Biofertilizer-Based Zinc Application Enhances Maize Growth, Gas Exchange Attributes, and Yield in Zinc-Deficient Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Soil and Location
2.2. Experimental Soil Characterization
2.3. Pot Preparation by Addition of Zn and AMF
2.4. Moisture Maintenance and Fertilizer Addition
2.5. Seeds Sowing and Thinning
2.6. Morphological Attributes
2.7. Yield Parameters
2.8. Gas Exchange Attributes
2.9. Total Soluble Protein (TSP)
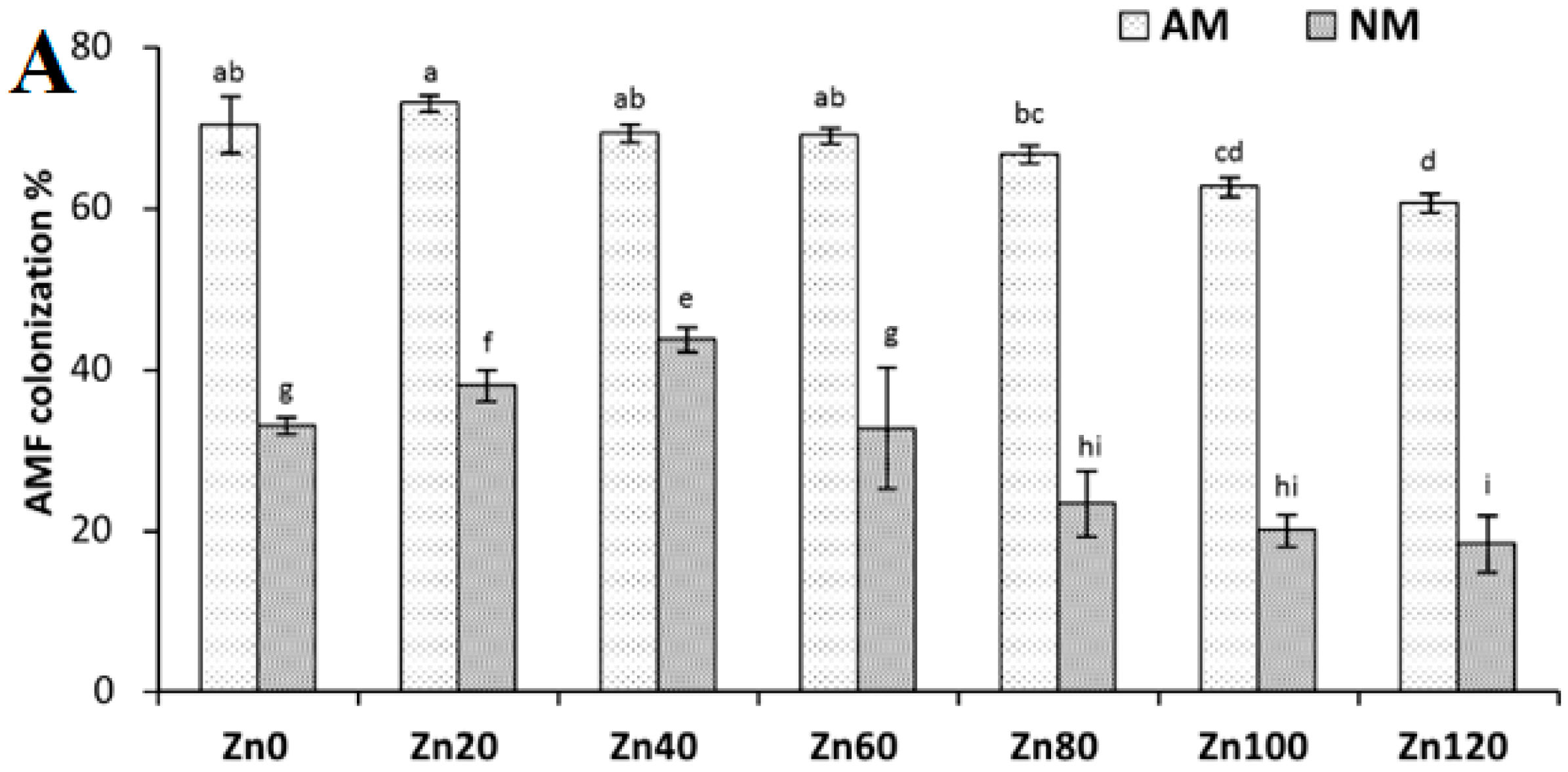
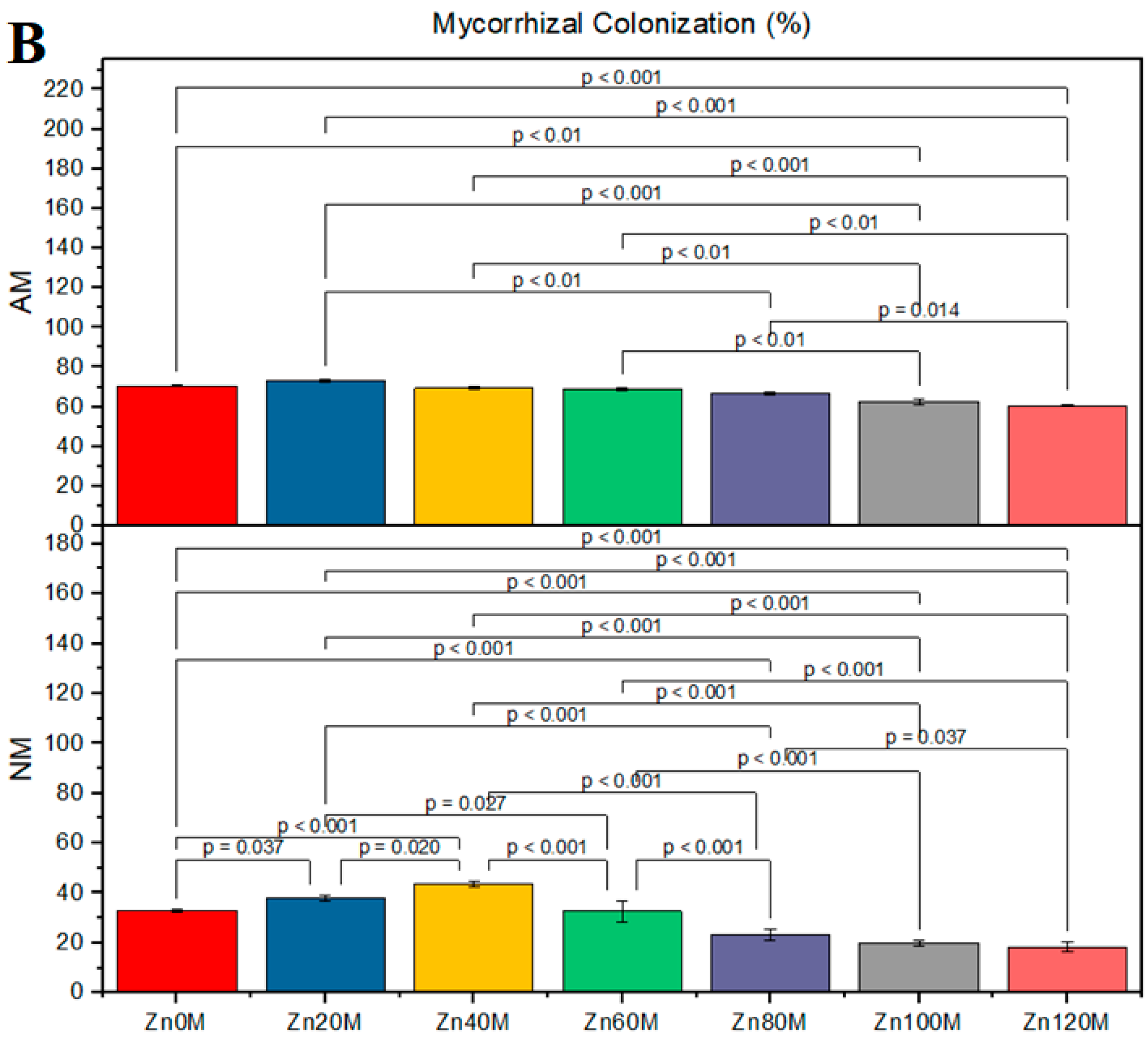
2.10. AMF Colonization Determination/Measurement
2.11. Statistical Analysis
3. Results
3.1. Root Colonization
3.2. Plant Height
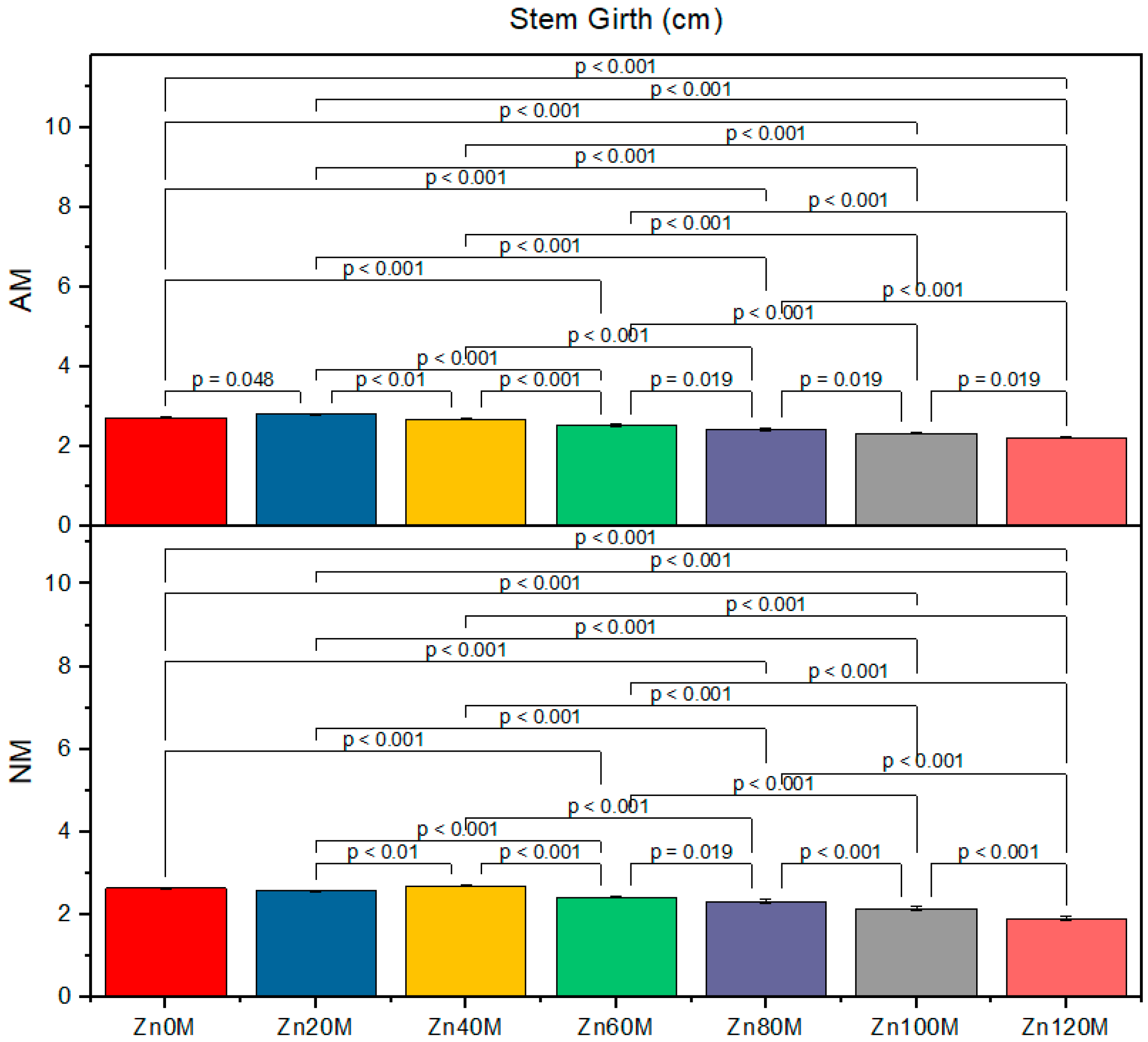
3.3. Stem Girth
3.4. Number of Leaves
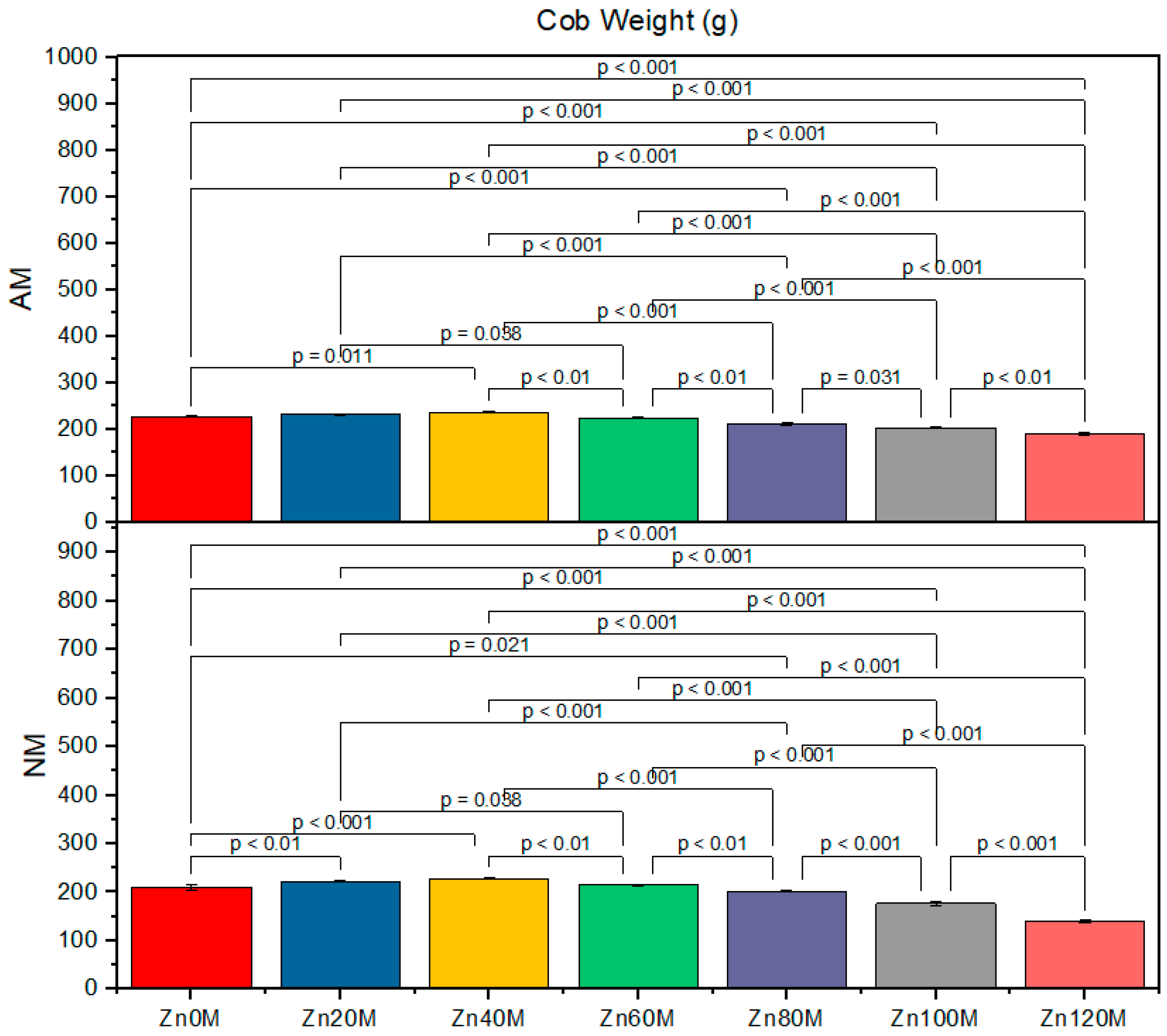
3.5. Cob Weight
3.6. Cob Length
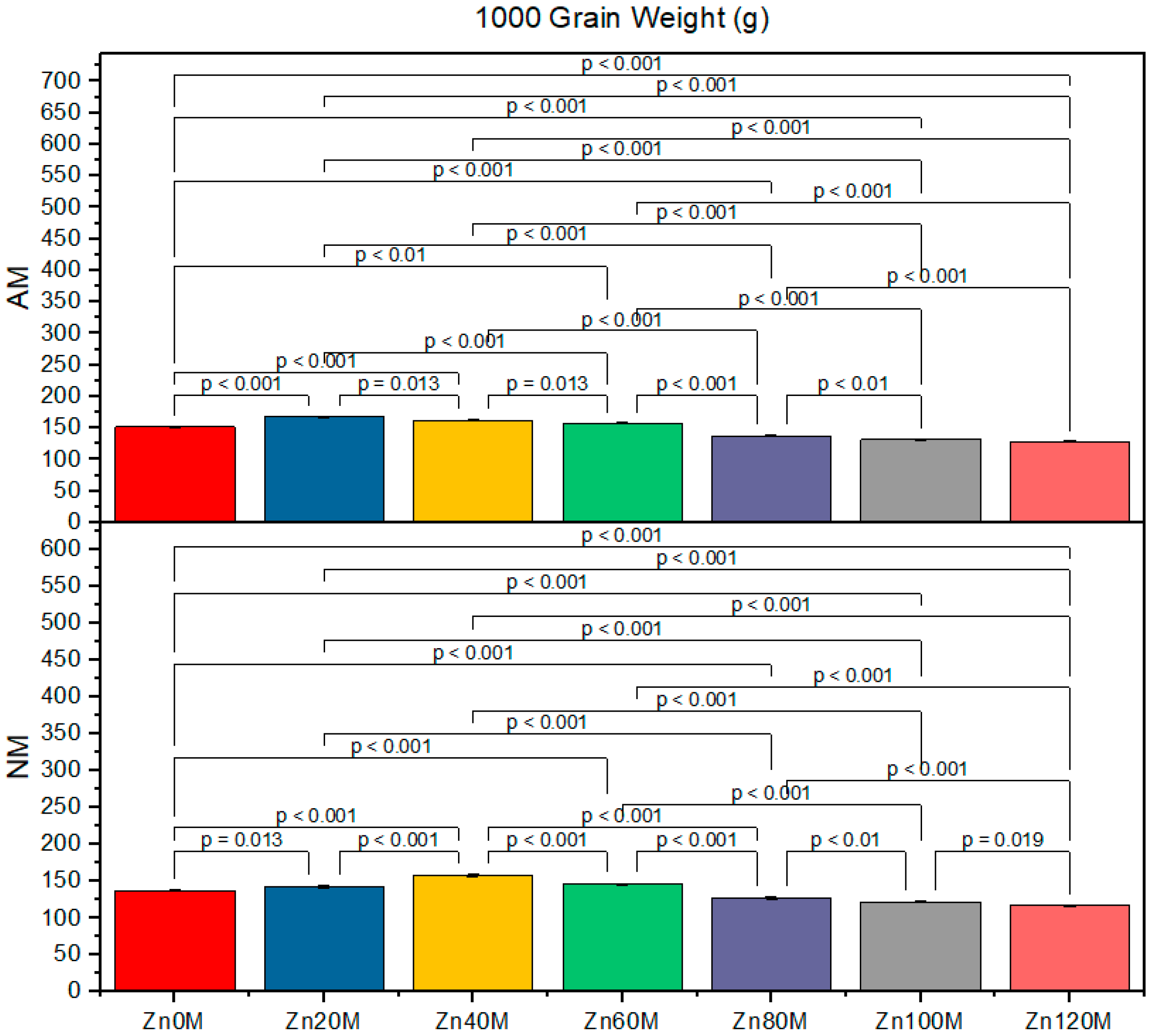
3.7. 1000-Grain Weight
3.8. Grain Yield
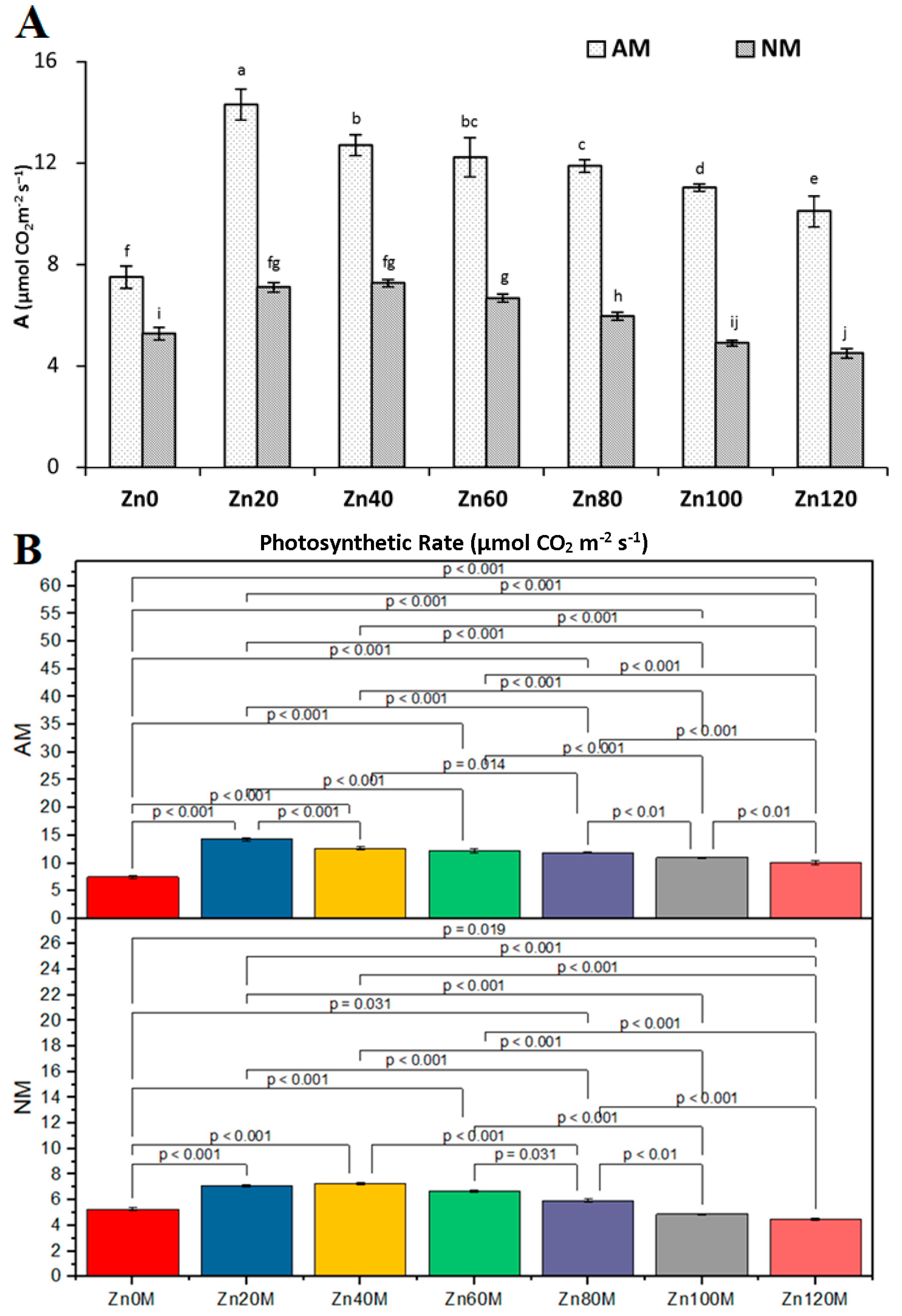
3.9. Photosynthetic Rate
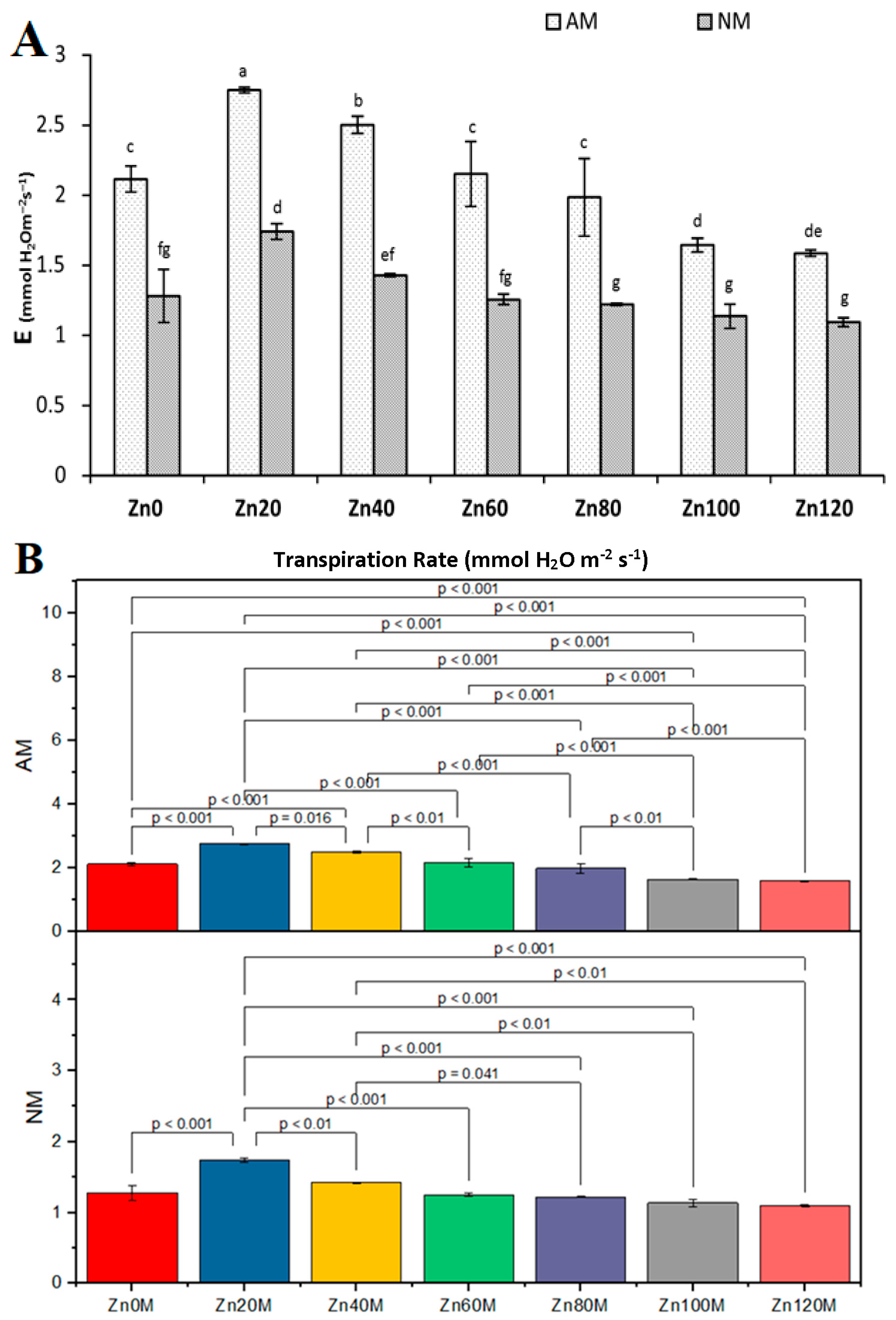
3.10. Transpiration Rate
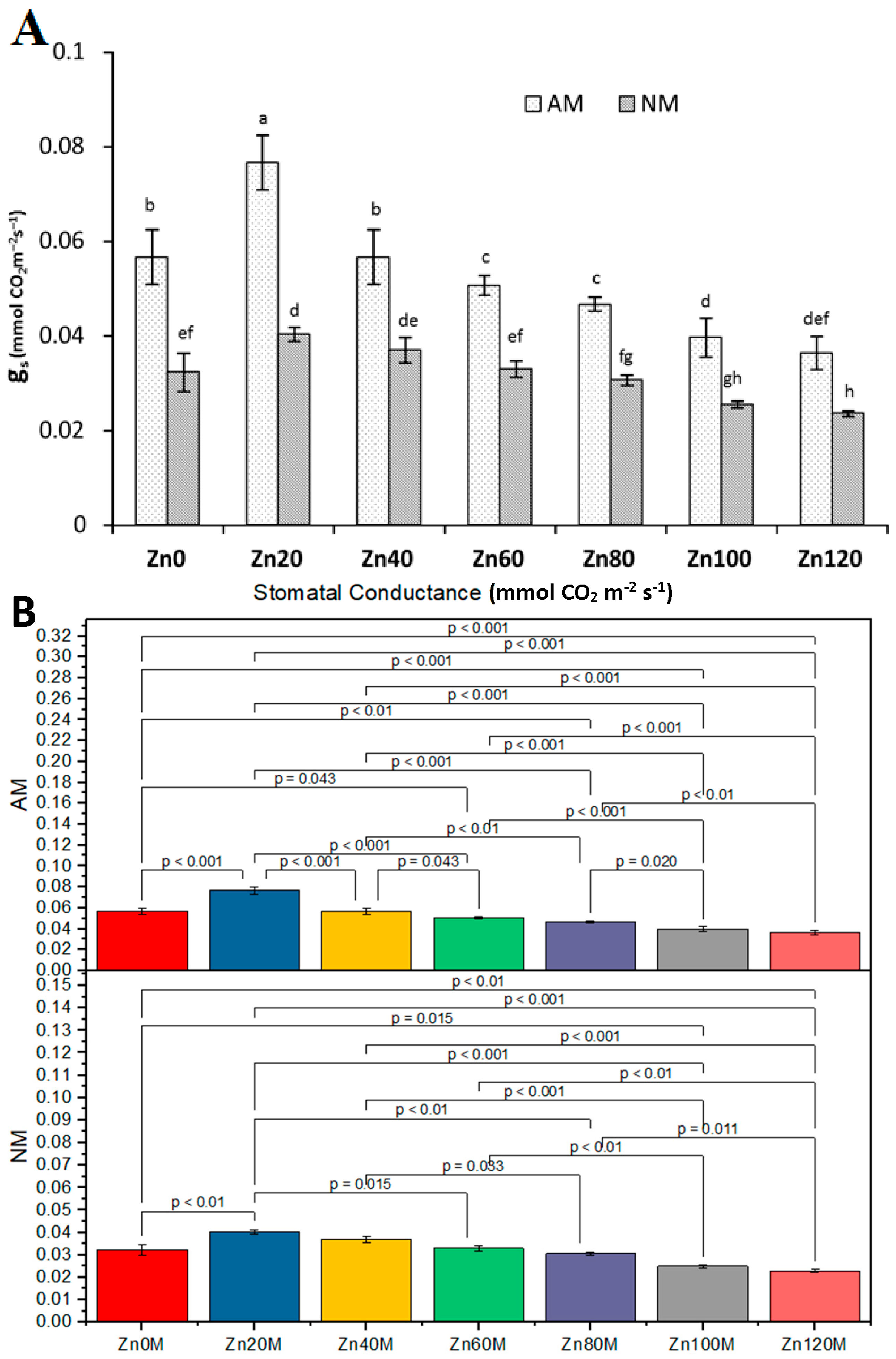
3.11. Stomatal Conductance
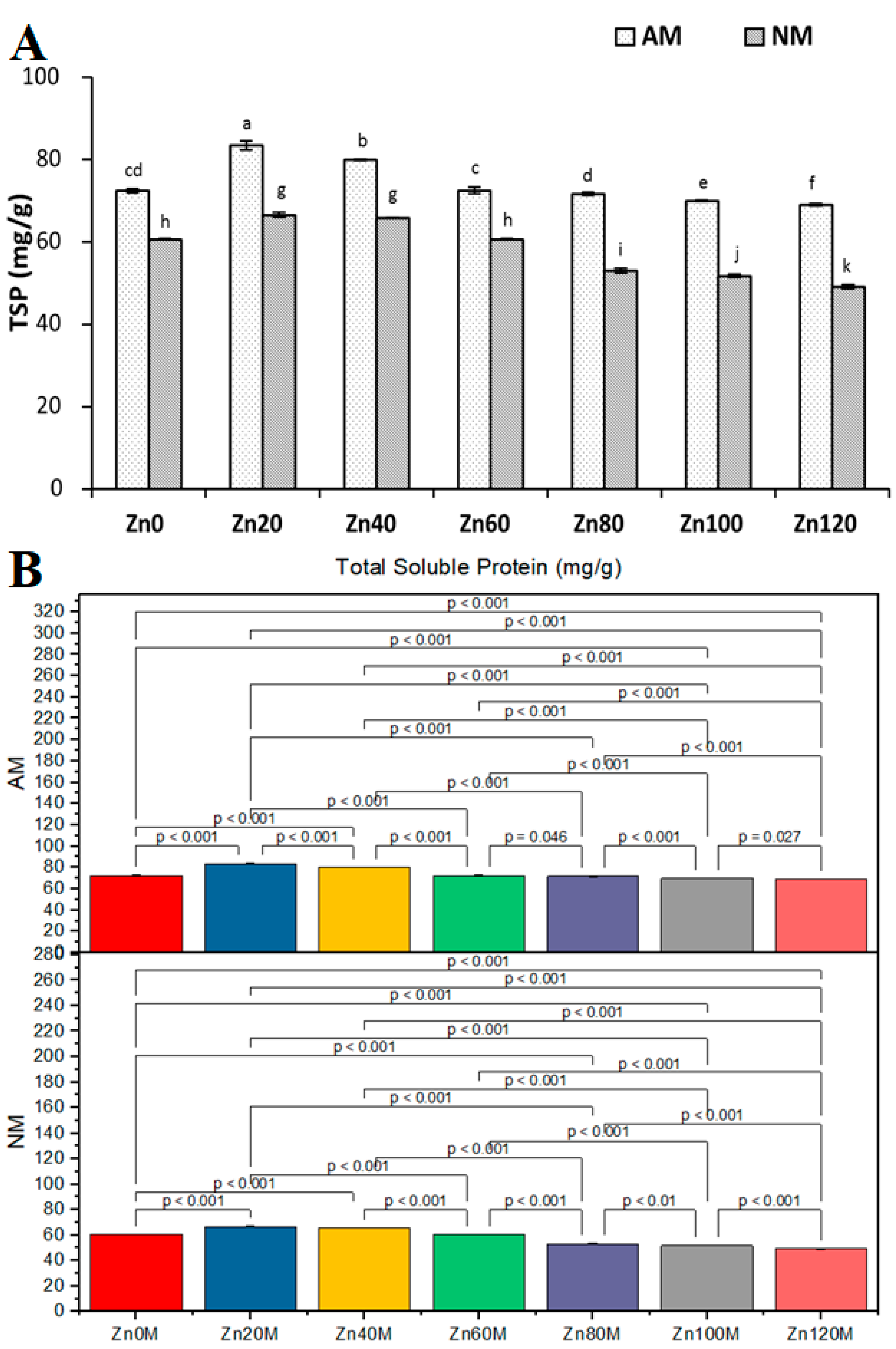
3.12. Total Soluble Protein Concentration in Leaves
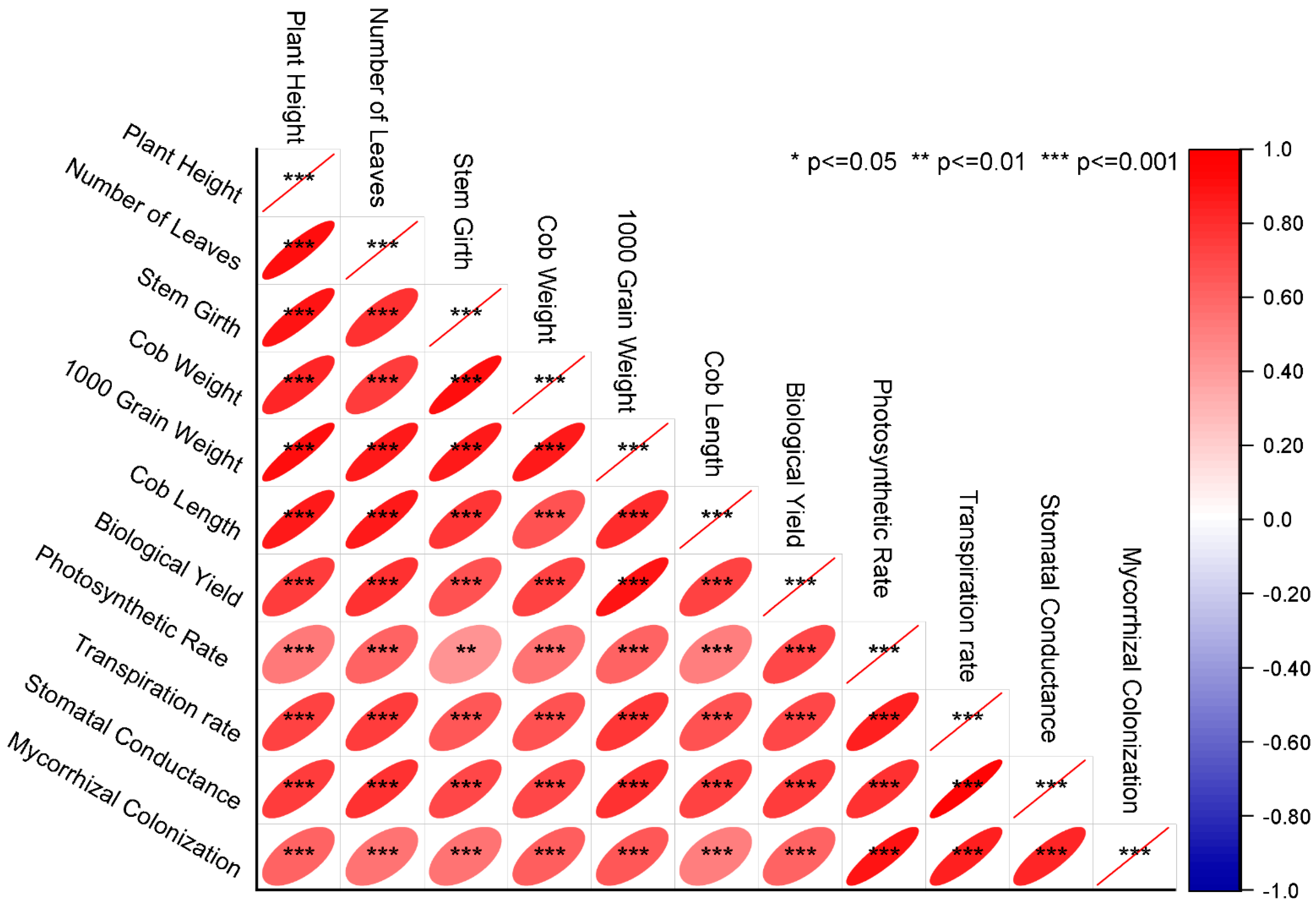
3.13. Pearson Correlation and Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanif, N.Q.; Akhtar, N. Nutritional Evaluation of Maize Plant Fodder Grown in Spring and Autumn Season in Punjab, Pakistan. J. Bioresour. Manag. 2020, 7, 74–93. [Google Scholar] [CrossRef]
- Tariq, M.; Iqbal, H. Maize in Pakistan—An overview. Kasetsart J. Nat. Sci. 2010, 44, 757–763. [Google Scholar]
- Rafiullah, A.; Tariq, M.; Khan, F.; Shah, A.H.; Fahad, S.; Wahid, F.; Ali, J.; Adnan, M.; Ahmad, M.; Irfan, M.; et al. Effect of micronutrients foliar supplementation on the production and eminence of plum. Qual. Assur. Saf. Crop. Foods 2020, 12, 32–40. [Google Scholar] [CrossRef]
- Tahir, F.A.; Ahamad, N.; Rasheed, M.K.; Danish, S. Effect of various application rate of zinc fertilizer with and without fruit waste biochar on the growth and Zn uptake in maize. Int. J. Biosci. 2018, 13, 159–166. [Google Scholar]
- Bibi, F.; Saleem, I.; Ehsan, S.; Jamil, S.; Ullah, H.; Mubashir, M.; Kiran, S.; Ahmad, I.; Irshad, I.; Saleem, M.; et al. Effect of various application rates of phosphorus combined with different zinc rates and time of zinc application on phytic acid concentration and zinc bioavailability in wheat. Agric. Nat. Resour. 2020, 54, 265–272. [Google Scholar]
- Imran, M.; Rehim, A.; Sarwar, N.; Hussain, S. Zinc bioavailability in maize grains in response of phosphorous-zinc interaction. J. Plant Nutr. Soil Sci. 2016, 179, 60–66. [Google Scholar] [CrossRef]
- Imran, M.; Rehim, A. Zinc fertilization approaches for agronomic biofortification and estimated human bioavailability of zinc in maize grain. Arch. Agron. Soil Sci. 2017, 63, 106–116. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M.; Meena, B.S.; Ram, H.; Parihar, C.M.; Kumar, S.; Yadav, M.R.; Meena, R.K.; Kumar, U.; Meena, V.K. Zinc management effects on quality and nutrient yield of fodder maize (Zea mays). Indian J. Agric. Sci. 2017, 87, 1013–1017. [Google Scholar]
- Obaid, E.A.; Al-hadethi, M.E.A. Effect of Foliar Application with Manganese and Zinc on Pomegranate Growth, Yield and Fruit Quality. J. Hortic. Sci. Ornam. Plants 2013, 5, 41–45. [Google Scholar]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; International Zinc Association: Brussels, Belgium, 2008; ISBN 978-90-8133-310-8. [Google Scholar]
- Iratkar, A.G.; Giri, J.D.; Kadam, M.M.; Giri, J.N.; Dabhade, M.B. Distribution of DTPA extractable micronutrients and their relationship with soil properties in soil of Parsori watershed of Nagpur district of Maharashtra. Asian J. Soil Sci. 2014, 9, 297–299. [Google Scholar]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef]
- Coccina, A.; Cavagnaro, T.R.; Pellegrino, E.; Ercoli, L.; McLaughlin, M.J.; Watts-Williams, S.J. The mycorrhizal pathway of zinc uptake contributes to zinc accumulation in barley and wheat grain. BMC Plant Biol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Danish, S.; Younis, U.; Akhtar, N.; Ameer, A.; Ijaz, M.; Nasreen, S.; Huma, F.; Sharif, S.; Ehsanullah, M. Phosphorus solubilizing bacteria and rice straw biochar consequence on maize pigments synthesis. Int. J. Biosci. 2015, 5, 31–39. [Google Scholar]
- Danish, S.; Zafar-ul-Hye, M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep. 2019, 9, 5999. [Google Scholar] [CrossRef]
- Zafar-Ul-Hye, M.; Hussain, N.M.; Danish, S.; Aslam, U.; Zahir, Z.A. Multi-Strain bacterial inoculation of enterobacter cloacae, serratia ficaria and burkholderia phytofirmans with fertilizers for enhancing resistance in wheat against salinity stress. Pak. J. Bot. 2019, 51, 1839–1846. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-Ul-Hye, M.; Hussain, S.; Riaz, M.; Qayyum, M.F. Mitigation of drought stress in maize through inoculation with drought tolerant ACC deaminase containing PGPR under axenic conditions. Pak. J. Bot. 2020, 52, 49–60. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Fahad, S.; Saud, S.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Drought stress alleviation by ACC deaminase producing Achromobacter xylosoxidans and Enterobacter cloacae, with and without timber waste biochar in maize. Sustainability 2020, 12, 6286. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Tahzeeb-ul-Hassan, M.; Abid, M.; Fahad, S.; Brtnicky, M.; Dokulilova, T.; Datta, R.; Danish, S. Potential role of compost mixed biochar with rhizobacteria in mitigating lead toxicity in spinach. Sci. Rep. 2020, 10, 69183. [Google Scholar] [CrossRef] [PubMed]
- Zafar-ul-Hye, M.; Tahzeeb-ul-Hassan, M.; Wahid, A.; Danish, S.; Khan, M.J.; Fahad, S.; Brtnicky, M.; Hussain, G.S.; Battaglia, M.L.; Datta, R. Compost mixed fruits and vegetable waste biochar with ACC deaminase rhizobacteria can minimize lead stress in mint plants. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Khan, R.I.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of Seaweed Extract on Productivity and Quality Attributes of Four Onion Cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Izhar Shafi, M.; Adnan, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z.; Danish, S.; Zafar-ul-Hye, M.; Brtnicky, M.; Datta, R. Application of Single Superphosphate with Humic Acid Improves the Growth, Yield and Phosphorus Uptake of Wheat (Triticum aestivum L.) in Calcareous Soil. Agronomy 2020, 10, 1224. [Google Scholar] [CrossRef]
- Ullah, A.; Ali, M.; Shahzad, K.; Ahmad, F.; Iqbal, S.; Habib, M.; Rahman, U.; Ahmad, S.; Iqbal, M.M. Impact of Seed Dressing and Soil Application of Potassium Humate on Cotton Plants Productivity and Fiber Quality. Plants 2020, 9, 1444. [Google Scholar] [CrossRef] [PubMed]
- Zafar-ul-hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; Brtnicky, M.; Kintl, A.; Hussain, M.S.; El-esawi, M.A. Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants 2020, 9, 1386. [Google Scholar] [CrossRef] [PubMed]
- Zafar-Ul-hye, M.; Naeem, M.; Danish, S.; Fahad, S.; Datta, R.; Abbas, M.; Rahi, A.A.; Brtnicky, M.; Holátko, J.; Tarar, Z.H.; et al. Alleviation of cadmium adverse effects by improving nutrients uptake in bitter gourd through cadmium tolerant rhizobacteria. Environments 2020, 7, 54. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Pathan, S.I.; Větrovský, T.; Giagnoni, L.; Datta, R.; Baldrian, P.; Nannipieri, P.; Renella, G. Microbial expression profiles in the rhizosphere of two maize lines differing in N use efficiency. Plant Soil 2018, 433, 401–413. [Google Scholar] [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press; Elsevier: New York, NY, USA, 2008; Volume 137, ISBN 9780123705266. [Google Scholar]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants {\textendash} A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef]
- Wang, F.; Jing, X.; Adams, C.A.; Shi, Z.; Sun, Y. Decreased {ZnO} nanoparticle phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ. Sci. Pollut. Res. 2018, 25, 23736–23747. [Google Scholar] [CrossRef] [PubMed]
- Jansa, J.; Mozafar, A.; Frossard, E. Long-distance transport of P and Zn through the hyphae of an arbuscular mycorrhizal fungus in symbiosis with maize. Agronomie 2003, 23, 481–488. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Smith, F.A.; McLaughlin, M.J.; Patti, A.F.; Cavagnaro, T.R. How important is the mycorrhizal pathway for plant Zn uptake? Plant Soil 2015, 390, 157–166. [Google Scholar] [CrossRef]
- Rhoades, J.D. Salinity: Electrical Conductivity and Total Dissolved Solids. In Methods of Soil Analysis, Part 3, Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 417–435. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Soil pH and lime requirement. In Methods of Soil Analysis; American Society of Agronomy: Madison, MI, USA, 1982; pp. 199–208. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; John Wiley & Sons, Ltd.: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Pratt, P.F. Potassium. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2; Norman, A.G., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1965; pp. 1022–1030. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis Part 3—Chemical Methods; Soil Science Society of America; American Society of Agronomy; John Wiley & Sons, Ltd.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, M. Chapter 37: Nitrogen-Total. Methods Soil Anal. Part Chem. Methods SSSA B. Ser. 1996, 5, 1085–1121. [Google Scholar]
- Danish, S.; Zafar-ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol. Plant. 2014, 152, 331–344. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Hussain, M.; Shaaban, M.; Núñez-delgado, A. Rhizobacteria with ACC-Deaminase Activity Improve Nutrient Uptake, Chlorophyll Contents and Early Seedling Growth of Wheat under PEG- Induced Osmotic Stress. Int. J. Agric. Biol. 2019, 21, 1212–1220. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- GIOVANNETTI, M.; MOSSE, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book International Co.: Singapore, 1997. [Google Scholar]
- Lee, Y.-J.; George, E. Contribution of Mycorrhizal Hyphae to the Uptake of Metal Cations by Cucumber Plants at Two Levels of Phosphorus Supply. Plant Soil 2005, 278, 361–370. [Google Scholar] [CrossRef]
- Zhu, Y.; Christie, P.; Laidlaw, A.S. Uptake of Zn by arbuscular mycorrhizal white clover from Zn-contaminated soil. Chemosphere 2001, 42, 193–199. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Patti, A.F.; Cavagnaro, T.R. Arbuscular mycorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 2013, 371, 299–312. [Google Scholar] [CrossRef]
- Lingua, G.; Franchin, C.; Todeschini, V.; Castiglione, S.; Biondi, S.; Burlando, B.; Parravicini, V.; Torrigiani, P.; Berta, G. Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environ. Pollut. 2008, 153, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, T.E.; Charvat, I. Heavy-Metal Stress and Developmental Patterns of Arbuscular Mycorrhizal Fungi. Appl. Environ. Microbiol. 2004, 70, 6643–6649. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuoka, H.; Hayashi, H. Isolation and identification of a phosphate deficiency-induced C-glycosylflavonoid that stimulates arbuscular mycorrhiza formation in melon roots. Mol. Plant Microbe Interact. 2002, 15, 334–340. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Luo, W.; Li, J.; Wu, F. Arbuscular mycorrhizal fungi increase both concentrations and bioavilability of Zn in wheat (Triticum aestivum L) grain on Zn-spiked soils. Appl. Soil Ecol. 2019, 135, 91–97. [Google Scholar] [CrossRef]
- Paneque, M.; María, J.; Rosa, D.; Aragón, C.; Kern, J.; Conte, P. Sewage sludge hydrochars: Properties and agronomic impact as related to different production conditions. Geophys. Res. Abstr. 2015, 17, 3–4. [Google Scholar]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2018, 222, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Abadi, V.A.J.M.; da Silva, J.A.T. Potential of arbuscular mycorrhizae and tall fescue in remediation of soils polluted with zinc. Chem. Ecol. 2020, 36, 122–137. [Google Scholar] [CrossRef]
- Monnet, F.; Vaillant, N.; Hitmi, A.; Sallanon, H. Photosynthetic activity of Lolium perenne as a function of endophyte status and zinc nutrition. Funct. Plant Biol. 2005, 32, 131–139. [Google Scholar] [CrossRef]
- Zinzala, V.N.; Narwade, A.V.; Karmakar, N.; Patel, P.B. Influence of Zinc Applications on Photosynthesis, Transpiration and Stomatal Conductance in Kharif Rice (Oryza sativa L.) Genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 150–168. [Google Scholar] [CrossRef]
- Liu, C.; Hu, C.; Tan, Q.; Sun, X.; Wu, S.; Zhao, X. Co-application of molybdenum and zinc increases grain yield and photosynthetic efficiency of wheat leaves. Plant Soil Environ. 2019, 65, 508–515. [Google Scholar] [CrossRef]
- Vassilev, A.; Nikolova, A.; Koleva, L.; Lidon, F. Effects of excess Zn on growth and photosynthetic performance of young bean plants. J. Phytol. 2011, 3, 58–62. [Google Scholar]
- Sagardoy, R.; Vázquez, S.; Florez-Sarasa, I.D.; Albacete, A.; Ribas-Carbó, M.; Flexas, J.; Abadía, J.; Morales, F. Stomatal and mesophyll conductances to {CO}2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol. 2010, 187, 145–158. [Google Scholar] [CrossRef]
- Tobin, A.J. Carbonic anhydrase from parsley leaves. J. Biol. Chem. 1970, 245, 2656–3666. [Google Scholar] [CrossRef]
- Ohki, K. Effect of Zinc Nutrition on Photosynthesis and Carbonic Anhydrase Activity in Cotton. Physiol. Plant. 1976, 38, 300–304. [Google Scholar] [CrossRef]
- Reed, M.I.; Graham, D. Carbonic anhydrase in plants: Distribution, properties and possible physiological functions. Prog. Phytochem. 1980, 7, 47–94. [Google Scholar]
- Nelson, E.B.; Cenedella, A.; Tolbert, N.E. Carbonic anhydrase in Chlamydomonas. Phytochemistry 1969, 8, 2305–2306. [Google Scholar] [CrossRef]
- Hatch, M.D.; Slack, C.R. Photosynthetic CO2-Fixation Pathways. Annu. Rev. Plant Physiol. 1970, 21, 141–162. [Google Scholar] [CrossRef]
- Sharma, P.N.; Tripathi, A.; Bisht, S.S. Zinc requirement for stomatal opening in cauliflower. Plant Physiol. 1995, 107, 751–756. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Hu, C.-Y.; Xiao, J.-X. Effects of arbuscular mycorrhizal inoculation on the growth, zinc distribution and photosynthesis of two citrus cultivars grown in low-zinc soil. Trees 2014, 28, 1427–1436. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Cavagnaro, T.R.; Watts-Williams, S.J. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: A physiological and molecular assessment. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]



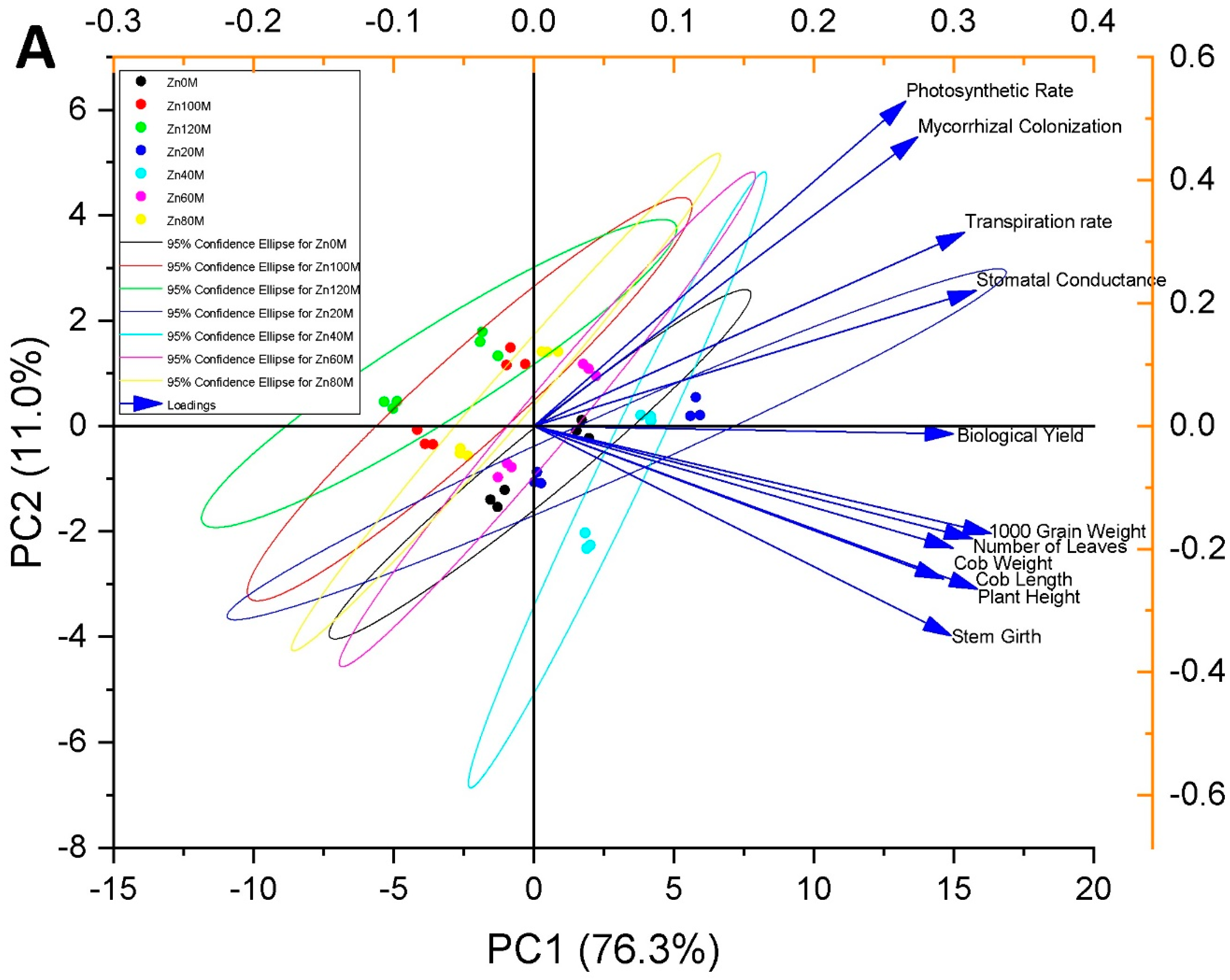
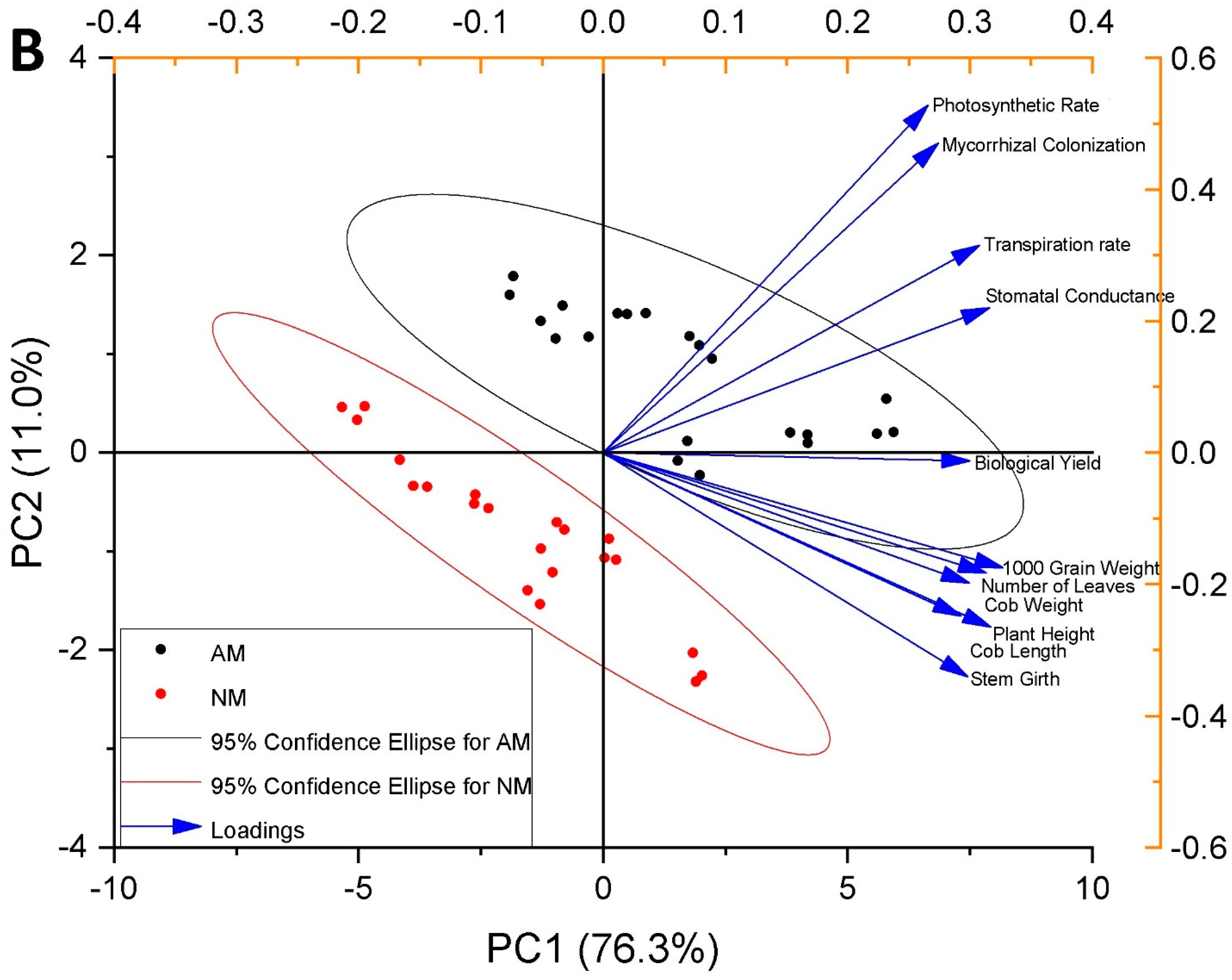
| Treatment | Plant Height (cm) | Stem Girth (cm) | No. of Leaves | |||
|---|---|---|---|---|---|---|
| AM | NM | AM | NM | AM | NM | |
| Control (Zn0) | 160c | 147d | 2.7ab | 2.6bc | 9.3c | 8.3de |
| Zn20 | 184a | 158c | 2.8a | 2.7cd | 12.6a | 9.3c |
| Zn40 | 182ab | 176b | 2.6b | 2.5b | 12.0a | 11.0b |
| Zn60 | 149d | 136e | 2.5d | 2.4e | 9.1cd | 8.5cde |
| Zn80 | 138ef | 118gh | 2.4e | 2.3f | 9.0cd | 8.0ef |
| Zn100 | 132ef | 113h | 2.3f | 2.1g | 8.3de | 7.3fg |
| Zn120 | 124g | 111fh | 2.2g | 1.9h | 7.3fg | 7.0f |
| AM | *** | *** | *** | |||
| Zinc | *** | *** | *** | |||
| AM*Zn | * | *** | ** | |||
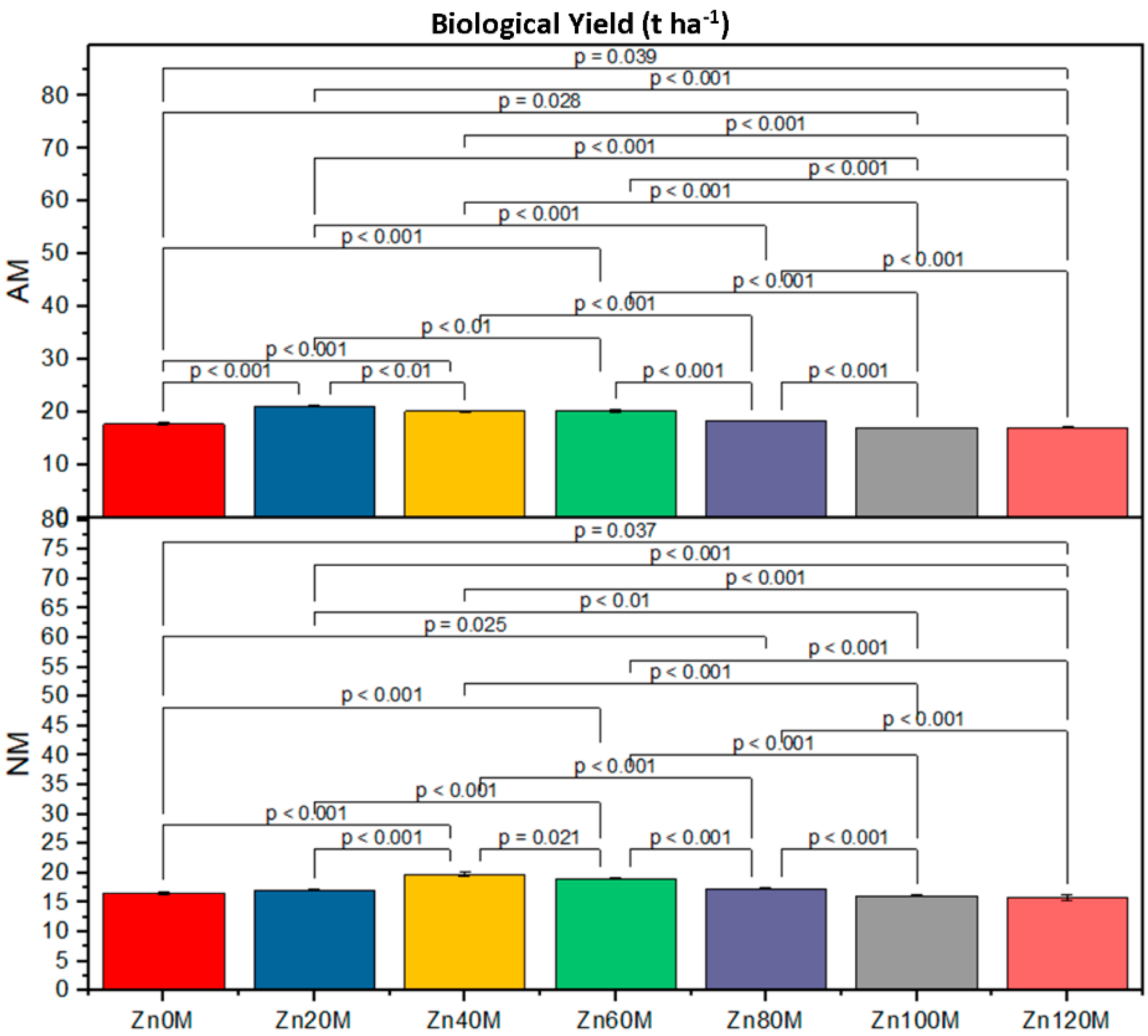
| Treatment | Cob Weight (g) | Cob Length (cm) | 1000-Grain Weight (g) | Biological Yield (t/ha) | ||||
|---|---|---|---|---|---|---|---|---|
| AM | NM | AM | NM | AM | NM | AM | NM | |
| Control (Zn0) | 227.6bc | 210.6d | 16.5a | 15.2ef | 152d | 138g | 4.17c | 3.79d |
| Zn20 | 232.6ab | 222.6c | 20.8a | 16.4d | 168a | 143f | 5.93a | 4.30c |
| Zn40 | 237.6a | 227.6bc | 17.7c | 19.5b | 163b | 158c | 5.21ab | 5.06b |
| Zn60 | 224.6c | 214.6d | 15.5e | 14.5f | 158c | 158c | 5.00c | 4.88e |
| Zn80 | 211.6d | 201.6e | 15.5e | 14.5f | 138g | 146e | 4.76ef | 4.03gh |
| Zn100 | 203.3e | 176g | 15.5e | 14.5f | 132h | 122i | 3.78ef | 16.13h |
| Zn120 | 190f | 139h | 14.5f | 13.4g | 128hi | 117j | 3.72fg | 3.82h |
| AM | *** | *** | *** | *** | ||||
| Zinc | *** | *** | *** | *** | ||||
| AM*Zn | *** | *** | *** | * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saboor, A.; Ali, M.A.; Ahmed, N.; Skalicky, M.; Danish, S.; Fahad, S.; Hassan, F.; Hassan, M.M.; Brestic, M.; EL Sabagh, A.; et al. Biofertilizer-Based Zinc Application Enhances Maize Growth, Gas Exchange Attributes, and Yield in Zinc-Deficient Soil. Agriculture 2021, 11, 310. https://doi.org/10.3390/agriculture11040310
Saboor A, Ali MA, Ahmed N, Skalicky M, Danish S, Fahad S, Hassan F, Hassan MM, Brestic M, EL Sabagh A, et al. Biofertilizer-Based Zinc Application Enhances Maize Growth, Gas Exchange Attributes, and Yield in Zinc-Deficient Soil. Agriculture. 2021; 11(4):310. https://doi.org/10.3390/agriculture11040310
Chicago/Turabian StyleSaboor, Abdul, Muhammad Arif Ali, Niaz Ahmed, Milan Skalicky, Subhan Danish, Shah Fahad, Fahmy Hassan, Mohamed M. Hassan, Marian Brestic, Ayman EL Sabagh, and et al. 2021. "Biofertilizer-Based Zinc Application Enhances Maize Growth, Gas Exchange Attributes, and Yield in Zinc-Deficient Soil" Agriculture 11, no. 4: 310. https://doi.org/10.3390/agriculture11040310
APA StyleSaboor, A., Ali, M. A., Ahmed, N., Skalicky, M., Danish, S., Fahad, S., Hassan, F., Hassan, M. M., Brestic, M., EL Sabagh, A., & Datta, R. (2021). Biofertilizer-Based Zinc Application Enhances Maize Growth, Gas Exchange Attributes, and Yield in Zinc-Deficient Soil. Agriculture, 11(4), 310. https://doi.org/10.3390/agriculture11040310