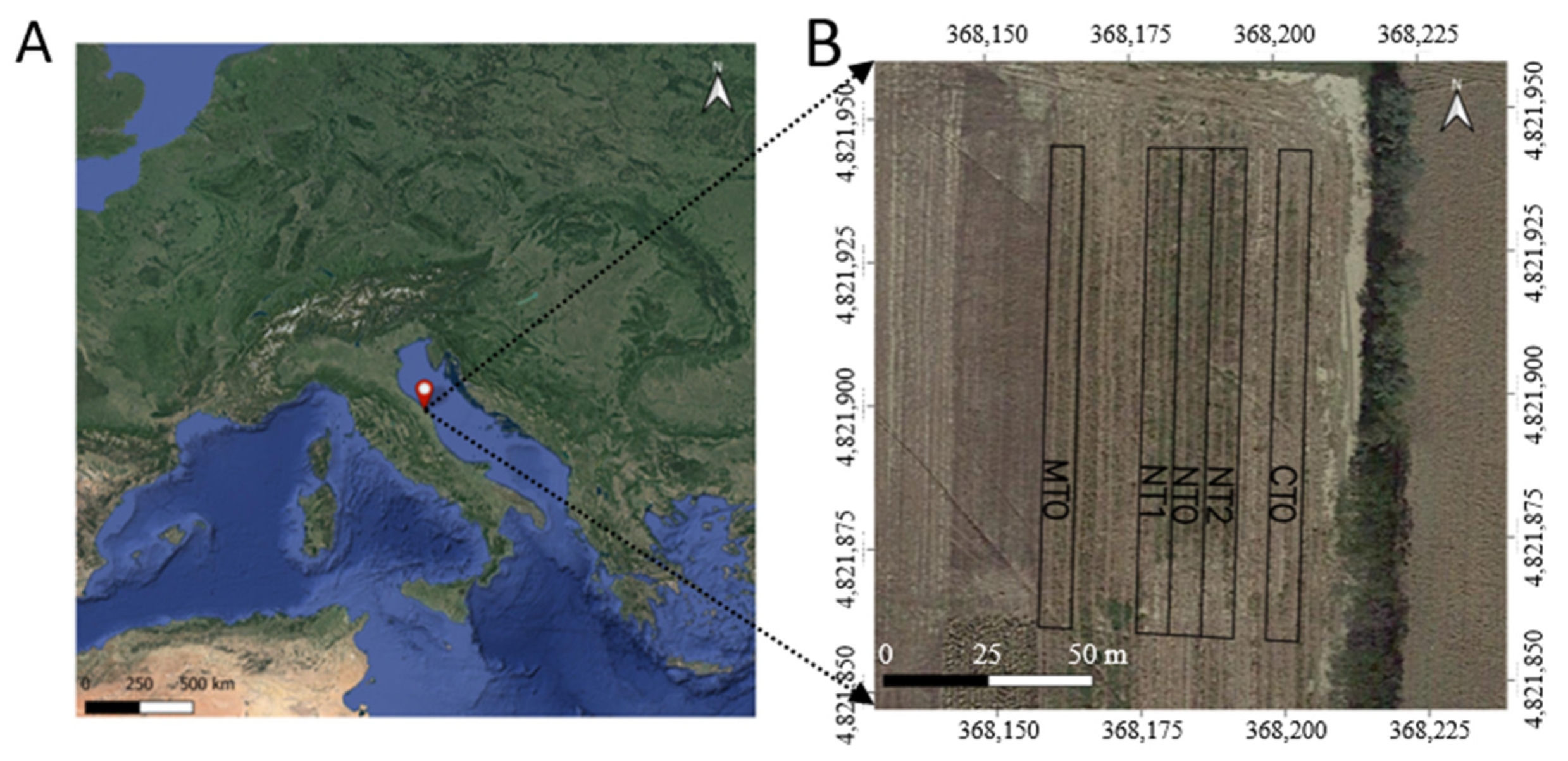
2.1. Experimental Site and Design
2.2. ATR-FTIR Measurements and Data Analysis
2.3. Statistical Analysis
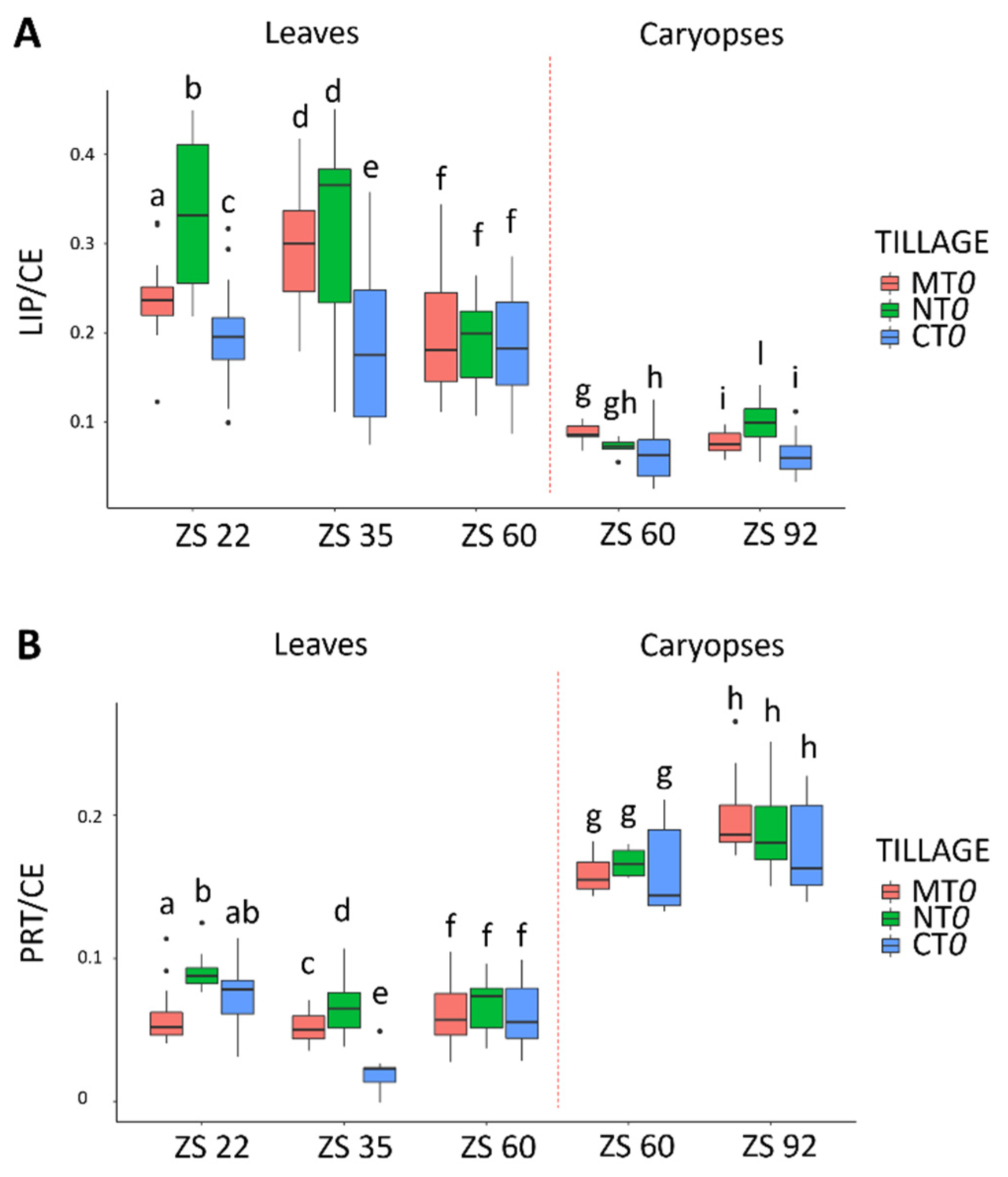
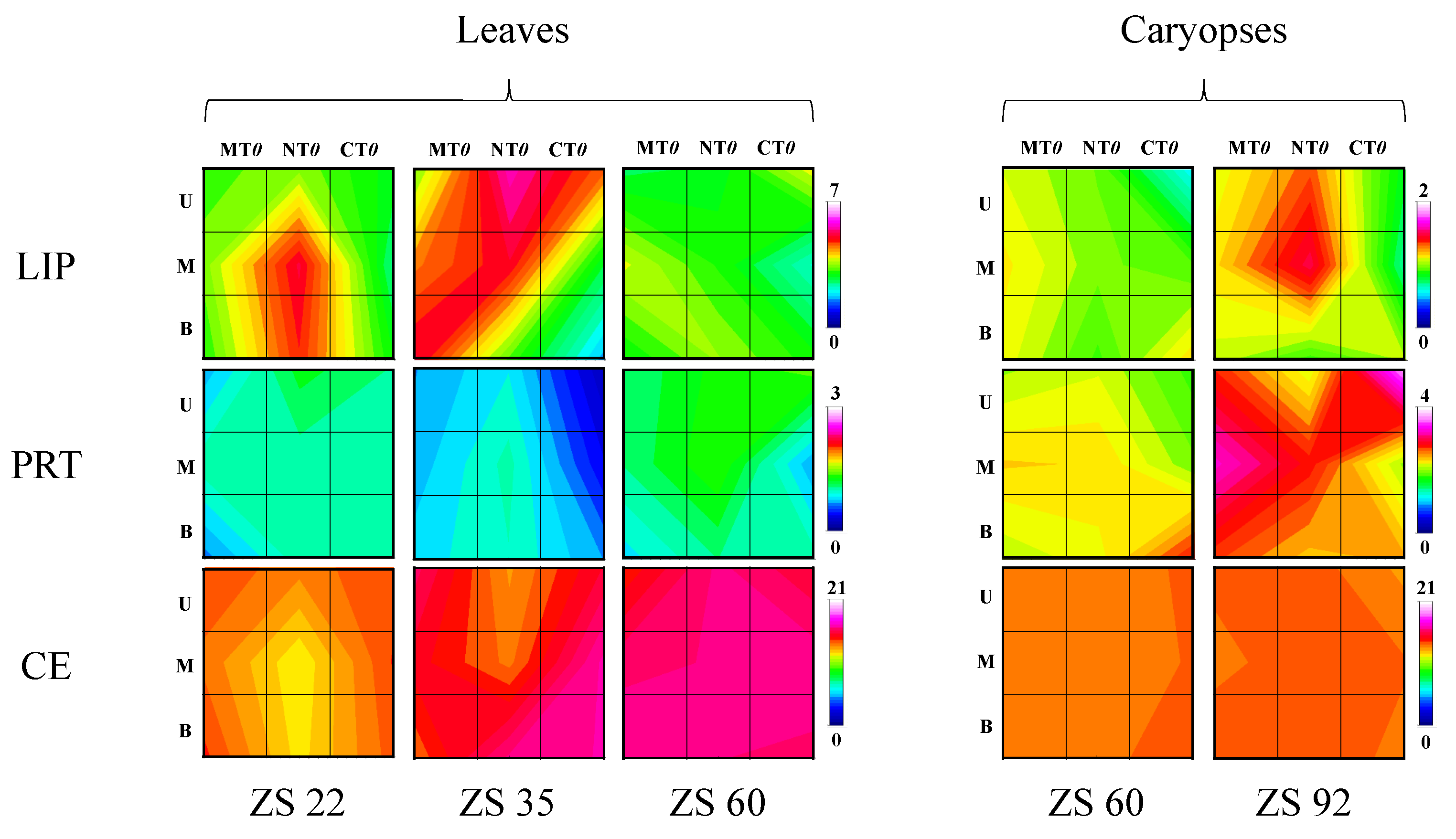
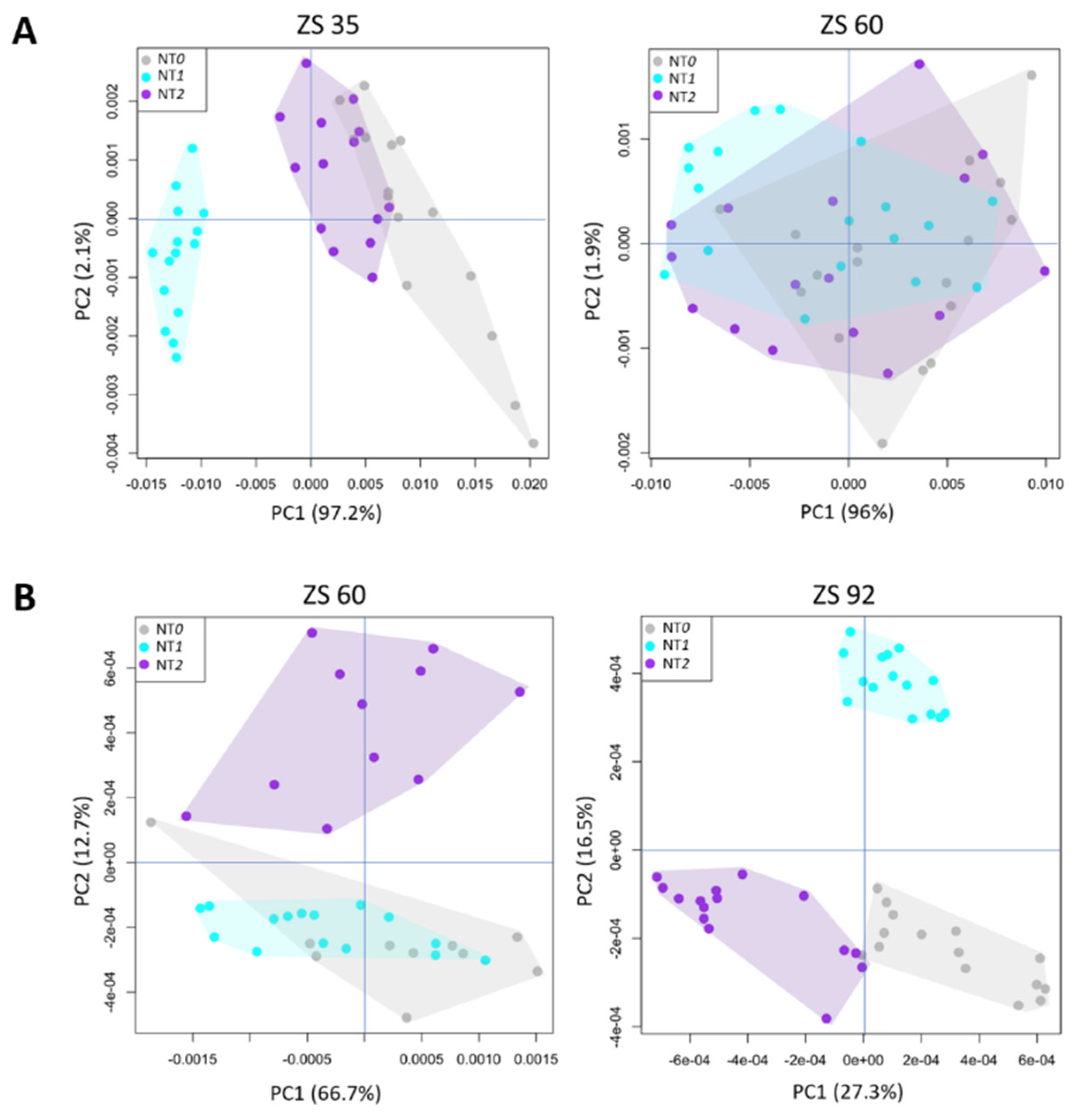
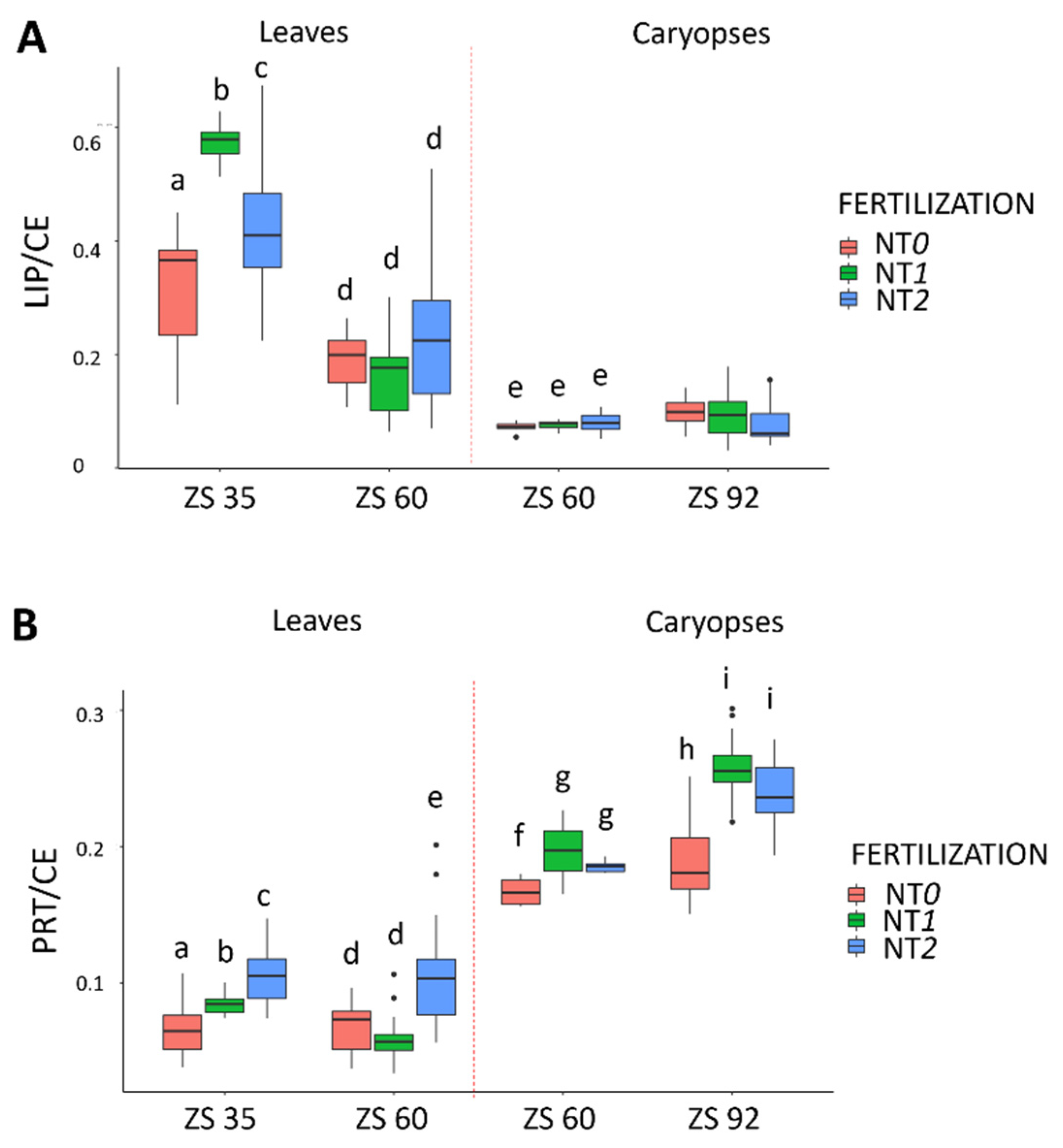
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2019: Highlights; ST/ESA/SER.A/423; Department of Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
- Bodirsky, B.L.; Popp, A.; Lotze-Campen, H.; Dietrich, J.P.; Rolinski, S.; Weindl, I.; Schmitz, C.; Müller, C.; Bonsch, M.; Humpenöder, F.; et al. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nat. Commun. 2014, 5, 3858. [Google Scholar] [CrossRef] [PubMed]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Iocola, I.; Bassu, S.; Farina, R.; Antichi, D.; Basso, B.; Bindi, M.; Dalla Marta, A.; Danuso, F.; Doro, L.; Ferrise, R.; et al. Can conservation tillage mitigate climate change impacts in Mediterranean cereal systems? A soil organic carbon assessment using long term experiments. Eur. J. Agron. 2017, 90, 96–107. [Google Scholar] [CrossRef]
- López-Garrido, R.; Madejón, E.; Murillo, J.M.; Moreno, F. Short and long-term distribution with depth of soil organic carbon and nutrients under traditional and conservation tillage in a Mediterranean environment (southwest Spain). Soil Use Manag. 2011, 27, 177–185. [Google Scholar] [CrossRef]
- Badagliacca, G.; Benítez, E.; Amato, G.; Badalucco, L.; Giambalvo, D.; Laudicina, V.A.; Ruisi, P. Long-term no-tillage application increases soil organic carbon, nitrous oxide emissions and faba bean (Vicia faba L.) yields under rain-fed Mediterranean conditions. Sci. Total Environ. 2018, 639, 350–359. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Tu, C.; Hoyt, G.D.; DeForest, J.L.; Hu, S. Long-term no-tillage and organic input management enhanced the diversity and stability of soil microbial community. Sci. Total Environ. 2017, 609, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gebbers, R.; Adamchuk, V.I. Precision Agriculture and Food Security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.V. Implementing Precision Agriculture in the 21st Century. J. Agric. Eng. Res. 2000, 76, 267–275. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Optimal Leaf Positions for SPAD Meter Measurement in Rice. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef] [PubMed]
- Mogili, U.R.; Deepak, B.B.V.L. Review on Application of Drone Systems in Precision Agriculture. Procedia Comput. Sci. 2018, 133, 502–509. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Perspectives for Remote Sensing with Unmanned Aerial Vehicles in Precision Agriculture. Trends Plant Sci. 2019, 24, 152–164. [Google Scholar] [CrossRef]
- Orsini, R.; Basili, D.; Belletti, M.; Bentivoglio, D.; Bozzi, C.A.; Chiappini, S.; Conti, C.; Galli, A.; Giorgini, E.; Fiorentini, M.; et al. Setting of a precision farming robotic laboratory for cropping system sustainability and food safety and security: Preliminary results. IOP Conf. Ser. Earth Environ. Sci. 2019, 275, 012021. [Google Scholar] [CrossRef]
- Sonmez, N.K.; Slater, B. Measuring Intensity of Tillage and Plant Residue Cover Using Remote Sensing. Eur. J. Remote Sens. 2016, 49, 121–135. [Google Scholar] [CrossRef]
- Orsini, R.; Fiorentini, M.; Zenobi, S. Testing vegetation index categories as influenced by soil management and nitrogen fertilization in cereal based cropping systems. In Proceedings of the 2019 IEEE International Workshop on Metrology for Agriculture and Forestry, Portici, Italy, 24–26 October 2019; pp. 13–18. [Google Scholar] [CrossRef]
- Fiorentini, M.; Zenobi, S.; Giorgini, E.; Basili, D.; Conti, C.; Pro, C.; Monaci, E.; Orsini, R. Nitrogen and chlorophyll status determination in durum wheat as influenced by fertilization and soil management: Preliminary results. PLoS ONE 2019, 14, e0225126. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Yin, X.; Savoy, H.J.; McClure, A.; Essington, M.E. Ammonia Volatilization Loss and Corn Nitrogen Nutrition and Productivity with Efficiency Enhanced UAN and Urea under No-tillage. Sci. Rep. 2019, 9, 6610. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Carville, D.; Dikeman, E.; Daigger, G.; Booth, G. Evaluation of two infrared instruments for determining protein content of hard red winter wheat. Cereal Chem. 1976, 53, 214–222. [Google Scholar]
- Polesello, A.; Giangiacomo, R.; Dull, G.G. Application of near infrared spectrophotometry to the nondestructive analysis of foods: A review of experimental results. Crit. Rev. Food Sci. Nutr. 1983, 18, 203–230. [Google Scholar] [CrossRef]
- Scotter, C.N.G. Non-destructive spectroscopic techniques for the measurement of food quality. Trends Food Sci. Technol. 1997, 8, 285–292. [Google Scholar] [CrossRef]
- Yu, P.; McKinnon, J.J.; Christensen, C.R.; Christensen, D.A. Using Synchrotron Transmission FTIR Microspectroscopy as a Rapid, Direct, and Nondestructive Analytical Technique to Reveal Molecular Microstructural–Chemical Features within Tissue in Grain Barley. J. Agric. Food Chem. 2004, 52, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Foca, G.; Lucisano, M.; Marchetti, A.; Pagani, M.A.; Tassi, L.; Ulrici, A. Classification of Cereal Flours by Chemometric Analysis of MIR Spectra. J. Agric. Food Chem. 2004, 52, 1062–1067. [Google Scholar] [CrossRef]
- Karoui, R.; Downey, G.; Blecker, C. Mid-Infrared Spectroscopy Coupled with Chemometrics: A Tool for the Analysis of Intact Food Systems and the Exploration of Their Molecular Structure–Quality Relationships—A Review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef]
- Siano, F.; Moccia, S.; Picariello, G.; Russo, G.; Sorrentino, G.; Di Stasio, M.; La Cara, F.; Volpe, M. Comparative Study of Chemical, Biochemical Characteristic and ATR-FTIR Analysis of Seeds, Oil and Flour of the Edible Fedora Cultivar Hemp (Cannabis sativa L.). Molecules 2018, 24, 83. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, C.; Amato, A.; Occhialini, S.; Critchley, A.T.; Totti, C.; Giorgini, E.; Conti, C.; Beolchini, F. Adsorption of indium by waste biomass of brown alga Ascophyllum nodosum. Sci. Rep. 2019, 9, 16763. [Google Scholar] [CrossRef] [PubMed]
- Seddaiu, G.; Iocola, I.; Farina, R.; Orsini, R.; Iezzi, G.; Roggero, P.P. Long term effects of tillage practices and N fertilization in rainfed Mediterranean cropping systems: Durum wheat, sunflower and maize grain yield. Eur. J. Agron. 2016, 77, 166–178. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). World Reference Base for Soil Resources. World Soil Resources Report 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzac, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Huck, C. A Review of Mid-Infrared and Near-Infrared Imaging: Principles, Concepts and Applications in Plant Tissue Analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Cisternas, I.; Velásquez, I.; Caro, A.; Rodríguez, A. Systematic literature review of implementations of precision agriculture. Comput. Electron. Agric. 2020, 176, 105626. [Google Scholar] [CrossRef]
- Sanches, G.M.; Magalhães, P.S.G.; Kolln, O.T.; Otto, R.; Rodrigues, F.; Cardoso, T.F.; Chagas, M.F.; Franco, H.C.J. Agronomic, economic, and environmental assessment of site-specific fertilizer management of Brazilian sugarcane fields. Geoderma Reg. 2021, 24, e00360. [Google Scholar] [CrossRef]
- Orsini, R.; Fiorentini, M.; Zenobi, S. Evaluation of Soil Management Effect on Crop Productivity and Vegetation Indices Accuracy in Mediterranean Cereal-Based Cropping Systems. Sensors 2020, 20, 3383. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, M.; Zenobi, S.; Orsini, R. Remote and Proximal Sensing Applications for Durum Wheat Nutritional Status Detection in Mediterranean Area. Agriculture 2021, 11, 39. [Google Scholar] [CrossRef]
- Tabaglio, V.; Gavazzi, C.; Menta, C. The Influence of No-Till, Conventional Tillage and Nitrogen Fertilization on Physico-Chemical and Biological Indicators After Three Years of Monoculture Barley. Ital. J. Agron. 2008, 3, 233. [Google Scholar] [CrossRef]
- De Sanctis, G.; Roggero, P.P.; Seddaiu, G.; Orsini, R.; Porter, C.H.; Jones, J.W. Long-term no tillage increased soil organic carbon content of rain-fed cereal systems in a Mediterranean area. Eur. J. Agron. 2012, 40, 18–27. [Google Scholar] [CrossRef]
- Notarstefano, V.; Sabbatini, S.; Conti, C.; Pisani, M.; Astolfi, P.; Pro, C.; Rubini, C.; Vaccari, L.; Giorgini, E. Investigation of human pancreatic cancer tissues by Fourier Transform Infrared Hyperspectral Imaging. J. Biophotonics 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Notarstefano, V.; Sabbatini, S.; Pro, C.; Belloni, A.; Orilisi, G.; Rubini, C.; Byrne, H.J.; Vaccari, L.; Giorgini, E. Exploiting fourier transform infrared and Raman microspectroscopies on cancer stem cells from oral squamous cells carcinoma: New evidence of acquired cisplatin chemoresistance. Analyst 2020, 145, 8038–8049. [Google Scholar] [CrossRef]
- Cortés, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Ge, Y.; Thomasson, J.A.; Sui, R. Remote sensing of soil properties in precision agriculture: A review. Front. Earth Sci. 2011, 5, 229–238. [Google Scholar] [CrossRef]
- Zhukov, A.V. Very Long-Chain Fatty Acids in Composition of Plant Membrane Lipids. Russ. J. Plant Physiol. 2018, 65, 784–800. [Google Scholar] [CrossRef]
- Pallardy, S.G.; Kozlowski, T.T. Physiology of Woody Plants; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-0-12-088765-1. [Google Scholar]
- Skylas, D.J.; Mackintosh, J.A.; Cordwell, S.J.; Basseal, D.J.; Walsh, B.J.; Harry, J.; Blumenthal, C.; Copeland, L.; Wrigley, C.W.; Rathmell, W. Proteome Approach to the Characterisation of Protein Composition in the Developing and Mature Wheat-grain Endosperm. J. Cereal Sci. 2000, 32, 169–188. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Xia, X.; Yan, Y.; Appels, R.; Mahmood, T.; He, Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J. Cereal Sci. 2014, 60, 11–24. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; Van Groenigen, L.J.; Lee, J.; Lundy, M.E.; Van Gestel, N.; Six, J.; Venterea, R.T.; Van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Li, S.-X.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]


| Months | Period (Years) | Total Rainfall (mm) | Average Air Temperature (°C) | |
|---|---|---|---|---|
| Tmin | Tmax | |||
| November | 2017–2018 | 124 | 7.9 | 11.1 |
| 1998–2018 | 93 | 8.7 | 15.3 | |
| December | 2017–2018 | 96 | 4.1 | 11.9 |
| 1998–2018 | 87 | 4.4 | 10.9 | |
| January | 2017–2018 | 29 | 5.2 | 12.8 |
| 1998–2018 | 54 | 3.2 | 9.6 | |
| February | 2017–2018 | 173 | 2.0 | 8.3 |
| 1998–2018 | 68 | 3.8 | 11.0 | |
| March | 2017–2018 | 143 | 5.7 | 13.3 |
| 1998–2018 | 85 | 6.6 | 14.9 | |
| April | 2017–2018 | 37 | 11.8 | 21.5 |
| 1998–2018 | 70 | 9.7 | 18.8 | |
| Mai | 2017–2018 | 95 | 14.9 | 23.7 |
| 1998–2018 | 73 | 13.7 | 23.5 | |
| June | 2017–2018 | 48 | 17.7 | 27.8 |
| 1998–2018 | 54 | 17.8 | 28.1 | |
| July | 2017–2018 | 57 | 20.5 | 30.8 |
| 1998–2018 | 36 | 20.2 | 30.8 | |
| Total | 2017–2018 | 802 | 10.0 | 17.9 |
| 1998–2018 | 838 | 11.4 | 20.0 | |
| Nitrogen Fertilization | Soil Tillage | |||||
|---|---|---|---|---|---|---|
| CT | MT | NT | ||||
| 0 kg N ha−1 (0) |  l-CT0 |  c-CT0 |  l-MT0 |  c-MT0 |  l-NT0 |  c-NT0 |
| 90 kg N ha−1 (1) | - | - | - | - |  l-NT1 |  c-NT1 |
| 180 kg N ha−1 (2) | - | - | - | - |  l-NT2 |  c-NT2 |
| Peak Position (cm−1) | Vibrational Mode | Biochemical Assignment | |
|---|---|---|---|
| Leaves | Caryopses | ||
| ~2917, ~2849 | ~2924, ~2855 | Symmetric and asymmetric stretching modes of CH2 moieties νsym CH2, νasym CH2 | Alkyl chains [30] |
| ~1736 | ~1746 | Stretching mode of carbonyl moiety ν C=O | Hemicellulose [31] |
| ~1625 | ~1648 | Stretching and bending modes of peptide linkage ν C=O, ν C-N, δ N-H | Amide I of proteins, pectin [30] |
| ~1463 | ~1539 | Bending mode of CH2 moieties δ CH2 | Alkyl chains [31] |
| ~1033, ~800 | ~998, ~762 | Vibrational modes of C-OH groups | Cellulose [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pro, C.; Basili, D.; Notarstefano, V.; Belloni, A.; Fiorentini, M.; Zenobi, S.; Alia, S.; Vignini, A.; Orsini, R.; Giorgini, E. A Spectroscopic Approach to Evaluate the Effects of Different Soil Tillage Methods and Nitrogen Fertilization Levels on the Biochemical Composition of Durum Wheat (Triticum turgidum subsp. durum) Leaves and Caryopses. Agriculture 2021, 11, 321. https://doi.org/10.3390/agriculture11040321
Pro C, Basili D, Notarstefano V, Belloni A, Fiorentini M, Zenobi S, Alia S, Vignini A, Orsini R, Giorgini E. A Spectroscopic Approach to Evaluate the Effects of Different Soil Tillage Methods and Nitrogen Fertilization Levels on the Biochemical Composition of Durum Wheat (Triticum turgidum subsp. durum) Leaves and Caryopses. Agriculture. 2021; 11(4):321. https://doi.org/10.3390/agriculture11040321
Chicago/Turabian StylePro, Chiara, Danilo Basili, Valentina Notarstefano, Alessia Belloni, Marco Fiorentini, Stefano Zenobi, Sonila Alia, Arianna Vignini, Roberto Orsini, and Elisabetta Giorgini. 2021. "A Spectroscopic Approach to Evaluate the Effects of Different Soil Tillage Methods and Nitrogen Fertilization Levels on the Biochemical Composition of Durum Wheat (Triticum turgidum subsp. durum) Leaves and Caryopses" Agriculture 11, no. 4: 321. https://doi.org/10.3390/agriculture11040321
APA StylePro, C., Basili, D., Notarstefano, V., Belloni, A., Fiorentini, M., Zenobi, S., Alia, S., Vignini, A., Orsini, R., & Giorgini, E. (2021). A Spectroscopic Approach to Evaluate the Effects of Different Soil Tillage Methods and Nitrogen Fertilization Levels on the Biochemical Composition of Durum Wheat (Triticum turgidum subsp. durum) Leaves and Caryopses. Agriculture, 11(4), 321. https://doi.org/10.3390/agriculture11040321








