Selective and Sensitive Quantification of Acetochlor and S-Metolachlor in Maize and Soybean Plant Samples by Gas Chromatography-Tandem Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
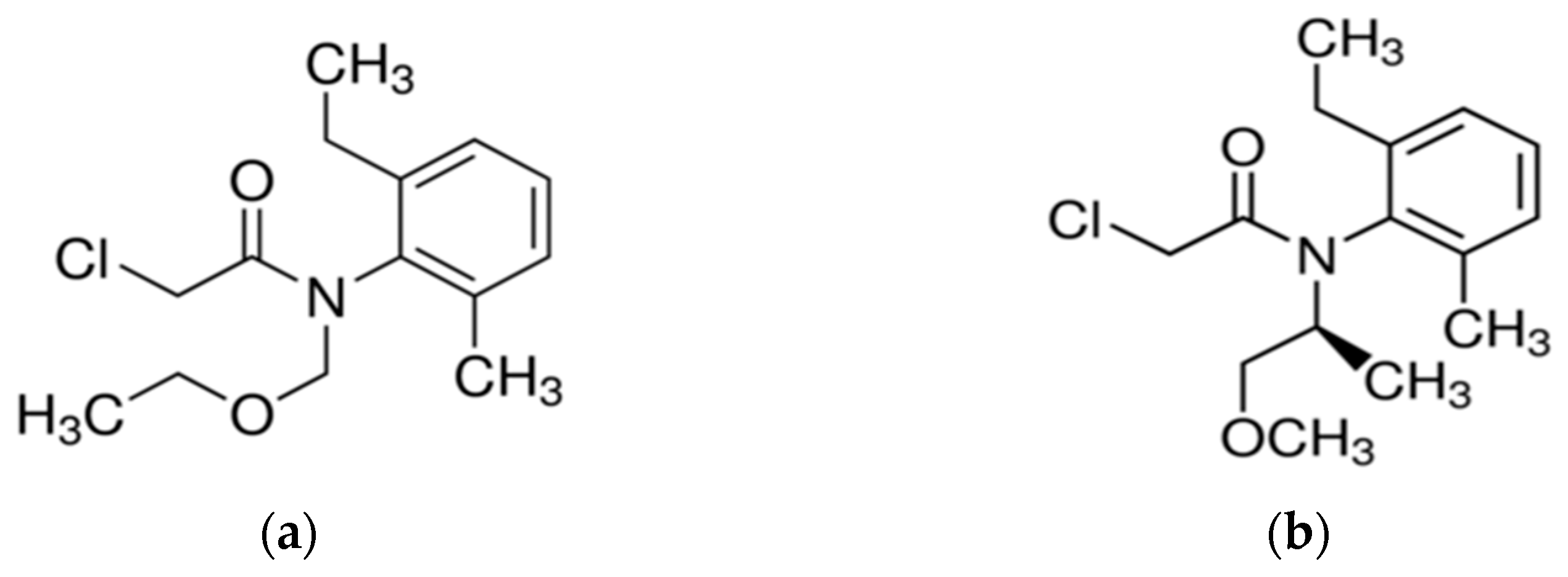
2.1. Chemicals and Materials
2.2. Instrumental
2.3. Sample Collection and Preparation
2.4. Accelerated Solvent Extraction Procedure
2.5. Method Validation
3. Results
3.1. Optimization of the ASE Procedure
3.1.1. Solvent Selection
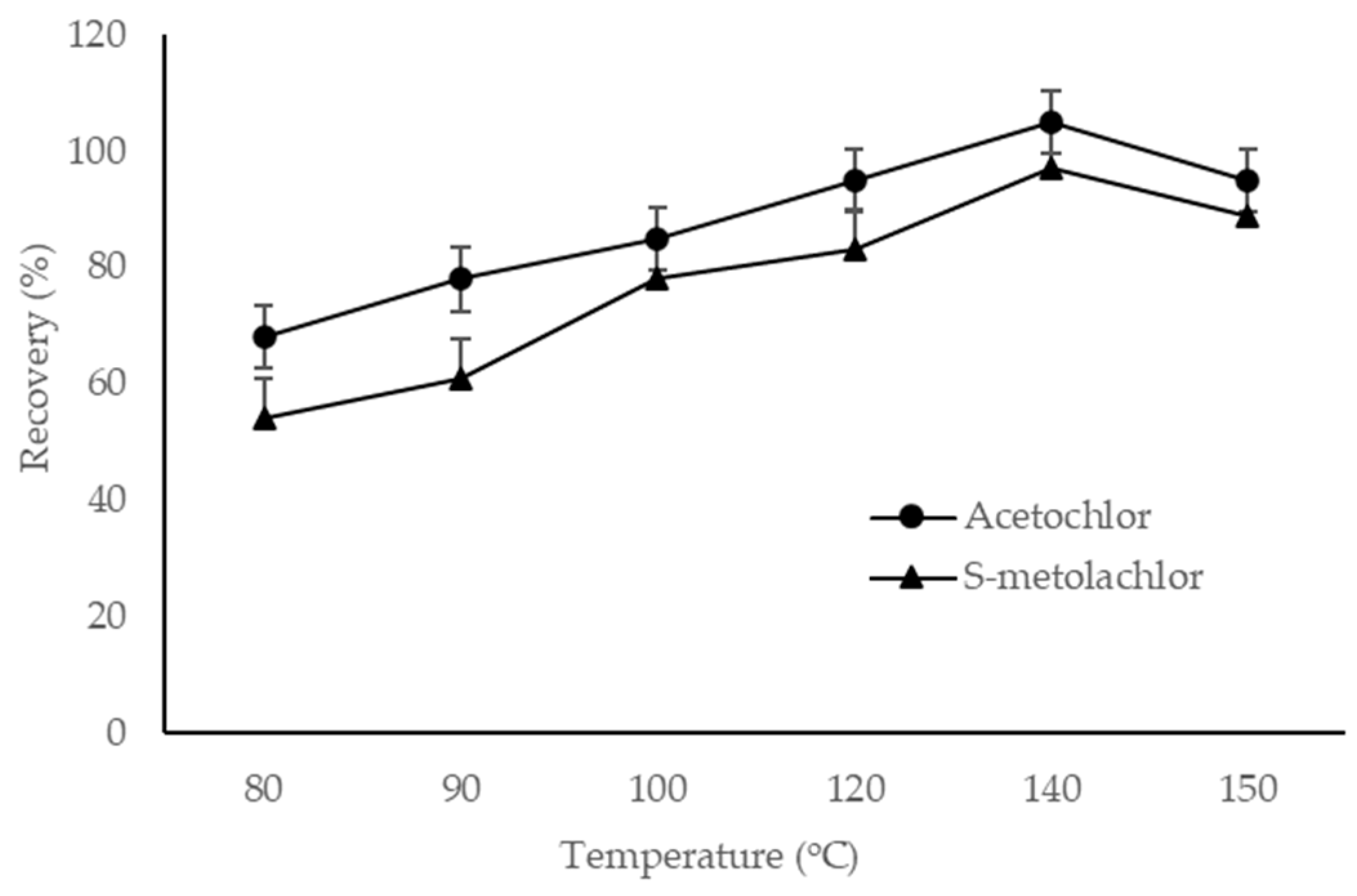
3.1.2. Effect of Temperature
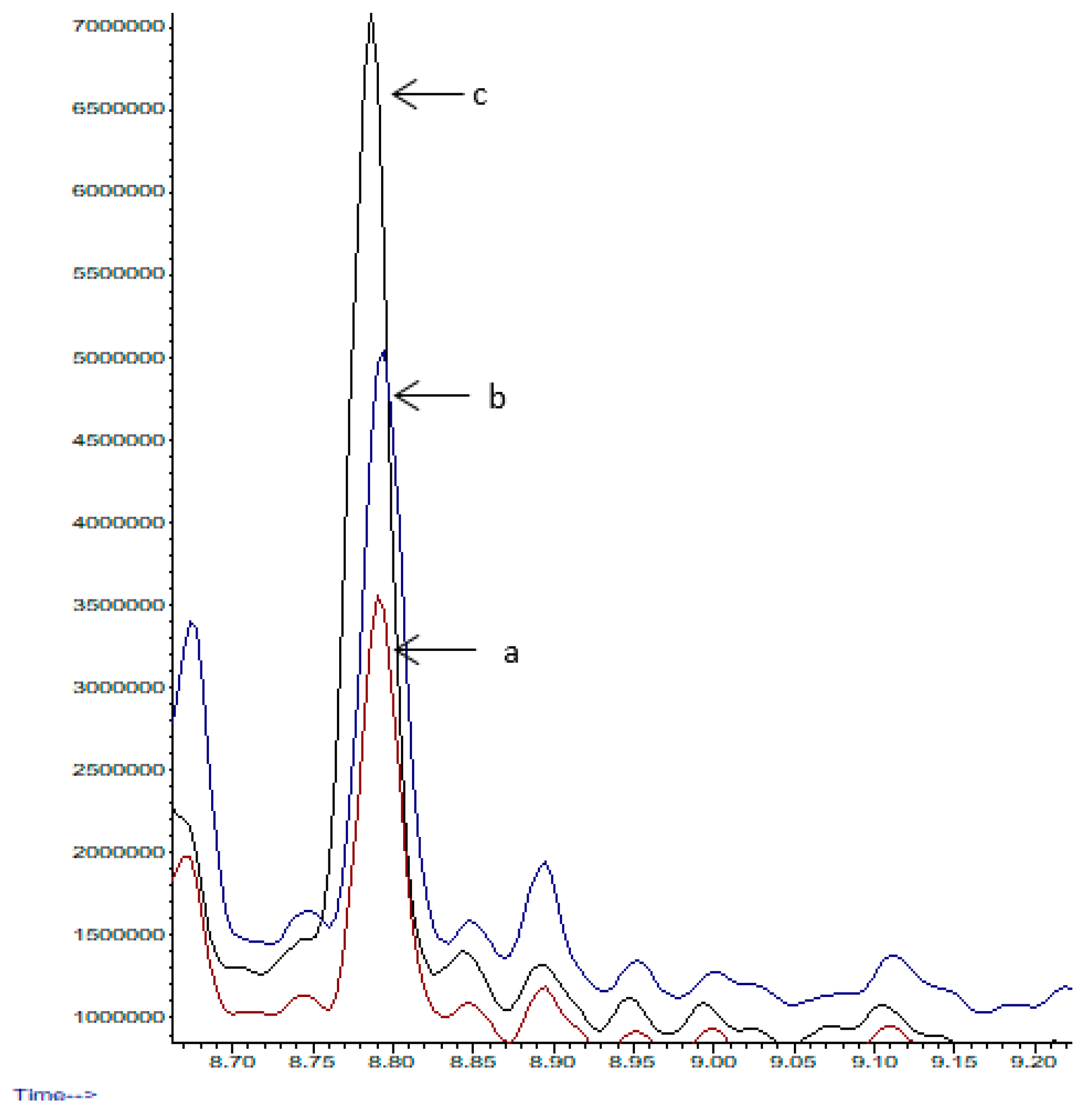
3.1.3. Effect of Exposure and Number of Extraction Cycles
3.2. Method Validation
3.3. Application to Real Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of Electricity and Heat from Biomass Waste using a Converted Aircraft Turbine AI-20. Processes 2021, 9, 364. [Google Scholar] [CrossRef]
- Harischandra, N.R.; Pallavi, M.S.; Bheemanna, M.; PavanKumar, K.; Reddy, V.C.S.; Udaykumar, N.R.; Paramasivam, M.; Yadav, S. Simultaneous determination of 79 pesticides in pigeonpea grains using GC-MS/MS and LC-MS/MS. Food Chem. 2021, 347. [Google Scholar] [CrossRef]
- Ju, C.; Zhang, H.; Wu, R.; Dong, S.; Yao, S.; Wang, F.; Cao, D.; Xu, S.; Fang, H.; Yu, Y. Upward translocation of acetochlor and atrazine in wheat plants depends on their distribution in roots. Sci. Total Environ. 2020, 703. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, P.; He, Y.; Xu, Z.; He, X.; Hu, Y.; Zhang, H.; He, L. Dissipation Dynamics and Residue of Four Herbicides in Paddy Fields using HPLC-MS/MS and GC-MS. Int. J. Environ. Res. Public Health 2019, 16, 236. [Google Scholar] [CrossRef]
- Pannacci, E.; Del Buono, D.; Bartucca, M.L.; Nasini, L.; Proietti, P.; Tei, F. Herbicide uptake and regrowth ability of Tall Fescue and Orchardgrass in s-metolachlor-contaminated leachates from sand pot experiment. Agriculture 2020, 10, 487. [Google Scholar] [CrossRef]
- Chang, X.; Liang, J.; Sun, Y.; Zhao, L.; Zhou, B.; Li, X.; Li, Y. Isolation, degradation performance and field application of the metolachlor-degrading fungus Penicillium oxalicum MET-F-1. Appl. Sci. 2020, 10, 8556. [Google Scholar] [CrossRef]
- PPDB (Pesticide Properties Data Base). Acetochlor, General Information. University of Hertfordshire, UK. 2020. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/12.htm (accessed on 15 December 2020).
- Janniche, G.S.; Mouvet, C.; Albrechtsen, H.J. Acetochlor sorption and degradation in limestone subsurface and aquifers. Pest. Manag. Sci. 2010, 66, 1287–1297. [Google Scholar] [CrossRef]
- Wu, X.; Li, M.; Long, Y.; Liu, R.; Yu, Y.; Fang, H.; Li, S. Effects of adsorption on degradation and bioavailability of metolachlor in soil. J. Soil Sci. Plant Nutr. 2011, 11, 83–97. [Google Scholar]
- Velisek, J.; Stara, A.; Zuskova, E.; Kubec, J.; Buric, M.; Kouba, A. Effects of s-metolachlor on early life stages of marbled crayfish. Pestic. Biochem. Physiol. 2019, 153, 87–94. [Google Scholar] [CrossRef]
- Zemolin, C.R.; Avila, L.; Cassol, G.; Massey, J.H.; Camargo, E. Environmental fate of S-Metolachlor: A review. Planta Daninha 2014, 32, 655–664. [Google Scholar] [CrossRef]
- PPDB (Pesticide Properties Data Base). S-metolachlor, General Information. University of Hertfordshire, UK. 2020. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/465.htm (accessed on 17 December 2020).
- Jiang, J.; Wu, S.; Liu, X.; Wang, Y.; An, X.; Cai, L.; Zhao, X. Effect of acetochlor on transcription of genes associated with oxidative stress, apoptosis, immunotoxicity and endocrine disruption in the early life stage of zebrafish. Environ. Toxicol. Pharmacol. 2015, 40, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Eu, Pesticide MRL Database. 2020. Available online: https://ec.europa.eu/food/plant/pesticides/max_residue_levels/eu_rules_en (accessed on 20 January 2021).
- Wang, D.; Wu, S. Development and validation of a GC-MS method for the simultaneous determination of acetochlor, fluorochloridone and pendimethalin in herbicide emulsifiable concentrate formulation. Acta Chromatograph. 2020, 32, 238–241. [Google Scholar] [CrossRef]
- Ping, H.; Pan, L.G.; Shu, X.L.; Lu, A.X. Multiresidue Determination of Thifensulfuron-Methyl, Atrazine and Acetochlor in Soybeans by High Performance Liquid Chromatography. Sens. Lett. 2011, 9, 1180–1183. [Google Scholar] [CrossRef]
- Wu, X.; Shen, S.; Yan, H.; Yuan, Y.; Chen, X. Efficient enrichment and analysis of atrazine and its degradation products in Chinese Yam using accelerated solvent extraction and pipette tip solid-phase extraction followed by UPLC–DAD. Food Chem. 2021, 337. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, C.; Gartner, M.; Mentler, A.; Fuerhacker, M. Comparision of four extraction methods for the analysis of 24 pesticides in soil samples with gas chromatography-mass spectrometry and liquid chromatography ion trap mass spectrometry. Talanta 2008, 75, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Pensabene, J.W.; Fiddler, W.; Donoghue, D.J. Supercritical fluid extraction of atrazine and other triazine herbicides from fortified and incurred eggs. J. Agric. Food Chem. 2000, 48, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Cheng, H. Rapid extraction and determination of atrazine and its degradation products from microporous mineral sorbents using microwave-assisted solvent extraction followed by ultra-HPLC-MS/MS. Microchim. Acta 2013, 180, 703–710. [Google Scholar] [CrossRef]
- Megersa, N. Hollow fiber liquid phase microextraction for race enrichment of the residues of atrazine and its major degradation products from environmental water and human urine samples. Anal. Methods 2015, 7, 9940–9948. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, A.J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Xu, X.Q.; Li, Q.L.; Yuan, J.D.; Wang, S.G.; Wang, W.S.; Lee, F.S.C.; Wang, X.R. Determination of three kinds of chloroacetanilide herbicides in Radix Pseudostellariae by Accelerated Solvent Extraction and Gas chromatography mass spectrometry. Chin. J Anal. Chem. 2007, 35, 206–210. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, B.; Xie, K.; Liu, Y.; Zhang, Y.; Liu, C.; Guo, Y.; Bu, X.; Zhang, G.; Zhang, T.; et al. Development of an Ase-Gs-ms/ms method for detecting dinitolmide and its metabolite 3-ANOT in eggs. J. Mass. Spectrom. 2018, 53, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Tomkins, D.F. Determination of acetochlor in technical and formulated products by capillary gas chromatography. Agric. Mat. 2001, 84, 317–322. [Google Scholar] [CrossRef]
- Blanchoud, H.; Alliot, F.; Chen, N.; Valdes, D. Rapid SPE-LCMS/MS analysis of atrazine, its by products, simazine and s-metolachlor in groundwater samples. Methodsx 2020, 7. [Google Scholar] [CrossRef]
- Bodur, S.; Borahan, T.; Ates, N.; Bakirdere, S. Sensitive determination of acetochlor, metolachlor and fenthion utilizing shaking assisted dispersive liquid-liquid microextraction prior to gas chromatography-mass spectrometry. B Environ. Contam. Toxicol. 2020, 105, 460–467. [Google Scholar] [CrossRef]
- Yokley, R.A.; Mayer, L.C.; Huang, S.B.; Vargo, J.D. Analytical method for the determination of metolaclor, acetochlor, alachlor, dimethenamid and their corresponding ethanesulfonic and oxanilic acid degrdates in water using SPE and LC/ESI-MS/MS. Anal. Chem. 2002, 74, 3754–3759. [Google Scholar] [CrossRef]
- Ning, M.; Jun, Y.; Meseret, A.; Langping, W.; Geoffrey, S.; Li, H.; Chen, Y.; Liu, J.; Mihuez, V.G. Accelerated solvent extraction combined with GC-MS: A convenient technique for the determination and compound specific stable isotope analysis of phthalates in mine tailings. Microchem. J. 2020, 153. [Google Scholar] [CrossRef]
- Sun, H.W.; Ge, X.S.; Lv, Y.K.; Wang, A.B. Application of accelerate solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feeds. J. Chrom. A 2012, 1237, 1–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Shi, R.; Su, Q.; Yao, L.; Li, P. Determination of acetanilide herbicides in cereal crops using accelerated solvent extraction, solid-phase extraction and gas chromatography-electron capture detector. J. Sep. Sci. 2011, 34, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Q.; Wang, L.; Shao, J.; Mei, W.; Wang, L. Development and application of a dispersive solid-phase extraction method for the simultaneous determination of chloroacetamide herbicide residues in soil by gas chromatography-tandem mass spectrometry (GC-MS/MS). Int. J. Environ. Anal. Chem. 2019, 99, 282–296. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Peng, C.; Li, X.; Xu, C.; Li, C. A rapid multi-residue determination method of herbicides in grain by GC-MS-SIM. J. Chromatogr. Sci. 2008, 46, 424–429. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Wang, L.; Han, B.; Wang, X.; Lee, F.S.-C. Analysis of chloroacetanilide herbicides in water samples by solid-phase microextraction coupled with gas chromatography–mass spectrometry. Anal. Chim. Acta 2007, 591, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, A.; Roman, K.; Roman, M.; Sliwinski, D.; Roman, M. Energy Efficiency of Maize production Technology: Evidence from polish Farms. Energies 2021, 14, 1–20. [Google Scholar]
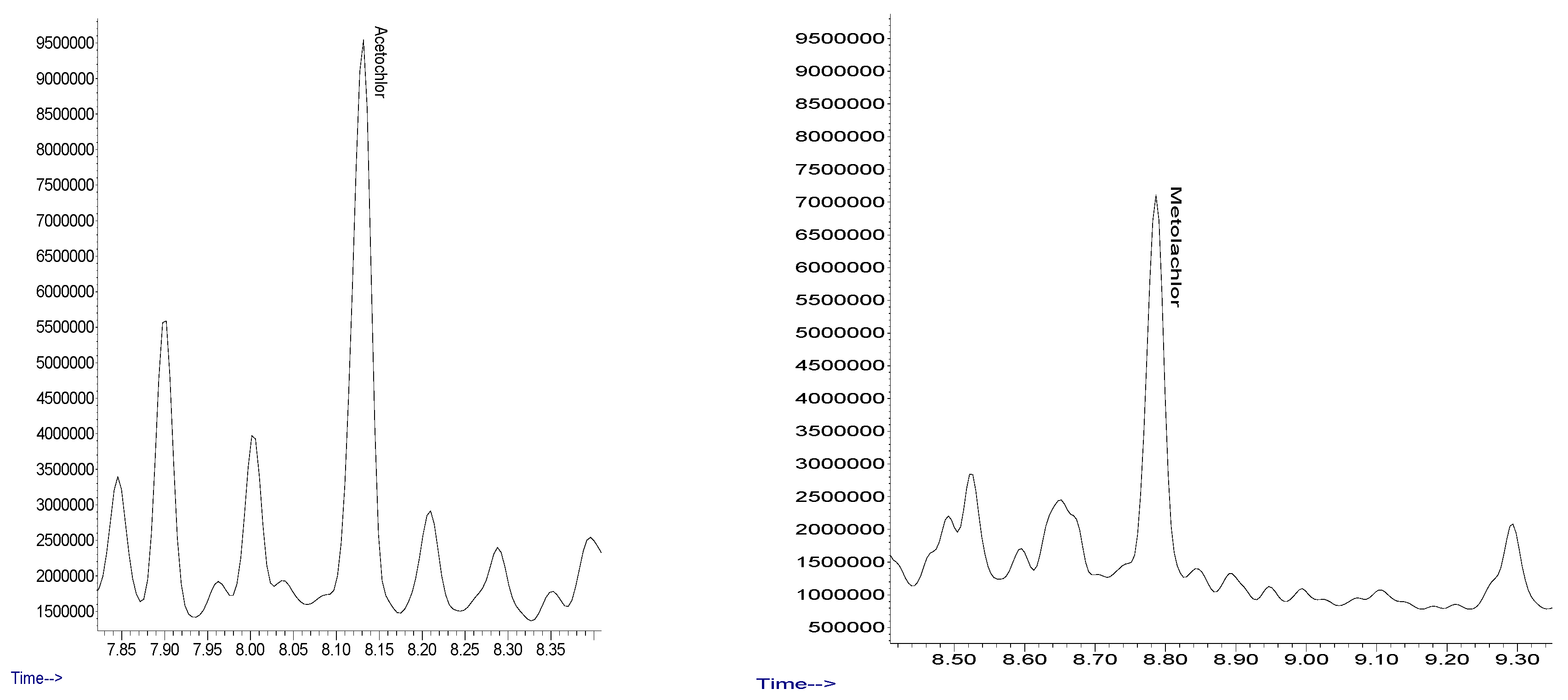

| Analyte | Retention Time | Qualifying Ions (m/z) | Quantifying Ions (m/z) |
|---|---|---|---|
| Acetochlor | 8.10 | 162.0, 146.0, 174.0 | 146.0 |
| s-metolachlor | 8.78 | 162.0, 238.0, 211.0 | 162.0 |
| Analyte | Correlation Coefficient (R2) | Linear Range (mg L−1) | LOD (ng g−1) | LOQ (ng g−1) | RSD (%) |
|---|---|---|---|---|---|
| Acetochlor | 0.993 | 0.001–0.05 | 0.2 | 0.67 | 1.7 |
| s-metolachlor | 0.997 | 0.001–0.05 | 0.07 | 0.19 | 6.9 |
| Analyte | Straw Sample n = 5 | R2 | Me | Mean Recovery (%) | Intraday RSD (%) | Interday RSD (%) | |
|---|---|---|---|---|---|---|---|
| 0.05 mg kg−1 | 0.1 mg kg−1 | ||||||
| Acetochlor | Maize | 0.996 | 0.87 | 85.5 | 104.7 | 1.2–3.4 | 2.6 |
| s-metolachlor | Soybean | 0.998 | 0.92 | 83.4 | 112.9 | 2.4–4.8 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cara, I.G.; Topa, D.; Raus, L.; Calistru, A.E.; Filipov, F.; Jitareanu, G. Selective and Sensitive Quantification of Acetochlor and S-Metolachlor in Maize and Soybean Plant Samples by Gas Chromatography-Tandem Mass Spectrometry. Agriculture 2021, 11, 283. https://doi.org/10.3390/agriculture11040283
Cara IG, Topa D, Raus L, Calistru AE, Filipov F, Jitareanu G. Selective and Sensitive Quantification of Acetochlor and S-Metolachlor in Maize and Soybean Plant Samples by Gas Chromatography-Tandem Mass Spectrometry. Agriculture. 2021; 11(4):283. https://doi.org/10.3390/agriculture11040283
Chicago/Turabian StyleCara, Irina Gabriela, Denis Topa, Lucian Raus, Anca Elena Calistru, Feodor Filipov, and Gerard Jitareanu. 2021. "Selective and Sensitive Quantification of Acetochlor and S-Metolachlor in Maize and Soybean Plant Samples by Gas Chromatography-Tandem Mass Spectrometry" Agriculture 11, no. 4: 283. https://doi.org/10.3390/agriculture11040283
APA StyleCara, I. G., Topa, D., Raus, L., Calistru, A. E., Filipov, F., & Jitareanu, G. (2021). Selective and Sensitive Quantification of Acetochlor and S-Metolachlor in Maize and Soybean Plant Samples by Gas Chromatography-Tandem Mass Spectrometry. Agriculture, 11(4), 283. https://doi.org/10.3390/agriculture11040283







