The Effect of Myco-Biocontrol Based Formulates on Yield, Physiology and Secondary Products of Organically Grown Basil
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material and Experimental Site
2.2. Experimental Design
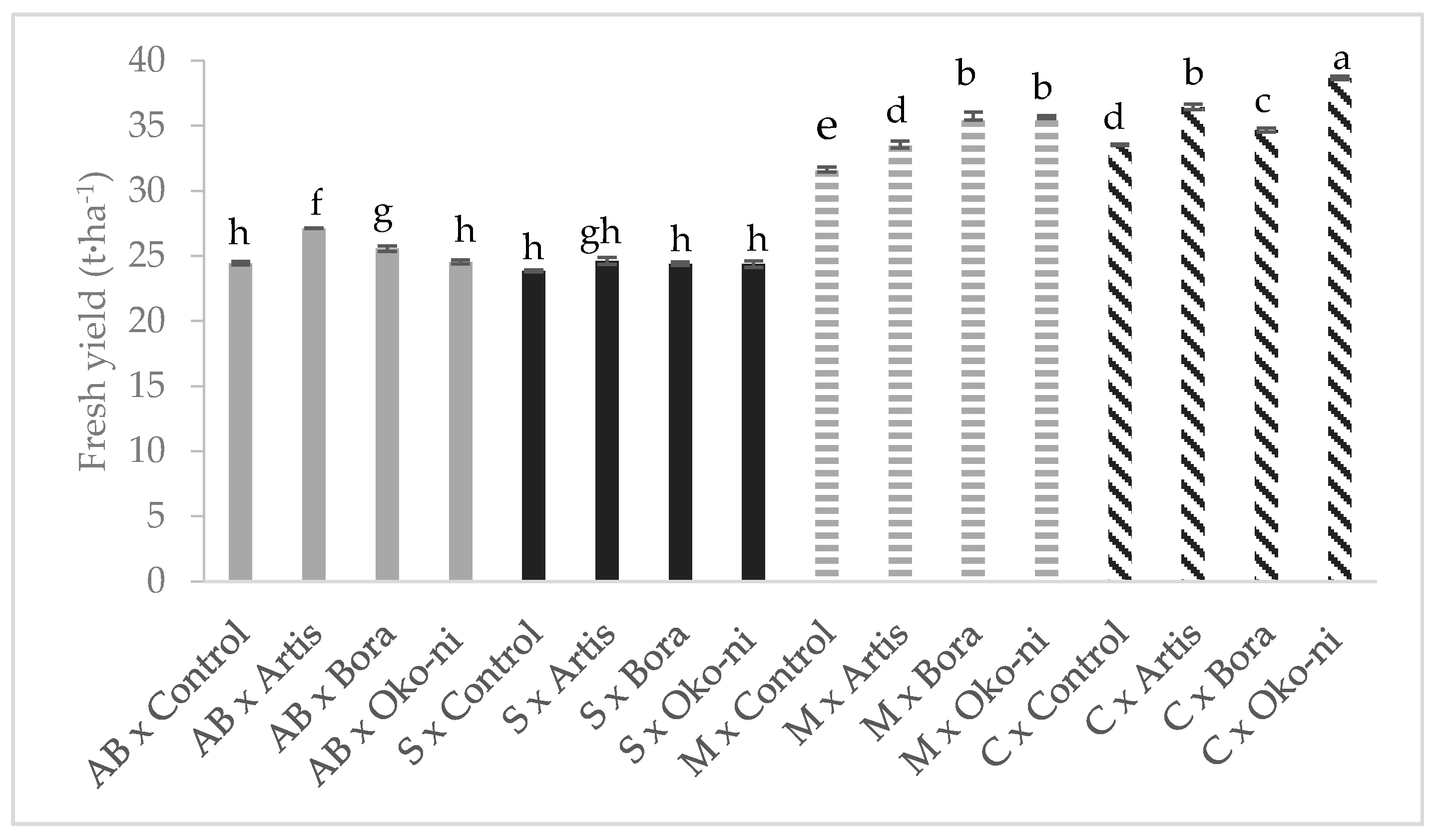
2.3. Yield Determination
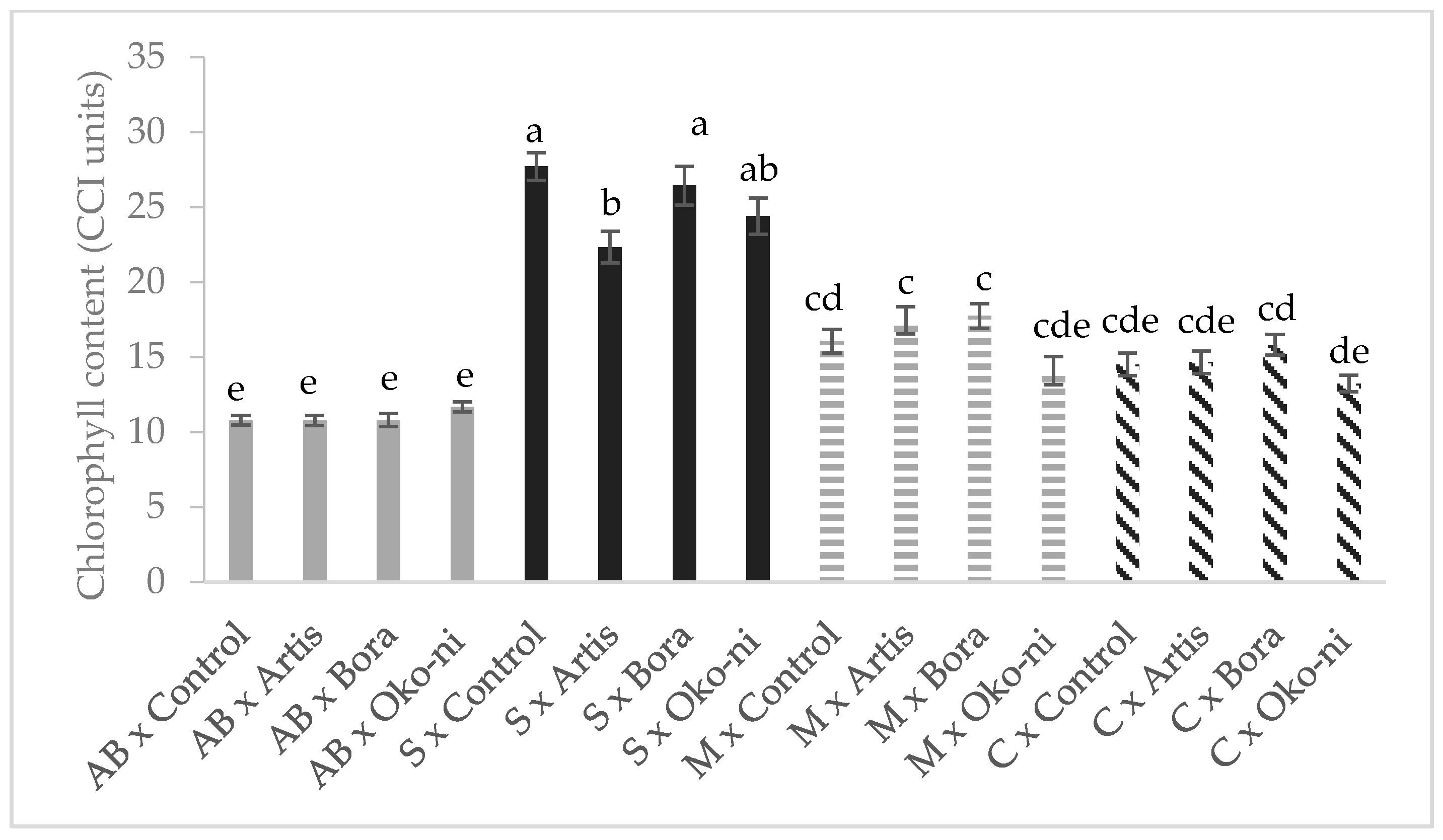
2.4. Total Chlorophyll Content Determination
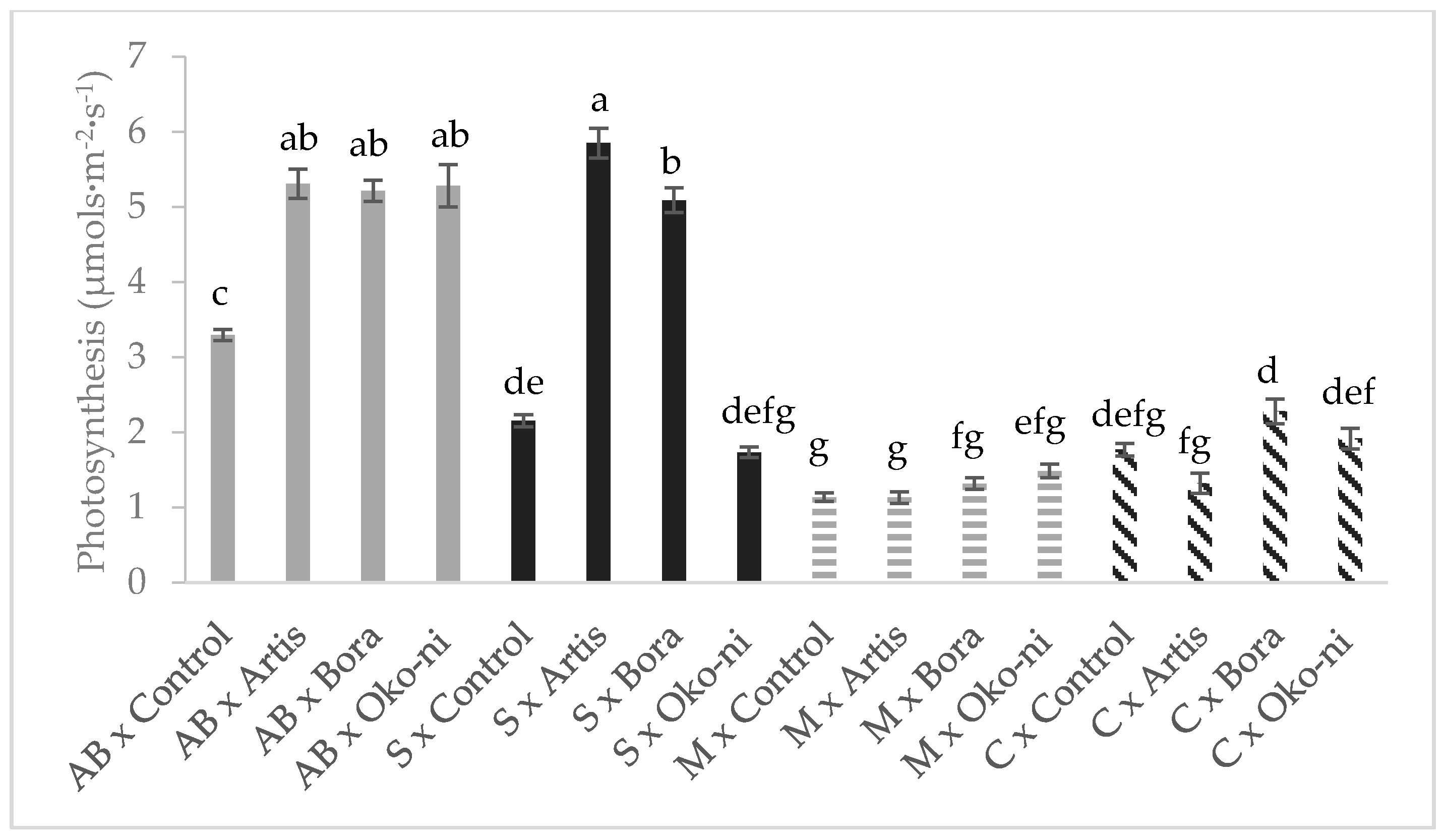
2.5. Photosynthesis Determination
2.6. Phenolic Compounds Extraction and Chromatographic Separation
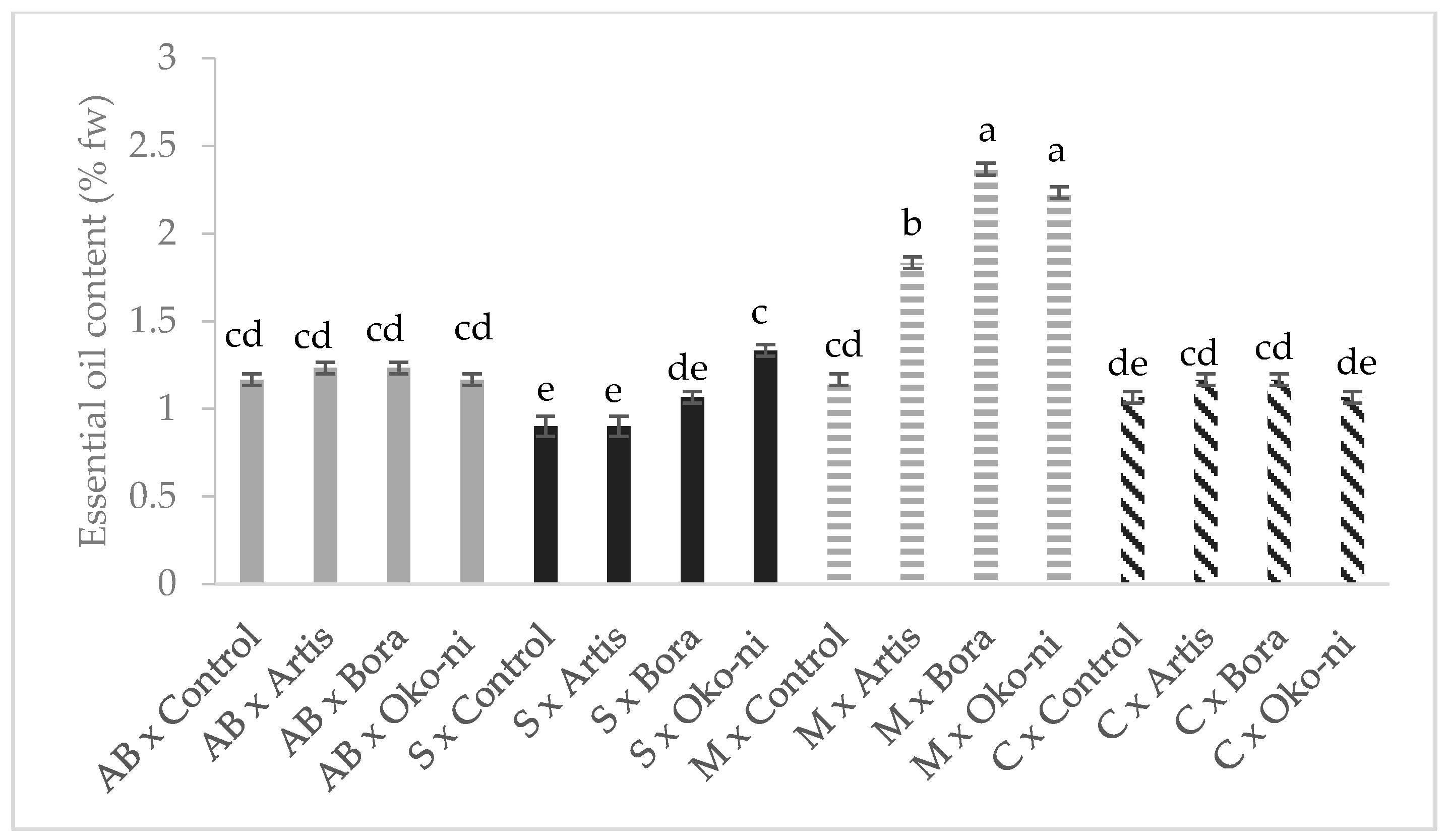
2.7. Essential Oil (EO) Extraction and Chromatographic Separation
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bączek, K.; Kosakowska, O.; Gniewosz, M.; Gientka, I.; Węglarz, Z. Sweet Basil (Ocimum basilicum L.) Productivity and raw material quality from organic cultivation. Agronomy 2019, 9, 279. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and oil composition of 38 basil (Ocimum basilicum L.) accessions grown in Mississippi. J. Agric. Food Chem. 2008, 56, 241–245. [Google Scholar] [CrossRef] [PubMed]
- De la Portilla, N.; Vaca, R.; Mora-Herrera, M.E.; Salinas, L.; Del Aguila, P.; Yañez-Ocampo, G.; Lugo, J. Soil amendment with biosolids and inorganic fertilizers: Effects on biochemical properties and oxidative stress in basil (Ocimum basilicum L.). Agronomy 2020, 10, 1117. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into composition of bioactive phenolic compounds in leaves and flowers of green and purple basil. Plants 2020, 9, 22. [Google Scholar] [CrossRef]
- Burducea, M.; Lobiuc, A.; Asandulesa, M.; Zaltariov, M.-F.; Burducea, I.; Popescu, S.M.; Zheljazkov, V.D. Effects of sewage sludge amendments on the growth and physiology of sweet basil. Agronomy 2019, 9, 548. [Google Scholar] [CrossRef]
- Ciriello, M.; Pannico, A.; El-Nakhel, C.; Formisano, L.; Cristofano, F.; Duri, L.G.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Colla, G.; et al. Sweet Basil Functional Quality as Shaped by Genotype and Macronutrient Concentration Reciprocal Action. Plants 2020, 9, 1786. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and Successive Harvests Interaction Affects Phenolic Acids and Aroma Profile of Genovese Basil for Pesto Sauce Production. Foods 2021, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.R.O.; Fernandes, Â.; Di Gioia, F.; Petropoulos, S.A.; Polyzos, N.; Dias, M.I.; Pinela, J.; Kostić, M.; Soković, M.D.; Ferreira, I.C.F.R.; et al. The Effect of Nitrogen Input on Chemical Profile and Bioactive Properties of Green- and Red-Colored Basil Cultivars. Antioxidants 2020, 9, 1036. [Google Scholar] [CrossRef]
- Eurostat, Organic Farming Statistics. Available online: https://ec.europa.eu/eurostat/statistcs-ecplained/index.php/organic_farming_statistics (accessed on 22 December 2020).
- Organic Trade Association. 2020. Available online: https://ota.com/news/press-releases/21328 (accessed on 19 December 2020).
- Merot, A.; Fermaud, M.; Gosme, M.; Smits, N. Effect of conversion to organic farming on pest and disease control in French vineyards. Agronomy 2020, 10, 1047. [Google Scholar] [CrossRef]
- Murariu, O.C.; Robu, T.; Ișan, E.; Irimia, L.; Murariu, F. Researches regarding pesticides and it’s metabolites dynamics in fruit in 2014 year marked in Romania and assessment of human health risks. J. Biotechnol. 2019, 305, s68–s69. [Google Scholar] [CrossRef]
- Murariu, F.; Voda, A.D.; Murariu, O.C. Researches on food safety assessment—Supporting a healthy lifestyle for the population from NE of Romania. J. Biotechnol. 2019, 305, s68. [Google Scholar] [CrossRef]
- Butu, M.; Stef, R.; Corneanu, M.; Butnariu, M. Mycoremediation: A Sustainable Approach for Pesticide Pollution Abatement. In Bioremediation and Biotechnology; Bhat, R., Hakeem, K., Dervash, M., Eds.; Springer: Cham, Switerland, 2020; Volume 2. [Google Scholar] [CrossRef]
- Butu, A.; Grozea, I.; Sarac, I.; Butnariu, M. Global Scenario of Remediation Techniques to Combat Pesticide Pollution. In Bioremediation and Biotechnology; Bhat, R., Hakeem, K., Dervash, M., Eds.; Springer: Cham, Switerland, 2020; Volume 2. [Google Scholar] [CrossRef]
- Murariu, O.C.; Isan, E.; Robu, T.; Irimia, L.M.; Dicu, L.; Ratu, R.N.; Murariu, F. Evaluation of the Presence of the Pesticide Residues and It’s Metabolites from Raw Materials Used as Sources for Ensuring a Healthy Nutrition for Athletes. In The Impact of Sport and Physical Education Science on Today’s Society; Hodorca, R.M., Onose, I., Eds.; Proceedings of I.C.U.; 2018; pp. 185–191. Available online: http://www.edlearning.it/ebook/BY23.pdf (accessed on 22 December 2020).
- Vodă, A.D.; Robu, T.; Robu, D.; Murariu, F.; Murariu, O.C. 2019—Residues of pesticides and it’s metabolites from vegetal products. J. Biotechnol. 2019, 305, s69. [Google Scholar] [CrossRef]
- COM (2020) 381. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0381 (accessed on 18 January 2021).
- Inglis, G.D.; Goettel, M.S.; Johnson, D.L. Influence of ultraviolet light protectants on persistence of the entomopathogenic fungus, Beauveria bassiana. Biol. Control 1995, 5, 581–590. [Google Scholar] [CrossRef]
- Mittal, N.; Saxena, G.; Mukerji, K.G. Biological control of root-knot nematode by nematode-destroying fungi. In From Ethnomycology to Fungal Biotechnology; Singh, J., Aneja, K.R., Eds.; Springer: Boston, MA, USA, 1999; Available online: https://doi.org/10.1007/978-1-4615-4815-7_15 (accessed on 22 December 2020).
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzon, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Pandey, A.; Singh, S.; Pillai, S. Biotechnological potential of Beauveria bassiana as a source of novel biocatalysts and metabolites. Crit. Rev. Biotechnol. 2020, 40, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- De Faria, M.R.; Wright, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Zafar, J.; Shoukat, R.F.; Zhang, Y.; Freed, S.; Xu, X.; Jin, F. Metarhizium anisopliae challenges immunity and demography of Plutella xylostella. Insects 2020, 11, 694. [Google Scholar] [CrossRef]
- Ugine, T.A.; Wraight, S.P.; Sanderson, J.P. Effects of manipulating spray-application parameters on efficacy of the entomopathogenic fungus Beauveria bassiana against western flower thrips, Frankliniella occidentalis, infesting greenhouse impatiens crops. Biocontrol Sci. Technol. 2007, 17, 193–219. [Google Scholar] [CrossRef]
- Jacobson, R.; Chandler, D.; Fenlon, J.; Russell, K. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Sci. Technol. 2001, 11, 391–400. [Google Scholar] [CrossRef]
- Ansari, M.; Brownbridge, M.; Shah, F.; Butt, T. Efficacy of entomopathogenic fungi against soil-dwelling life stages of western flower thrips, Frankliniella occidentalis, in plant-growing media. Entomol. Exp. Appl. 2008, 127, 80–87. [Google Scholar] [CrossRef]
- Prova, A.; Akanda, A.M.; Islam, S.; Hossain, M.M. Characterization of Sclerotinia sclerotiorum, an emerging fungal pathogen causing blight in Hyacinth Bean (Lablab purpureus). Plant Pathol. J. 2018, 34, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M.; Sreenivasaprasad, S.; Muthumeenakshi, S.; Rogers, C.W.; Challen, M.P. Use of Coniothyrium minitansas a biocontrol agent and some molecular aspects of sclerotial mycoparasitism. Eur. J. Plant Pathol. 2008, 121, 323–330. [Google Scholar] [CrossRef]
- Behie, S.W.; Zelisko, P.; Bidochka, M.J. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 2012, 336, 1576–1577. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Doni, F.; Isahak, A.; Zain, C.R.C.M.; Ariffin, S.M.; Mohamad, W.N.W.; Yusoff, W.M.W. Formulation of Trichoderma sp. SL2 inoculants using different carriers for soil treatment in rice seedling growth. Springerplus 2014, 3, 532. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Parvu, M.; Parvu, E.A. Chemical constituents of three Allium species from Romania. Molecules 2013, 18, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Burducea, M.; Zheljazkov, V.D.; Dincheva, I.; Lobiuc, A.; Teliban, G.C.; Stoleru, V.; Zamfirache, M.M. Fertilization modifies the essential oil and physiology of basil varieties. Ind. Crop Prod. 2018, 121, 282–293. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/ Mass Spectrometry, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Khan, M.S.I.; Shahid, N.; Aaliya, K. Bottlenecks in commercialisation and future prospects of PGPR. Appl. Soil Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
- Whipps, J.M.; Gerlagh, M. Biology of Coniothyrium minitans and its potential for use in disease biocontrol. Mycol. Res. 1992, 96, 897–907. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.E.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Morris, P.F.; Bone, E.; Tyler, B.M. Chemotropic and contact responses of Phytophthora sojae hyphae to soybean isoflavonoids and artificial substrates. Plant Physiol. 1998, 117, 1171–1178. [Google Scholar] [CrossRef]
- Lombardi, N.; Vitale, S.; Turra, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; d’Errico, G.; et al. Root exudates of stressed plants stimulate and attract Trichoderma soil fungi. Mol. Plant Microbe Interact. 2018, 31, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Szczałba, M.; Kopta, T.; Gazstoł, M.; Sezkara, A. Comprehensive insight into arbuscular mycorrhizal fungi, Trichoderma spp. and plant multilevel interactions with emphasis on biostimulation of horticultural crops. J. Appl. Microbiol. 2019, 127, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, G.M.; Shabana, Y.M.; Ismail, Y.M.; Rashad, Y.M. Trichoderma harzianum: A biocontrol agent against Bipolaris oryzae. Mycopathologia 2007, 164, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Teliban, G.C.; Stoleru, V.; Burducea, M.; Lobiuc, A.; Munteanu, N.; Popa, L.D.; Caruso, G. Biochemical, physiological and yield characteristics of red basil as affected by cultivar and fertilization. Agriculture 2020, 10, 48. [Google Scholar] [CrossRef]
- Golubkina, N.; Logvinenko, L.; Novitsky, M.; Zamana, S.; Sokolov, S.; Molchanova, A.; Shevchuk, O.; Sekara, A.; Tallarita, A.; Caruso, G. Yield, Essential Oil and Quality Performances of Artemisia dracunculus, Hyssopus officinalis and Lavandula angustifolia as Affected by Arbuscular Mycorrhizal Fungi under Organic Management. Plants 2020, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Onofrei, V.; Benchennouf, A.; Jancheva, M.; Loupassaki, S.; Ouaret, W.; Burducea, M.; Lobiuc, A.; Teliban, G.C.; Robu, T. Ecological foliar fertilization effects on essential oil composition of sweet basil (Ocimum basilicum L.) cultivated in a field system. Sci. Hortic. 2018, 239, 104–113. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, X.R.; Guo, Q.S.; Cao, L.P.; Qin, Q.; Li, C.; Zhao, M.; Wang, W.M. Plant morphology, physiological characteristics, accumulation of secondary metabolites and antioxidant activities of Prunella vulgaris L. under UV solar exclusion. Biol. Res. 2019, 52, 17. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Zhou, W.; Lee, W.; Han, M.S.; Na, M.; Bae, J.S. Anti-inflammatory effects of hyperoside in human endothelial cells and in mice. Inflammation 2015, 38, 784–799. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancírová, M.; Ulrichová, J.; Kren, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Quercetin feeding protects plants against oxidative stress. F1000Research 2016, 5, 2430. [Google Scholar] [CrossRef]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Identification and tissue-specific expression of rutin biosynthetic pathway genes in Capparis spinosa elicited with salicylic acid and methyl jasmonate. Sci. Rep. 2020, 10, 8884. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defense and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Boaro, C.S.F.; Vieira, M.A.R.; Campos, F.G.; Ferreira, G.; De-la-Cruz-Chacón, I.; Marques, M.O.M. Factors influencing the production and chemical composition of essential oils in aromatic plants from Brazil. In Essential Oil Research; Malik, S., Ed.; Springer: Cham, Switerland, 2019; pp. 19–47. [Google Scholar]
- Banchio, E.; Bogino, P.C.; Zygadlo, J.; Giordano, W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008, 36, 766–771. [Google Scholar] [CrossRef]
- Banchio, E.; Xie, X.; Zhang, H.; Pare, P.W. Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J. Agric. Food Chem. 2009, 57, 653–657. [Google Scholar] [CrossRef]
- Milanos, S.; Elsharif, S.A.; Janzen, D.; Buettner, A.; Villmann, C. Metabolic products of linalool and modulation of GABAA receptors. Front. Chem. 2017, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Barboza, I.N.; Bezerra, C.D.M.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Liao, P.C.; Yang, T.S.; Chou, J.C.; Chen, J.; Lee, S.C.; Kuo, Y.H.; Ho, C.L.; Chao, L.K.P. Anti-inflammatory activity of neral and geranial isolated from fruits of Litsea cubeba Lour. J. Funct. Foods 2015, 19, 248–258. [Google Scholar] [CrossRef]
| Month | Temperature of Air (°C) | Humidity (%) | Precipitation (mm) | |||
|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |
| April | 15.3 | 10.7 | 60 | 66 | 6.4 | 6.9 |
| May | 18.9 | 16.6 | 61 | 77 | 10.9 | 74.9 |
| June | 21.3 | 22.7 | 72 | 59 | 161.9 | 8.4 |
| July | 21.9 | 22.0 | 77 | 67 | 136.4 | 3.8 |
| August | 23.0 | 22.1 | 67 | 67 | 5.6 | 35.1 |
| Treatment | Fresh Yield (t·ha−1) | Essential Oil Content (%) | Assimilatory Pigments (CCI) | Photosynthesis Rate (µmols·m−2·s−1) |
|---|---|---|---|---|
| Cultivar | ||||
| ‘Aromat de Buzau’ | 25.41 ± 0.33 c | 1.20 ± 0.02 b | 11.01 ± 0.18 d | 4.77 ± 0.12 a |
| ‘Serafim’ | 24.31 ± 0.12 d | 1.05 ± 0.06 c | 25.21 ± 0.59 a | 3.70 ± 0.19 b |
| ‘Macedon’ | 34.14 ± 0.52 b | 1.90 ± 0.14 a | 16.34 ± 0.45 b | 1.27 ± 0.04 d |
| ‘Cuisoare’ | 35.82 ± 0.59 a | 1.12 ± 0.02 c | 14.55 ± 0.35 c | 1.82 ± 0.07 c |
| Myco-biocontrol formulates | ||||
| Control | 28.36 ± 1.24 c | 1.08 ± 0.04 c | 17.26 ± 0.33 ab | 2.09 ± 0.08 b |
| Artis® | 30.42 ± 1.38 b | 1.28 ± 0.10 b | 16.30 ± 0.34 ab | 3.40 ± 0.23 a |
| Bora® | 30.09 ± 1.49 b | 1.45 ± 0.16 a | 17.70 ± 0.43 a | 3.47 ± 0.18 a |
| Öko-ni® | 30.81 ± 1.86 a | 1.45 ± 0.14 a | 15.85 ± 0.33 b | 2.60 ± 0.17 b |
| Treatment | Caffeic Acid | Hyperoside | Isoquercitrin | Rutin | Quercitrin |
|---|---|---|---|---|---|
| Cultivar | |||||
| ‘Aromat de Buzau’ | 40.57 ± 7.53 a | tr | 324.22 ± 7.52 c | 468.18 ± 8.81 b | 57.14 ± 1.50 a |
| ‘Serafim’ | 38.50 ± 6.85 b | tr | 122.44 ± 9.41 d | 227.35 ± 19.55 c | 21.77 ± 0.99 d |
| ‘Macedon’ | 33.78 ± 5.98 c | 83.04 ± 8.30 | 413.09 ± 14.04 a | 483.55 ± 59.50 c | 35.21 ± 1.69 b |
| ‘Cuisoare’ | 11.47 ± 6.05 d | tr | 342.17 ± 15.66 b | 1057.08 ± 71.54 a | 31.11 ± 3.21 c |
| Myco-biocontrol formulates | |||||
| Control | tr | 18.45 ± 9.64 c | 284.78 ± 31.42 bc | 506.48 ± 83.75 c | 32.70 ± 4.02 b |
| Artis® | 35.99 ± 6.50 c | 26.30 ± 13.75 a | 354.11 ± 34.19 a | 664.82 ± 125.96 a | 36.90 ± 3.29 a |
| Bora® | 37.86 ± 6.69 b | 22.78 ± 11.90 b | 279.03 ± 36.41 c | 487.89 ± 88.50 d | 37.15 ± 3.96 a |
| Öko-ni® | 51.37 ± 1.84 a | 23.19 ± 12.11 b | 291.31 ± 33.18 b | 571.21 ± 103.00 b | 36.82 ± 5.50 a |
| No | Name | Class | RIcalc | RIlit | Control | Artis® | Bora® | Öko-ni® |
|---|---|---|---|---|---|---|---|---|
| 1 | Eucalyptol (1,8-Cineole) | Bicyclic monoterpenoids | 1031 | 1030 | 0.19 | 0.55 | 0.38 | 0.26 |
| 2 | cis-β-Ocimene | Acyclic monoterpenes | 1040 | 1037 | 0.12 | 0.15 | 0.17 | tr |
| 3 | β-Linalool | Acyclic monoterpenoids | 1095 | 1096 | 18.49 | 21.37 | 23.05 | 20.39 |
| 4 | cis-β-Thujone | Bicyclic monoterpenoids | 1101 | 1102 | 0.18 | 0.20 | 0.16 | 0.20 |
| 5 | trans-β-Thujone | Bicyclic monoterpenoids | 1112 | 1114 | 0.11 | 0.11 | 0.08 | 0.12 |
| 6 | (Z)-Epoxy-ocimene | Monocyclic monoterpenoids | 1128 | 1132 | 0.12 | 0.16 | 0.24 | 0.18 |
| 7 | Camphor | Bicyclic monoterpenoids | 1141 | 1145 | 0.70 | 0.79 | 0.80 | 0.90 |
| 8 | Methyl chavicol | Phenolic monoterpenoids | 1195 | 1196 | 47.14 | 49.49 | 49.11 | 45.80 |
| 9 | Bornyl acetate | Bicyclic monoterpenoids | 1284 | 1285 | tr | 0.31 | 0.22 | 0.36 |
| 10 | trans-Linalool oxide acetate | Acyclic monoterpenoids | 1287 | 1288 | 0.91 | 0.62 | 0.67 | 0.78 |
| 11 | Neryl acetate | Acyclic monoterpenoids | 1359 | 1361 | tr | tr | 0.35 | 0.18 |
| 12 | Geranyl acetate | Acyclic monoterpenoids | 1379 | 1381 | 0.19 | tr | tr | tr |
| 13 | β-Elemene | Monocyclic sesquiterpenes | 1389 | 1390 | 4.47 | 3.74 | 3.66 | 3.26 |
| 14 | Methyl eugenol | Phenolic monoterpenoids | 1402 | 1403 | 0.78 | 0.62 | 0.46 | 0.89 |
| 15 | β-Caryophyllene | Bicyclic sesquiterpenes | 1417 | 1419 | 0.68 | 0.48 | 0.53 | 0.61 |
| 16 | α-Guaiene | Bicyclic sesquiterpenes | 1436 | 1439 | 0.86 | 0.75 | 0.77 | 0.74 |
| 17 | cis-Muurola-3,5-diene | Bicyclic sesquiterpenes | 1448 | 1450 | 0.10 | tr | 0.17 | tr |
| 18 | trans-Muurola-3,5-diene | Bicyclic sesquiterpenes | 1451 | 1453 | 0.16 | tr | 0.14 | tr |
| 19 | Humulene (α-Caryophyllene) | Monocyclic sesquiterpenes | 1454 | 1454 | 0.46 | 0.45 | 0.39 | 0.43 |
| 20 | trans-Muurola-4(14),5-diene | Bicyclic sesquiterpenes | 1465 | 1466 | 0.32 | 0.25 | 0.25 | 0.26 |
| 21 | Germacrene D | Monocyclic sesquiterpenes | 1481 | 1481 | 4.10 | 3.37 | 2.36 | 3.82 |
| 22 | Bicyclogermacrene | Bicyclic sesquiterpenes | 1500 | 1501 | 0.72 | 0.48 | 0.49 | 0.50 |
| 23 | α-Bulnesene | Bicyclic sesquiterpenes | 1510 | 1509 | 1.72 | 1.39 | 1.30 | 1.56 |
| 24 | γ-Cadinene | Bicyclic sesquiterpenes | 1513 | 1513 | 2.08 | 1.52 | 1.85 | 1.81 |
| 25 | cis-Muurol-5-en-4β-ol | Bicyclic sesquiterpenoids | 1551 | 1552 | 0.25 | 0.16 | 0.18 | 0.23 |
| 26 | Elemicin | Phenolic monoterpenoids | 1155 | 1557 | 1.13 | 0.82 | 0.76 | 1.26 |
| 27 | cis-Muurol-5-en-4α-ol | Bicyclic sesquiterpenoids | 1559 | 1561 | 8.86 | 5.37 | 5.27 | 9.10 |
| 28 | 1,10-di-epi-Cubenol | Bicyclic sesquiterpenoids | 1618 | 1619 | 0.33 | 0.37 | 0.69 | 0.31 |
| 29 | 1-epi-Cubenol | Bicyclic sesquiterpenoids | 1627 | 1628 | 0.78 | 0.88 | 0.72 | 0.89 |
| 30 | epi-α-Cadinol | Bicyclic sesquiterpenoids | 1638 | 1640 | 3.07 | 4.61 | 3.80 | 4.17 |
| tr ≥ 0.03 | ||||||||
| Acyclic monoterpenes | 0.12 | 0.15 | 0.17 | tr | ||||
| Acyclic monoterpenoids | 19.58 | 21.99 | 24.06 | 21.35 | ||||
| Monocyclic monoterpenoids | 0.12 | 0.16 | 0.24 | 0.18 | ||||
| Bicyclic monoterpenoids | 1.17 | 1.95 | 1.63 | 1.84 | ||||
| Phenolic monoterpenoids | 49.05 | 50.92 | 50.33 | 47.95 | ||||
| Monocyclic sesquiterpenes | 9.03 | 7.56 | 6.42 | 7.51 | ||||
| Bicyclic sesquiterpenes | 6.64 | 4.88 | 5.50 | 5.46 | ||||
| Bicyclic sesquiterpenoids | 13.29 | 11.39 | 10.66 | 14.71 | ||||
| No | Name | Class | RIcalc | RIlit | Control | Artis® | Bora® | Öko-ni® |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | Bicyclic monoterpenes | 932 | 939 | tr | 0.08 | 0.06 | 0.10 |
| 2 | Sabinene | Bicyclic monoterpenes | 969 | 974 | 0.09 | 0.11 | 0.17 | 0.21 |
| 3 | β-Myrcene | Acyclic monoterpenes | 988 | 990 | 0.16 | 0.18 | tr | 0.11 |
| 4 | Limonene | Monocyclic monoterpenes | 1024 | 1028 | 0.13 | 0.15 | 0.17 | 0.17 |
| 5 | Eucalyptol (1,8-Cineole) | Bicyclic monoterpenoids | 1031 | 1030 | 3.31 | 4.04 | 3.75 | 3.70 |
| 6 | cis-β-Ocimene | Acyclic monoterpenes | 1041 | 1037 | tr | tr | 0.16 | 0.16 |
| 7 | Fenchone | Bicyclic monoterpenoids | 1083 | 1085 | 0.23 | 0.24 | 0.24 | 0.24 |
| 8 | Terpinolene | Monocyclic monoterpenes | 1086 | 1088 | 0.16 | 0.16 | 0.15 | 0.15 |
| 9 | β-Linalool | Acyclic monoterpenoids | 1095 | 1096 | 52.74 | 54.12 | 50.77 | 51.14 |
| 10 | Camphor | Bicyclic monoterpenoids | 1141 | 1145 | 1.10 | 1.25 | 1.16 | 1.15 |
| 11 | α-Terpineol | Monocyclic monoterpenoids | 1188 | 1188 | 1.13 | tr | 1.23 | 1.22 |
| 12 | endo-Fenchyl acetate | Bicyclic monoterpenoids | 1220 | 1221 | 0.24 | 0.30 | 0.37 | 0.37 |
| 13 | cis-Carveol | Monocyclic monoterpenoids | 1229 | 1229 | 0.23 | 0.20 | 0.23 | 0.22 |
| 14 | Geranial | Acyclic monoterpenoids | 1266 | 1267 | 0.31 | 0.28 | 0.31 | 0.31 |
| 15 | Bornyl acetate | Bicyclic monoterpenoids | 1254 | 1285 | 0.45 | 0.36 | 0.53 | 0.52 |
| 16 | Eugenol | Phenolic monoterpenoids | 1356 | 1358 | 8.96 | 9.12 | 10.22 | 10.70 |
| 17 | α-Copaene | Tricyclic sesquiterpenes | 1375 | 1376 | 0.19 | 0.20 | 0.20 | 0.19 |
| 18 | β-Elemene | Monocyclic sesquiterpenes | 1389 | 1390 | 7.44 | 6.27 | 6.41 | 6.33 |
| 19 | Methyl eugenol | Phenolic monoterpenoids | 1403 | 1403 | 0.32 | 0.30 | 0.41 | 0.41 |
| 20 | β-Caryophyllene | Bicyclic sesquiterpenes | 1417 | 1419 | 1.32 | 1.48 | 1.59 | 1.57 |
| 21 | α-trans-Bergamotene | Bicyclic sesquiterpenes | 1433 | 1434 | 1.91 | 2.00 | 1.09 | 1.08 |
| 22 | α-Guaiene | Bicyclic sesquiterpenes | 1436 | 1439 | 1.68 | 1.46 | 1.53 | 1.50 |
| 23 | Humulene (α-Caryophyllene) | Monocyclic sesquiterpenes | 1454 | 1454 | 0.79 | 0.69 | 0.71 | 0.70 |
| 24 | Germacrene D | Monocyclic sesquiterpenes | 1481 | 1481 | 5.56 | 5.30 | 5.84 | 5.77 |
| 25 | β-Selinene | Bicyclic sesquiterpenes | 1489 | 1490 | 0.40 | 0.31 | 0.27 | 0.27 |
| 26 | Bicyclogermacrene | Bicyclic sesquiterpenes | 1500 | 1501 | tr | 0.38 | 0.49 | 0.57 |
| 27 | α-Bulnesene | Bicyclic sesquiterpenes | 1509 | 1509 | 3.21 | 2.77 | 2.87 | 2.83 |
| 28 | γ-Cadinene | Bicyclic sesquiterpenes | 1513 | 1513 | 1.54 | 1.44 | 1.58 | 1.56 |
| 29 | 1,10-di-epi-Cubenol | Bicyclic sesquiterpenoids | 1618 | 1628 | 0.62 | 0.54 | 0.65 | 0.64 |
| 30 | epi-α-Cadinol | Bicyclic sesquiterpenoids | 1638 | 1640 | 4.78 | 4.24 | 4.79 | 4.73 |
| tr ≥ 0.03 | ||||||||
| Acyclic monoterpenes | 0.16 | 0.18 | 0.16 | 0.27 | ||||
| Acyclic monoterpenoids | 53.04 | 54.40 | 51.08 | 51.45 | ||||
| Monocyclic monoterpenes | 0.29 | 0.31 | 0.32 | 0.32 | ||||
| Monocyclic monoterpenoids | 1.36 | 0.20 | 1.46 | 1.44 | ||||
| Bicyclic monoterpenes | 0.09 | 0.19 | 0.23 | 0.30 | ||||
| Bicyclic monoterpenoids | 5.32 | 6.18 | 6.05 | 5.97 | ||||
| Phenolic monoterpenoids | 9.28 | 9.41 | 10.64 | 11.10 | ||||
| Monocyclic sesquiterpenes | 13.80 | 12.26 | 12.97 | 12.81 | ||||
| Bicyclic sesquiterpenes | 10.06 | 9.84 | 9.41 | 9.37 | ||||
| Bicyclic sesquiterpenoids | 5.40 | 4.78 | 5.44 | 5.37 | ||||
| Tricyclic sesquiterpenes | 0.19 | 0.20 | 0.20 | 0.19 | ||||
| No | Name | Class | RIcalc | RIlit | Control | Artis® | Bora® | Öko-ni® |
|---|---|---|---|---|---|---|---|---|
| 1 | cis-β-Ocimene | Acyclic monoterpenes | 1041 | 1037 | 0.41 | 0.31 | 0.52 | 0.37 |
| 2 | β-Linalool | Acyclic monoterpenoids | 1095 | 1096 | 3.73 | 0.94 | 1.51 | 1.60 |
| 3 | cis-β-Thujone | Bicyclic monoterpenoids | 1101 | 1102 | 0.56 | tr | tr | tr |
| 4 | trans-β-Thujone | Bicyclic monoterpenoids | 1112 | 1114 | 0.22 | tr | tr | tr |
| 5 | Camphor | Bicyclic monoterpenoids | 1141 | 1145 | 0.54 | tr | tr | tr |
| 6 | (Z)-Isocitral | Acyclic monoterpenoids | 1163 | 1164 | 1.04 | 1.50 | 1.18 | 1.19 |
| 7 | (E)- Isocitral | Acyclic monoterpenoids | 1179 | 1180 | 1.40 | 1.59 | 1.52 | 1.55 |
| 8 | Methyl chavicol | Phenolic monoterpenoids | 1195 | 1196 | 1.70 | 0.63 | 0.92 | 1.07 |
| 9 | Nerol | Acyclic monoterpenoids | 1227 | 1229 | 6.82 | 9.27 | 7.95 | 6.10 |
| 10 | Neral | Acyclic monoterpenoids | 1235 | 1238 | 22.56 | 28.27 | 26.06 | 28.37 |
| 11 | Geraniol | Acyclic monoterpenoids | 1251 | 1252 | 1.90 | 2.33 | 2.68 | 2.51 |
| 12 | Geranial | Acyclic monoterpenoids | 1265 | 1267 | 30.90 | 34.05 | 31.02 | 34.34 |
| 13 | Neryl acetate | Acyclic monoterpenoids | 1359 | 1361 | 0.93 | 1.10 | 0.95 | 0.88 |
| 14 | Geranyl acetate | Acyclic monoterpenoids | 1379 | 1381 | 0.55 | tr | tr | tr |
| 15 | α-Copaene | Tricyclic sesquiterpenes | 1375 | 1376 | tr | 0.42 | 0.29 | 0.35 |
| 16 | β-Elemene | Monocyclic sesquiterpenes | 1389 | 1390 | 0.47 | tr | tr | tr |
| 17 | Methyl eugenol | Phenolic monoterpenoids | 1403 | 1403 | 1.17 | tr | 0.37 | 0.35 |
| 18 | β-Caryophyllene | Bicyclic sesquiterpenes | 1417 | 1419 | 7.73 | 6.49 | 7.52 | 6.48 |
| 19 | α-trans-Bergamotene | Bicyclic sesquiterpenes | 1433 | 1434 | 1.58 | 1.74 | 2.01 | 1.94 |
| 20 | Humulene (α-Caryophyllene) | Monocyclic sesquiterpenes | 1453 | 1454 | 1.29 | 1.07 | 1.18 | 1.10 |
| 21 | (E)-β-Farnesene | Acyclic sesquiterpenes | 1455 | 1456 | 1.15 | 0.97 | 1.23 | 1.15 |
| 22 | Sesquisabinene | Bicyclic sesquiterpenes | 1457 | 1459 | 0.16 | tr | 0.15 | tr |
| 23 | Germacrene D | Monocyclic sesquiterpenes | 1481 | 1481 | 2.03 | 1.31 | 2.64 | 2.05 |
| 24 | (Z)-γ-Bisabolene | Monocyclic sesquiterpenes | 1514 | 1515 | 0.40 | tr | 0.31 | tr |
| 25 | (E)-γ-Bisabolene | Monocyclic sesquiterpenes | 1528 | 1530 | 8.97 | 6.28 | 7.99 | 6.75 |
| 26 | epi-α-Cadinol | Bicyclic sesquiterpenoids | 1638 | 1640 | 0.44 | tr | tr | tr |
| tr ≥ 0.03 | ||||||||
| Acyclic monoterpenes | 0.41 | 0.31 | 0.52 | 0.37 | ||||
| Acyclic monoterpenoids | 69.84 | 79.05 | 72.87 | 76.53 | ||||
| Bicyclic monoterpenoids | 1.33 | 0.00 | 0.00 | 0.00 | ||||
| Phenolic monoterpenoids | 2.88 | 0.63 | 1.29 | 1.42 | ||||
| Acyclic sesquiterpenes | 1.15 | 0.97 | 1.23 | 1.15 | ||||
| Monocyclic sesquiterpenes | 13.15 | 8.66 | 12.12 | 9.91 | ||||
| Bicyclic sesquiterpenes | 9.47 | 8.23 | 9.68 | 8.42 | ||||
| Bicyclic sesquiterpenoids | 0.44 | tr | tr | tr | ||||
| Tricyclic sesquiterpenes | tr | 0.42 | 0.29 | 0.35 | ||||
| No | Name | Class | RIcalc | RIlit | Control | Artis® | Bora® | Öko-ni® |
|---|---|---|---|---|---|---|---|---|
| 1 | Sabinene | Bicyclic monoterpenes | 969 | 974 | tr | tr | tr | tr |
| 2 | Sylvestrene | Monocyclic monoterpenes | 1026 | 1030 | 0.08 | tr | tr | 0.10 |
| 3 | Eucalyptol (1,8-Cineole) | Bicyclic monoterpenoids | 1031 | 1030 | 2.74 | 1.54 | 1.56 | 1.44 |
| 4 | cis-β-Ocimene | Acyclic monoterpenes | 1041 | 1037 | 0.45 | 0.20 | 0.21 | 0.40 |
| 5 | Terpinolene | Monocyclic monoterpenes | 1086 | 1088 | tr | 0.00 | 0.00 | 0.00 |
| 6 | β-Linalool | Acyclic monoterpenoids | 1095 | 1096 | 45.58 | 45.83 | 44.44 | 38.49 |
| 7 | cis-β-Thujone | Bicyclic monoterpenoids | 1101 | 1102 | tr | tr | tr | tr |
| 8 | trans-β-Thujone | Bicyclic monoterpenoids | 1112 | 1114 | tr | tr | tr | tr |
| 9 | (Z)-Epoxy-ocimene | Monocyclic monoterpenoids | 1128 | 1132 | 0.52 | 0.37 | 0.38 | 0.35 |
| 10 | Camphor | Bicyclic monoterpenoids | 1141 | 1145 | 0.28 | tr | 0.51 | 0.49 |
| 11 | α-Terpineol | Monocyclic monoterpenoids | 1188 | 1188 | 1.11 | tr | tr | tr |
| 12 | Methyl chavicol | Phenolic monoterpenoids | 1195 | 1196 | tr | 1.60 | 1.62 | 1.43 |
| 13 | cis-Carveol | Monocyclic monoterpenoids | 1229 | 1229 | 0.46 | 0.20 | 0.20 | 0.22 |
| 14 | Geranial | Acyclic monoterpenoids | 1266 | 1267 | 0.58 | 0.26 | 0.26 | 0.30 |
| 15 | Bornyl acetate | Bicyclic monoterpenoids | 1284 | 1285 | 1.55 | 1.63 | 1.65 | 1.51 |
| 16 | trans-Linalool oxide acetate | Acyclic monoterpenoids | 1287 | 1288 | 0.13 | 0.12 | 0.13 | 0.19 |
| 17 | Eugenol | Phenolic monoterpenoids | 1356 | 1358 | 12.59 | 15.98 | 16.19 | 13.16 |
| 18 | α-Copaene | Tricyclic sesquiterpenes | 1375 | 1376 | tr | tr | tr | tr |
| 19 | β-Elemene | Monocyclic sesquiterpenes | 1389 | 1390 | 3.86 | 3.33 | 3.37 | 4.62 |
| 20 | Methyl eugenol | Phenolic monoterpenoids | 1403 | 1403 | 0.38 | 0.40 | 0.40 | 0.53 |
| 21 | β-Caryophyllene | Bicyclic sesquiterpenes | 1417 | 1419 | 0.35 | tr | tr | tr |
| 22 | α-trans-Bergamotene | Bicyclic sesquiterpenes | 1433 | 1434 | 4.74 | 5.70 | 5.77 | 7.56 |
| 23 | α-Guaiene | Bicyclic sesquiterpenes | 1436 | 1439 | 0.89 | 0.80 | 0.81 | 1.06 |
| 24 | cis-Muurola-3,5-diene | Bicyclic sesquiterpenes | 1448 | 1450 | 0.31 | 0.30 | 0.31 | tr |
| 25 | trans-Muurola-3,5-diene | Bicyclic sesquiterpenes | 1451 | 1452 | tr | tr | tr | tr |
| 26 | Humulene (α-Caryophyllene) | Monocyclic sesquiterpenes | 1453 | 1454 | 0.72 | 0.61 | 0.61 | 0.86 |
| 27 | trans-Muurola-4(14),5-diene | Bicyclic sesquiterpenes | 1466 | 1466 | 0.47 | 0.49 | 0.50 | 0.66 |
| 28 | Germacrene D | Monocyclic sesquiterpenes | 1481 | 1481 | 4.83 | 3.93 | 3.98 | 4.23 |
| 29 | Bicyclogermacrene | Bicyclic sesquiterpenes | 1500 | 1501 | 0.67 | 0.59 | 0.60 | 0.77 |
| 30 | α-Bulnesene | Bicyclic sesquiterpenes | 1509 | 1509 | 1.44 | 1.30 | 1.31 | 1.86 |
| 31 | γ-Cadinene | Bicyclic sesquiterpenes | 1513 | 1513 | 2.76 | 2.91 | 2.95 | 3.58 |
| 32 | β-Sesquiphellandrene | Monocyclic sesquiterpenes | 1522 | 1522 | 0.22 | 0.24 | 0.25 | 0.38 |
| 33 | trans-Nerolidol | Acyclic sesquiterpenoids | 1561 | 1563 | 0.12 | tr | tr | tr |
| 34 | 5-epi-7-epi-α-Eudesmol | Bicyclic sesquiterpenoids | 1605 | 1607 | 1.09 | 0.75 | 0.76 | 1.21 |
| 35 | 1,10-di-epi-Cubenol | Bicyclic sesquiterpenoids | 1618 | 1628 | 1.15 | 1.07 | 1.08 | 1.53 |
| 36 | epi-α-Cadinol | Bicyclic sesquiterpenoids | 1638 | 1640 | 8.52 | 8.24 | 8.34 | 11.32 |
| tr ≥ 0.03 | ||||||||
| Acyclic monoterpenes | 0.45 | 0.20 | 0.21 | 0.40 | ||||
| Acyclic monoterpenoids | 46.28 | 46.21 | 44.82 | 38.98 | ||||
| Monocyclic monoterpenes | 0.08 | 0.00 | 0.00 | 0.10 | ||||
| Monocyclic monoterpenoids | 2.09 | 0.57 | 0.58 | 0.57 | ||||
| Bicyclic monoterpenes | tr | tr | tr | tr | ||||
| Bicyclic monoterpenoids | 4.58 | 3.17 | 3.72 | 3.44 | ||||
| Phenolic monoterpenoids | 12.98 | 17.97 | 18.21 | 15.12 | ||||
| Acyclic sesquiterpenoids | 0.12 | tr | tr | tr | ||||
| Monocyclic sesquiterpenes | 9.64 | 8.10 | 8.21 | 10.09 | ||||
| Bicyclic sesquiterpenes | 11.64 | 12.08 | 12.24 | 15.50 | ||||
| Bicyclic sesquiterpenoids | 10.76 | 10.05 | 10.18 | 14.06 | ||||
| Tricyclic sesquiterpenes | tr | tr | tr | tr | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teliban, G.-C.; Burducea, M.; Zheljazkov, V.D.; Dincheva, I.; Badjakov, I.; Munteanu, N.; Mihalache, G.; Cojocaru, A.; Popa, L.-D.; Stoleru, V. The Effect of Myco-Biocontrol Based Formulates on Yield, Physiology and Secondary Products of Organically Grown Basil. Agriculture 2021, 11, 180. https://doi.org/10.3390/agriculture11020180
Teliban G-C, Burducea M, Zheljazkov VD, Dincheva I, Badjakov I, Munteanu N, Mihalache G, Cojocaru A, Popa L-D, Stoleru V. The Effect of Myco-Biocontrol Based Formulates on Yield, Physiology and Secondary Products of Organically Grown Basil. Agriculture. 2021; 11(2):180. https://doi.org/10.3390/agriculture11020180
Chicago/Turabian StyleTeliban, Gabriel-Ciprian, Marian Burducea, Valtcho D. Zheljazkov, Ivayla Dincheva, Ilian Badjakov, Neculai Munteanu, Gabriela Mihalache, Alexandru Cojocaru, Lorena-Diana Popa, and Vasile Stoleru. 2021. "The Effect of Myco-Biocontrol Based Formulates on Yield, Physiology and Secondary Products of Organically Grown Basil" Agriculture 11, no. 2: 180. https://doi.org/10.3390/agriculture11020180
APA StyleTeliban, G.-C., Burducea, M., Zheljazkov, V. D., Dincheva, I., Badjakov, I., Munteanu, N., Mihalache, G., Cojocaru, A., Popa, L.-D., & Stoleru, V. (2021). The Effect of Myco-Biocontrol Based Formulates on Yield, Physiology and Secondary Products of Organically Grown Basil. Agriculture, 11(2), 180. https://doi.org/10.3390/agriculture11020180









