Effects of Carbon Ion Beam Irradiation on Phenotypic Variations and Biochemical Parameters in Early Generations of Soybean Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Irradiation Treatments
2.2. Measurement of Biochemical Parameters
2.3. Statistics Analysis
3. Results
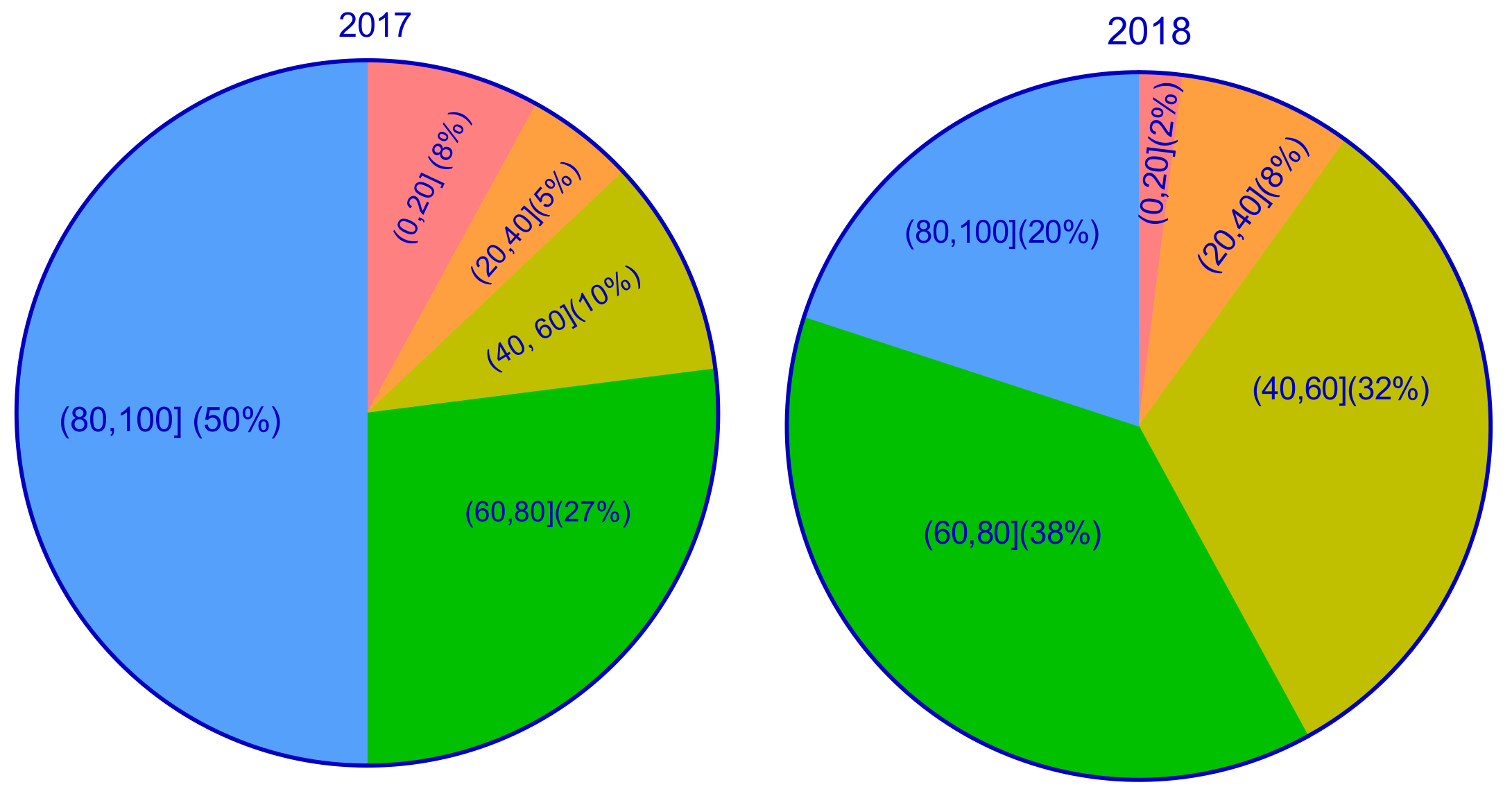
3.1. Effects of CIBR on Emergence Rate and Survival Rate in the M1 and M2 Generations
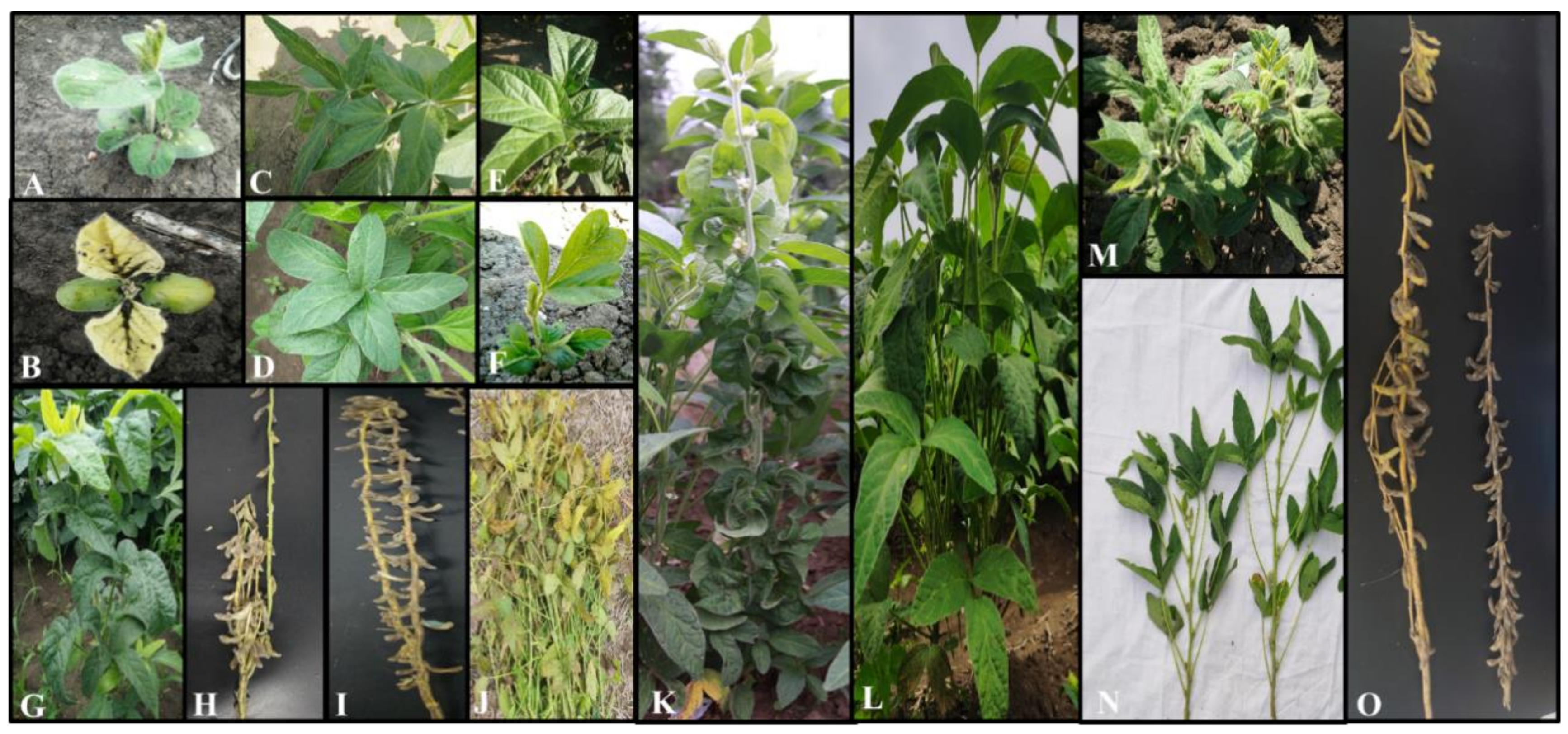
3.2. Screening for Visual Morphological Traits Mutants
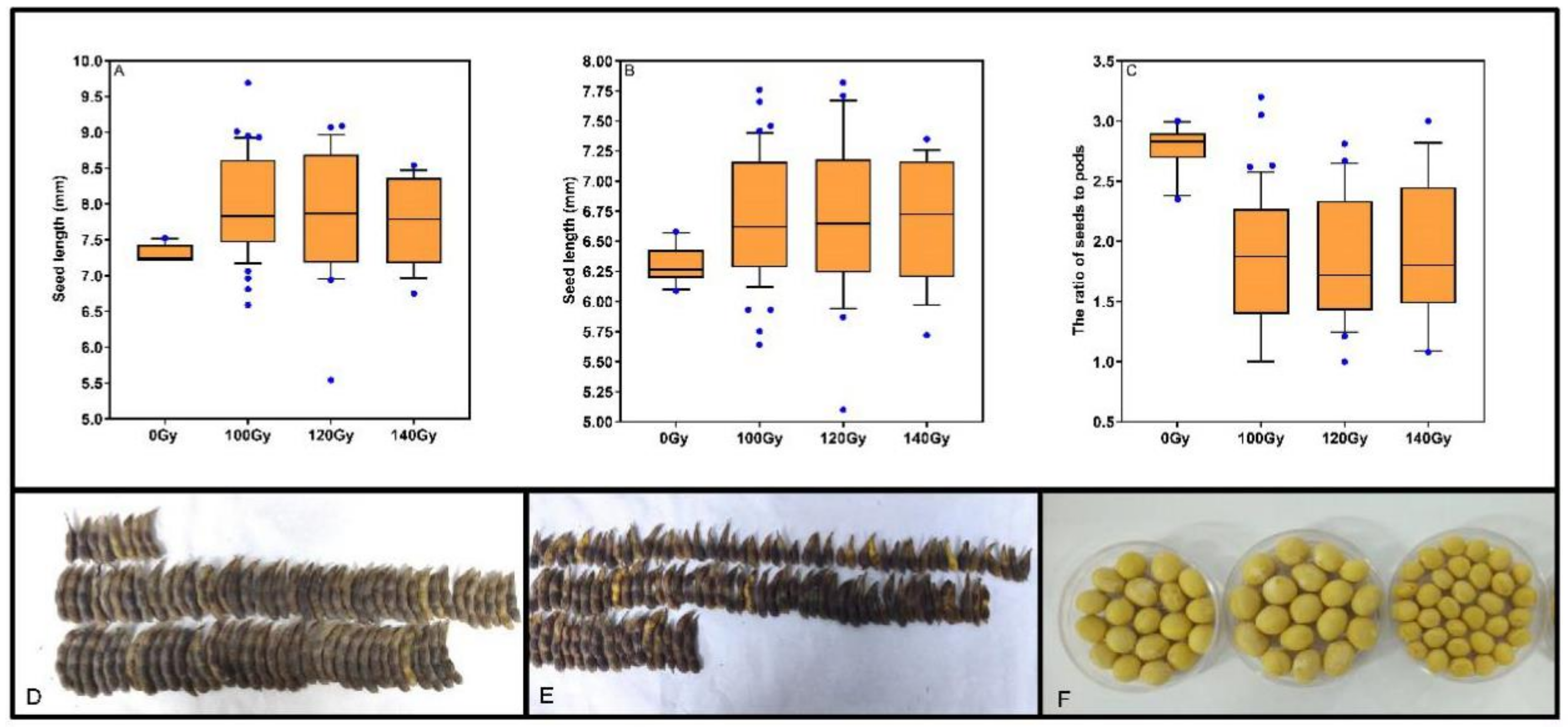
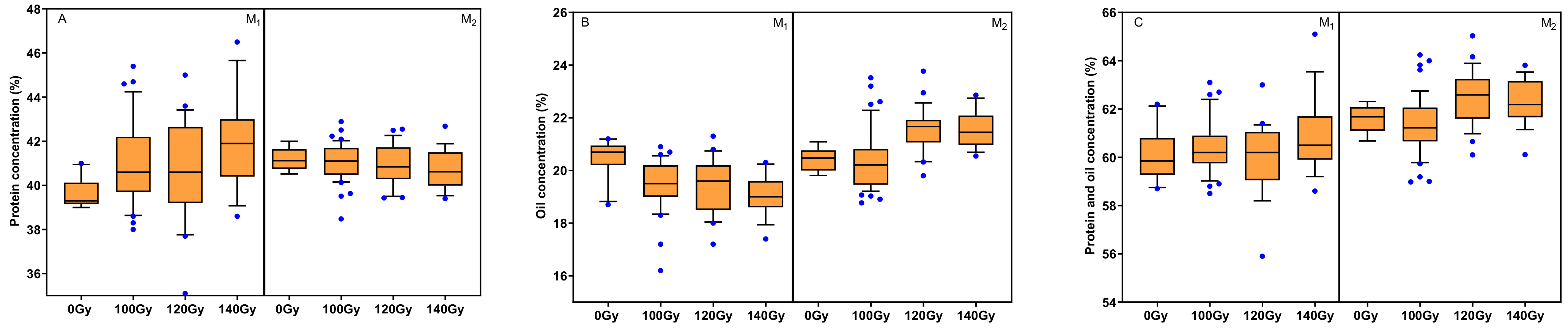
3.3. Effects of Carbon Ion Beam Radiation on Seeds Size and Seed Composition
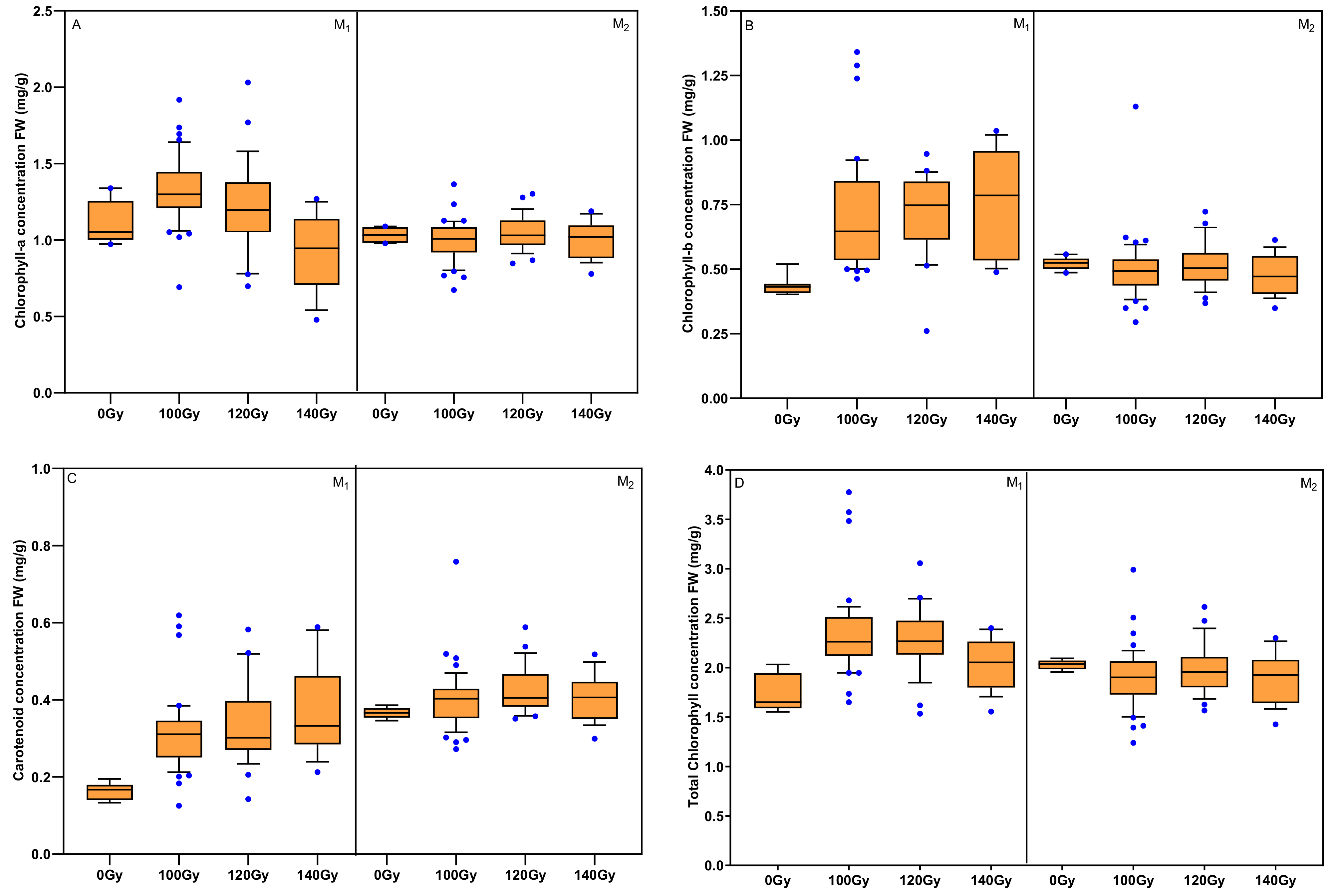
3.4. Effects of Carbon Ion Beam Radiation on Chlorophyll Concentration
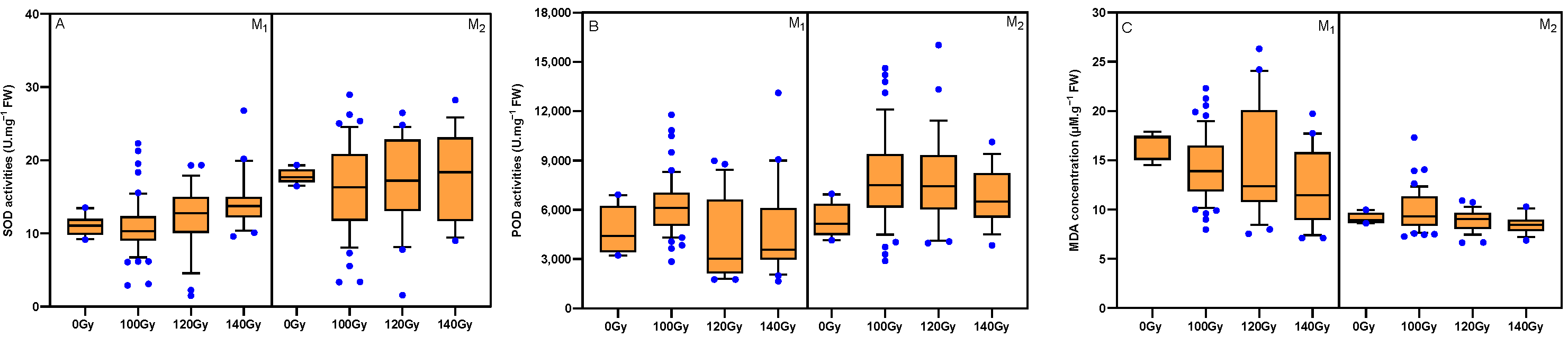
3.5. Effects of Carbon Ion Beam Radiation on Antioxidant Enzyme Activities and MDA Concentration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef]
- Singh, M.P.; Kumar, S. Genetics and Plant Breeding; APH Publishing Corporation: New Delhi, India, 2009. [Google Scholar]
- Novak, F.J.; Brunner, H. Plant breeding: Induced mutation technology for crop improvement. IAEA Bull. 1992, 4, 25–33. [Google Scholar]
- Lee, D.K.; Kim, Y.S.; Kim, J.K. Determination of the optimal condition for Ethyl methane sulfonate-mediated mutagenesis in a Korean commercial rice, Japonica cv. Dongjin. Appl. Biol. Chem. 2017, 60, 241–247. [Google Scholar] [CrossRef]
- Espina, M.J.; Sabbir Ahmed, C.M.; Angelina, B.; Ekundayo, A.; Zeinab, Y.; Prakash, A.; Vince, P.; Ali, T. Development and phenotypic screening of an ethyl methane sulfonate mutant population in soybean. Front. Plant. Sci. 2018, 9, 394. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Ma, Y.; Wei, Z.; Hong, H.; Liu, Z.; Lei, J.; Liu, Y.; Guan, R.; Guo, Y.; et al. Development and utilization of a new chemically-induced soybean library with a high mutation density. J. Integr. Plant Biol. 2017, 59, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Mba, C.; Afza, R.; Bado, S.; Jain, S.M. Induced Mutagenesis in Plants Using Physical and Chemical Agents. In Plant Cell Culture; Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 111–130. [Google Scholar]
- Stadler, L.J. Mutations in Barley Induced by X-Rays and Radium. Science 1928, 68, 186–187. [Google Scholar] [CrossRef]
- Bolon, Y.-T.; Haun, W.J.; Xu, W.W.; Grant, D.; Stacey, M.G.; Nelson, R.T.; Gerhardt, D.J.; Jeddeloh, J.A.; Stacey, G.; Muehlbauer, G.J.; et al. Phenotypic and Genomic Analyses of a Fast Neutron Mutant Population Resource in Soybean. Plant Physiol. 2011, 156, 240–253. [Google Scholar] [CrossRef]
- Ha, B.-K.; Lee, K.J.; Velusamy, V.; Kim, J.-B.; Kim, S.H.; Ahn, J.-W.; Kang, S.-Y.; Kim, D.S. Improvement of soybean through radiation-induced mutation breeding techniques in Korea. Plant Genet. Res. 2014, 12, S54–S57. [Google Scholar] [CrossRef]
- Kraft, G. Tumor therapy with heavy charged particles. Prog. Part. Nucl. Phys. 2000, 45, S473–S544. [Google Scholar] [CrossRef]
- Zhao, S.G.; Tang, M.L.; Wang, J.; Wang, T.; Wang, S.C.; Wu, Y.J.; Yu, Z.L. Mutagenic effects of BM302:Go112 induced by low-energy ion beam implantation. Plasma. Sci. Technol. 2007, 9, 508–512. [Google Scholar]
- Becker, D.; Razskazovskii, Y.; Callaghan, M.U.; Sevilla, M.D. Electron Spin Resonance of DNA Irradiated with a Heavy-Ion Beam (16O8+): Evidence for Damage to the Deoxyribose Phosphate Backbone. Radiat. Res. 1996, 146, 361. [Google Scholar] [CrossRef]
- Hase, Y.; Nozawa, S.; Narumi, I.; Oono, Y. Effects of ion beam irradiation on size of mutant sector and genetic damage in Arabidopsis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 391, 14–19. [Google Scholar] [CrossRef]
- Tanaka, A.; Shikazono, N.; Hase, Y. Studies on Biological Effects of Ion Beams on Lethality, Molecular Nature of Mutation, Mutation Rate, and Spectrum of Mutation Phenotype for Mutation Breeding in Higher Plants. J. Radiat. Res. 2010, 51, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhou, L.; Li, W.; Du, Y.; Yu, L.; Feng, H.; Mu, J.; Chen, Y. Mutagenic effects of carbon ion beam irradiations on dry Lotus japonicus seeds. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2016, 383, 123–128. [Google Scholar] [CrossRef]
- Shikazono, N.; Tanaka, A.; Watanabe, H.; Tano, S. Rearrangements of the DNA in carbon ion-induced mutants of Arabidopsis thaliana. Genet. 2001, 157, 379–387. [Google Scholar]
- Shikazono, N.; Yokota, Y.; Tanaka, A.; Watanabe, H.; Tano, S. Molecular analysis of carbon ion-induced mutations in Arabidopsis thaliana. Genes Genet. Syst. 1998, 73, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hase, Y.; Tanaka, A.; Shikazono, N.; Degi, K.; Shimizu, A.; Morishita, T. Mutagenic effects of ion beam irradiation on rice. Breed. Sci. 2009, 59, 169–177. [Google Scholar] [CrossRef]
- Mikuriya, S.; Kasai, M.; Nakashima, K.; Natasia; Hase, Y.; Yamada, T.; Abe, J.; Kanazawa, A. Frequent generation of mutants with coincidental changes in multiple traits via ion-beam irradiation in soybean. Genes Genet. Syst. 2017, 92, 153–161. [Google Scholar] [CrossRef][Green Version]
- Roychowdhury, R.; Datta, S.; Gupta, P.; Tah, J. Analysis of Genetic Parameters on Mutant Populations of Mungbean (Vigna radiata L.) after Ethyl Methane Sulphonate Treatment. Not. Sci. Biol. 2012, 4, 137–143. [Google Scholar] [CrossRef]
- Alikamanoglu, S.; Yaycili, O.; Sen, A. Effect of gamma radiation on growth factors, biochemical parameters, and accumulation of trace elements in soybean plants (Glycine maxl. Merrill). Biol. Trace. Elem. Res. 2011, 141, 283–293. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.M.; Chongling, Y.; Islam, K.S. Changes in growth and antioxidant enzymes activities during cadmium stress in the mangrove plant Kandelia candel (L.) Druce. Aes. Bioflux. 2010, 2, 15–24. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Kocsy, G.; Laurie, R.; Szalai, G.; Szilágyi, V.; Galiba, G.; De Ronde, J.A.; Simon-Sarkadi, L. Genetic manipulation of proline levels affects antioxidants in soybean subjected to simultaneous drought and heat stresses. Physiol. Plant. 2005, 124, 227–235. [Google Scholar] [CrossRef]
- Moussa, H. Low dose of gamma irradiation enhanced drought tolerance in soybean. Bulg. J. Agric. Sci. 2011, 17, 63–72. [Google Scholar] [CrossRef]
- Jan, S.; Parween, T.; Siddiqi, T.O.; Mahmooduzzafar. Gamma radiation effects on growth and yield attributes of Psoralea corylifolia L. with reference to enhanced production of psoralen. Plant Growth Regul. 2010, 64, 163–171. [Google Scholar]
- Maity, J.; Mishra, D.; Chakraborty, A.; Saha, A.; Santra, S.; Chanda, S. Modulation of some quantitative and qualitative characteristics in rice (Oryza sativa L.) and mung (Phaseolus mungo L.) by ionizing radiation. . Radiat. Phys. Chem. 2005, 74, 391–394. [Google Scholar]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Haq, Z.-U.; Abbas, M. Laser light and magnetic field stimulation effect on biochemical, enzymes activities and chlorophyll contents in soybean seeds and seedlings during early growth stages. J. Photochem. Photobiol. B Biol. 2016, 165, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Parween, T.; Siddiqi, T.; Mahmooduzzafar. Effect of gamma radiation on morphological, biochemical, and physiological aspects of plants and plant products. Environ. Rev. 2012, 20, 17–39. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Einhellig, F.A.; Rasmussen, J.A. Effects of three phenolic acids on chlorophyll content and growth of soybean and grain sorghum seedlings. J. Chem. Ecol. 1979, 5, 815–824. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yu, L.X.; Li, Y.S.; Du, Y.; Li, W.J.; Liu, X.B. Preliminary investigation of acceptable heavy ion beam irradiation dosage treated to soybean seed. Soyb. Sci. 2013, 32, 587–590, (Chinese with English Summary). [Google Scholar]
- Kaul, M.L.H.; Bhan, A.K. Mutagenic effectiveness and efficiency of EMS, DES and gamma-rays in rice. Theor. Appl. Genet. 1977, 50, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.R.; Ahmed, O.H.; Saamin, S.; Majid, N.M.A. Gamma radio sensitivity study on chili (Capsicum annuum); Technical report. Am. J. Appl. Sci. 2008, 5, 67–70. [Google Scholar]
- Chaudhuri, S.K. A simple and reliable method to detect gamma irradiated lentil (Lens culinaris Medik.) seeds by germination efficiency and seedling growth test. Radiat. Phys. Chem. 2002, 64, 131–136. [Google Scholar] [CrossRef]
- Patil, A.; Tawaresp, S.P.; Oak, M.D.; Tamhankar, S.A.; Rao, V.S. Improvement of oil quality in soybean [Glycine max (L.) Merrill] by mutation breeding. J. Am. Oil. Chem. Soc. 2007, 84, 1117–1124. [Google Scholar] [CrossRef]
- Adams, W.W.; Winter, K.; Schreiber, U.; Schramel, P. Photosynthesis and Chlorophyll Fluorescence Characteristics in Relationship to Changes in Pigment and Element Composition of Leaves of Platanus occidentalis L. during Autumnal Leaf Senescence. Plant Physiol. 1990, 92, 1184–1190. [Google Scholar] [CrossRef]
- Sakowska, K.; Alberti, G.; Genesio, L.; Peressotti, A.; Vedove, G.D.; Gianelle, D.; Colombo, R.; Rodeghiero, M.; Panigada, C.; Juszczak, R.; et al. Leaf and canopy photosynthesis of a chlorophyll deficient soybean mutant. Plant Cell Environ. 2018, 41, 1427–1437. [Google Scholar] [CrossRef]
- Arase, S.; Hase, Y.; Abe, J.; Kasai, M.; Yamada, T.; Kitamura, K.; Narumi, I.; Tanaka, A.; Kanazawa, A. Optimization of ion-beam irradiation for mutagenesis in soybean: Effects on plant growth and production of visibly altered mutants. Plant Biotechnol. 2011, 28, 323–329. [Google Scholar] [CrossRef]
- Jamil, Y.; Perveen, R.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He–Ne laser-induced changes in germination, thermodynamic parameters, internal energy, enzyme activities and physiological attributes of wheat during germination and early growth. Laser Phys. Lett. 2013, 10, 45606. [Google Scholar] [CrossRef]
- Aly, A.; El-Beltagi, H. Influence of ionizing irradiation on the antioxidant enzymes of Viciafaba L. Grasas Aceites 2010, 61, 288–294. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, C.-K.; Tu, B.-J.; Li, Y.-S.; Zhang, Q.-Y.; Liu, X.-B. Effects of Carbon Ion Beam Irradiation on Phenotypic Variations and Biochemical Parameters in Early Generations of Soybean Plants. Agriculture 2021, 11, 98. https://doi.org/10.3390/agriculture11020098
Wang X, Liu C-K, Tu B-J, Li Y-S, Zhang Q-Y, Liu X-B. Effects of Carbon Ion Beam Irradiation on Phenotypic Variations and Biochemical Parameters in Early Generations of Soybean Plants. Agriculture. 2021; 11(2):98. https://doi.org/10.3390/agriculture11020098
Chicago/Turabian StyleWang, Xue, Chang-Kai Liu, Bing-Jie Tu, Yan-Sheng Li, Qiu-Ying Zhang, and Xiao-Bing Liu. 2021. "Effects of Carbon Ion Beam Irradiation on Phenotypic Variations and Biochemical Parameters in Early Generations of Soybean Plants" Agriculture 11, no. 2: 98. https://doi.org/10.3390/agriculture11020098
APA StyleWang, X., Liu, C.-K., Tu, B.-J., Li, Y.-S., Zhang, Q.-Y., & Liu, X.-B. (2021). Effects of Carbon Ion Beam Irradiation on Phenotypic Variations and Biochemical Parameters in Early Generations of Soybean Plants. Agriculture, 11(2), 98. https://doi.org/10.3390/agriculture11020098




