Evaluation of the Nutrients Uptake by Tomato Plants in Different Phenological Stages Using an Electrical Conductivity Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
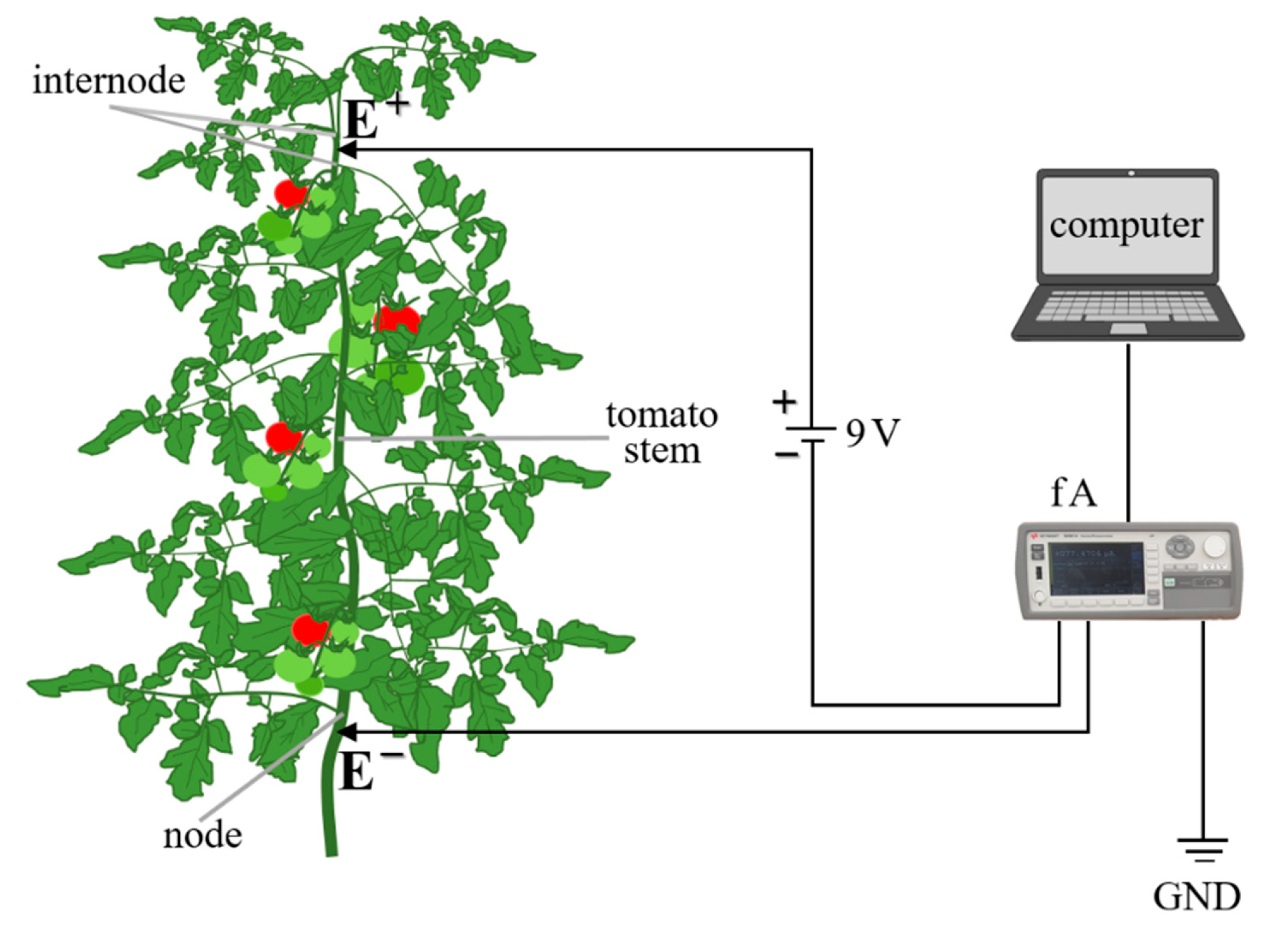
2.2. The Measurements of Nutrient Electrical Signals through Tomato
2.3. Determination of Electrical Conductivity through Plants
2.4. Monitoring and Recording the Electrical Signals
2.5. Statistical Analysis
3. Results and Discussion
3.1. Electrical Signals of the Nutrients
3.2. Electrical Conductivity in the Phenological Stages
3.2.1. Vegetative Stage
3.2.2. Budding Stage
3.2.3. Flowering Stage
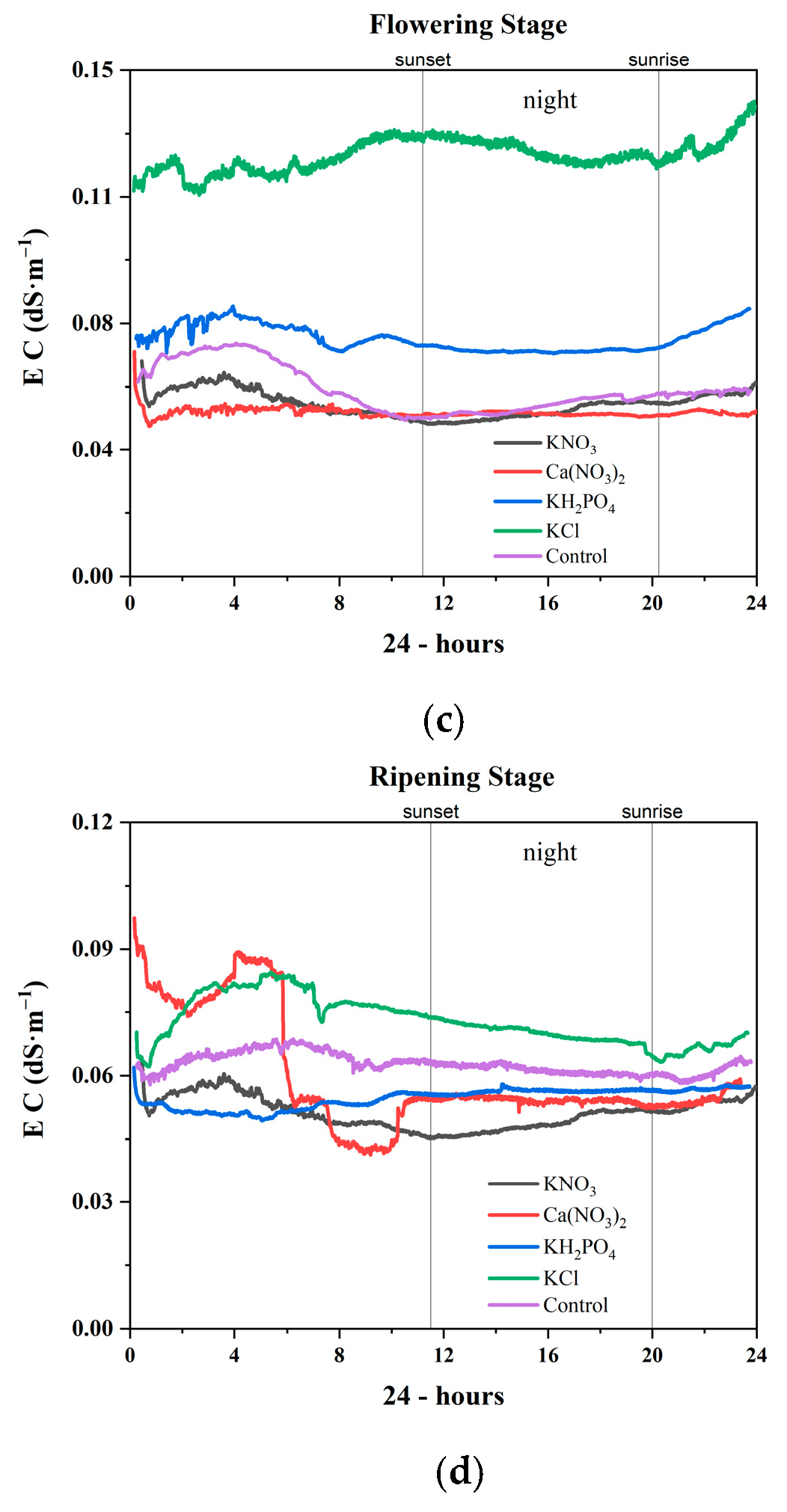
3.2.4. Ripening Stage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, E. Chapter 1—Introduction, Definition and Classification of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 3–5. ISBN 978-0-12-384905-2. [Google Scholar]
- Giehl, R.F.; Von Wirén, N. Root Nutrient Foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodale, I.; Stancu, A. Reversible and Irreversible Processes in Drying and Wetting of Soil. Materials 2019, 13, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachtman, D.P.; Shin, R. Nutrient Sensing and Signaling: NPKS. Annu. Rev. Plant Biol. 2007, 58, 47–69. [Google Scholar] [CrossRef]
- Ho, C.-H.; Lin, S.-H.; Hu, H.-C.; Tsay, Y.-F. CHL1 Functions as a Nitrate Sensor in Plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutiérrez, R.A. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482. [Google Scholar] [CrossRef] [Green Version]
- Ohkubo, Y.; Tanaka, M.; Tabata, R.; Ogawa-Ohnishi, M.; Matsubayashi, Y. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat. Plants 2017, 3, 17029. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2010, 62, 1399–1409. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.; Friedel, S.; Von Wirén, N. Plasticity of the Arabidopsis Root System under Nutrient Deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Canales, J.; Contreras-López, O.; Álvarez, J.M.; Gutiérrez, R.A. Nitrate induction of root hair density is mediated by TGA1/TGA4 and CPC transcription factors in Arabidopsis thaliana. Plant J. 2017, 92, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Shahzad, Z.; Amtmann, A. Food for thought: How nutrients regulate root system architecture. Curr. Opin. Plant Biol. 2017, 39, 80–87. [Google Scholar] [CrossRef]
- Ddamulira, G.; Idd, R.; Namazzi, S.; Kalali, F.; Mundingotto, J.; Maphosa, M. Nitrogen and Potassium Fertilizers Increase Cherry Tomato Height and Yield. J. Agric. Sci. 2019, 11, 48. [Google Scholar] [CrossRef]
- Benton, J.J. Plant Nutrition and Soil Fertility Manual; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-0-429-13081-6. [Google Scholar]
- Pineda-Pineda, J.; Ramírez-Arias, A.; Sánchez del Castillo, F.; Castillo-González, A.M.; Valdez-Aguilar, L.A.; Vargas-Canales, J.M. Extraction and Nutrient Efficiency during the Vegetative Growth of Tomato under Hydroponics Conditions. In Proceedings of the Acta Horticulturae, Leuven, Belgium, 30 April 2011; International Society for Horticultural Science (ISHS); pp. 997–1005. [Google Scholar]
- de Moraes, C.C.; Factor, T.L.; de Araújo, H.S.; Purquerio, L.F.V. Plant growth and nutrient accumulation in two tomato hybrids under tropical conditions. Aust. J. Crop Sci. 2018, 12, 1419–1425. [Google Scholar] [CrossRef]
- Adams, P. Mineral nutrition. In The Tomato Crop: A Scientific Basis for Improvement; Atherton, J., Rudich, J., Eds.; Springer: Dordrecht, The Netherlands, 1986; pp. 281–334. ISBN 978-0-412-25120-7. [Google Scholar]
- Petropoulos, S.A.; Fernandes, Â.; Xyrafis, E.; Polyzos, N.; Antoniadis, V.; Barros, L.; Ferreira, I.C. The Optimization of Nitrogen Fertilization Regulates Crop Performance and Quality of Processing Tomato (Solanum lycopersicum L. cv. Heinz 3402). Agronomy 2020, 10, 715. [Google Scholar] [CrossRef]
- Choi, W.-G.; Hilleary, R.; Swanson, S.J.; Kim, S.-H.; Gilroy, S. Rapid, Long-Distance Electrical and Calcium Signaling in Plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Hlaváčková, V.; Krchňák, P.; Nauš, J.; Novák, O.; Špundová, M.; Strnad, M. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta 2006, 225, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Gallé, A.; Lautner, S.; Flexas, J.; Fromm, J. Environmental stimuli and physiological responses: The current view on electrical signalling. Environ. Exp. Bot. 2015, 114, 15–21. [Google Scholar] [CrossRef]
- Grams, T.E.E.; Koziolek, C.; Lautner, S.; Matyssek, R.; Fromm, J. Distinct roles of electric and hydraulic signals on the reaction of leaf gas exchange upon re-irrigation in Zea mays L. Plant. Cell Environ. 2007, 30, 79–84. [Google Scholar] [CrossRef]
- Rios-Rojas, L.; Tapia, F.; Gurovich, L.A. Electrophysiological assessment of water stress in fruit-bearing woody plants. J. Plant Physiol. 2014, 171, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Mihalache, G.; Peres, C.I.; Bodale, I.; Achitei, V.; Gheorghitoaie, M.V.; Teliban, G.C.; Cojocaru, A.; Butnariu, M.; Muraru, V.; Stoleru, V. Tomato Crop Performances under Chemical Nutrients Monitored by Electric Signal. Agronomy 2020, 10, 1915. [Google Scholar] [CrossRef]
- Lam, V.P.; Kim, S.J.; Park, J.S. Optimizing the Electrical Conductivity of a Nutrient Solution for Plant Growth and Bioactive Compounds of Agastache rugosa in a Plant Factory. Agronomy 2020, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Abou-Hadid, A.; Abd-Elmoniem, E.; El-Shinawy, M.; Abou-Elsoud, M. Electrical Conductivity Effect on Growth and Mineral Composition of Lettuce Plants in Hydroponic System. Acta Hortic. 1996, 59–66. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in a hydroponic system. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef] [PubMed]
- Putranta, H.; Permatasari, A.K.; Sukma, T.A.; Dwandaru, W.S.B. The Effect of PH, Electrical Conductivity, and Nitrogen (N) in the Soil at Yogyakarta Special Region on Tomato Plant Growth. TEM J. 2019, 8, 860–865. [Google Scholar]
- Stoleru, V.; Munteanu, N.; Sellitto, V.M. New Approach of Organic Vegetable Systems; Aracne: Rome, Italy, 2014; ISBN 978-88-548-7847-1. [Google Scholar]
- Acostaquezada, P.G.; Riofrío-Cuenca, T.; Rojas, J.; Vilanova, S.; Plazas, M.; Prohens, J. Phenological growth stages of tree tomato (Solanum betaceum Cav.), an emerging fruit crop, according to the basic and extended BBCH scales. Sci. Hortic. 2016, 199, 216–223. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018; ISBN 978-3-95547-071-5. [Google Scholar]
- Pearson’s Correlation Coefficient. Encyclopedia of Public Health; Kirch, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1090–1091. ISBN 978-1-4020-5614-7. [Google Scholar]
- Stiles, W.; Jörgensen, I. The Measurement of Electrical Conductivity as a Method of Investigation in Plant Physiology. New Phytol. 1914, 13, 226–242. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, Ø.G. Chapter 2—Electrolytics. In Bioimpedance and Bioelectricity Basics, 3rd ed.; Grimnes, S., Martinsen, Ø.G., Eds.; Academic Press: Oxford, UK, 2015; pp. 9–36. ISBN 978-0-12-411470-8. [Google Scholar]




| Treatments | Phenological Stages | ||||
|---|---|---|---|---|---|
| Vegetative | Budding | Flowering | Ripening | ||
| EC ± 4 σ | EC ± σ | EC ± σ | EC ± σ | ||
| KNO3 | 1 Daylight | 0.220 ± 0.0078a 5 | 0.053 ± 0.0037c | 0.051 ± 0.0045bc | 0.060 ± 0.0040ab |
| 2 Night | 0.201 ± 0.0159a | 0.049 ± 0.0009c | 0.050 ± 0.0025c | 0.064 ± 0.0007b | |
| 3 Day | 0.211 ± 0.0155a | 0.051 ± 0.0033c | 0.051 ± 0.0037c | 0.062 ± 0.0033b | |
| Ca(NO3)2 | Daylight | 0.224 ± 0.0058a | 0.049 ± 0.0104c | 0.049 ± 0.0010c | 0.062 ± 0.0161ab |
| Night | 0.216 ± 0.0066a | 0.036 ± 0.0014d | 0.048 ± 0.0005c | 0.054 ± 0.0012c | |
| Day | 0.220 ± 0.0073a | 0.043 ± 0.0102c | 0.049 ± 0.0009c | 0.058 ± 0.0124b | |
| KH2PO4 | Daylight | 0.176 ± 0.0097b | 0.090 ± 0.0026b | 0.072 ± 0.0036b | 0.053 ± 0.0020b |
| Night | 0.194 ± 0.0137a | 0.087 ± 0.0015b | 0.069 ± 0.0032b | 0.057 ± 0.0004c | |
| Day | 0.185 ± 0.0146b | 0.089 ± 0.0024b | 0.070 ± 0.0038b | 0.055 ± 0.0023b | |
| KCl | Daylight | 0.141 ± 0.0086c | 0.124 ± 0.0053a | 0.096 ± 0.0122a | 0.078 ± 0.0037a |
| Night | 0.133 ± 0.0074b | 0.126 ± 0.0029a | 0.114 ± 0.0018a | 0.068 ± 0.0022a | |
| Day | 0.137 ± 0.0089d | 0.125 ± 0.0044a | 0.105 ± 0.0129a | 0.073 ± 0.0056a | |
| Control | Daylight | 0.152 ± 0.0098c | 0.051 ± 0.0020c | 0.057 ± 0.0084bc | 0.065 ± 0.0019ab |
| Night | 0.144 ± 0.0041b | 0.047 ± 0.0036c | 0.052 ± 0.0023c | 0.061 ± 0.0012a | |
| Day | 0.148 ± 0.0087c | 0.049 ± 0.0036c | 0.055 ± 0.0068c | 0.063 ± 0.0024ab[IB1] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodale, I.; Mihalache, G.; Achiţei, V.; Teliban, G.-C.; Cazacu, A.; Stoleru, V. Evaluation of the Nutrients Uptake by Tomato Plants in Different Phenological Stages Using an Electrical Conductivity Technique. Agriculture 2021, 11, 292. https://doi.org/10.3390/agriculture11040292
Bodale I, Mihalache G, Achiţei V, Teliban G-C, Cazacu A, Stoleru V. Evaluation of the Nutrients Uptake by Tomato Plants in Different Phenological Stages Using an Electrical Conductivity Technique. Agriculture. 2021; 11(4):292. https://doi.org/10.3390/agriculture11040292
Chicago/Turabian StyleBodale, Ilie, Gabriela Mihalache, Vladut Achiţei, Gabriel-Ciprian Teliban, Ana Cazacu, and Vasile Stoleru. 2021. "Evaluation of the Nutrients Uptake by Tomato Plants in Different Phenological Stages Using an Electrical Conductivity Technique" Agriculture 11, no. 4: 292. https://doi.org/10.3390/agriculture11040292
APA StyleBodale, I., Mihalache, G., Achiţei, V., Teliban, G.-C., Cazacu, A., & Stoleru, V. (2021). Evaluation of the Nutrients Uptake by Tomato Plants in Different Phenological Stages Using an Electrical Conductivity Technique. Agriculture, 11(4), 292. https://doi.org/10.3390/agriculture11040292









