Abstract
The development of sustainable plant production systems involves a search for different alternatives to chemical fertilizers. The aim of the present study is to compare growth and physiological effects of vermicompost on Dracocephalum moldavica plants in controlled conditions, using two types of commercially available substrates. The intention is to determine whether nondestructively measured photosynthesis-related parameters are useful for monitoring the physiological status of plants. The plants were cultivated in two base substrates without or with the addition of mineral fertilizer, as well as an amendment with vermicompost at a 20% or 30% rate in the conditions of an automated greenhouse. The biomass accumulation for control plants of D. moldavica was identical in peat substrate and commercial garden soil. The average growth increase by mineral fertilizer was 25% for D. moldavica plants grown in peat and 15% for plants grown in soil. Substrate amendment with 20% vermicompost resulted in an 114% average increase in biomass for plants grown in peat and a 98% average increase for plants grown in soil, but for plants at 30% the amendment rate increase was 148% and 68%, for peat and soil, respectively. Consequently, the addition of an identical amount of vermicompost resulted in a poorer growth response of plants in commercial garden soil as a substrate in comparison to peat, but an increase in the amendment rate from 20% to 30% resulted in some growth inhibition for these plants. Chlorophyll concentration was positively affected by the vermicompost amendment in a concentration-dependent manner, but this effect during a cultivation period appeared relatively late. Large differences were found between the three groups of fluorescence-derived parameters, with variable levels of predictability with respect to the differences in plant yield due to the pronounced variation in correlation through time. It is concluded that the incorporation of vermicompost for the cultivation of D. moldavica, even in substrate mixes with relatively high and balanced composition of plant-available nutrients, benefits plant growth, physiological status and biomass yield, but it is necessary to explore interactions between vermicompost and other substrates leading to possible changes in quality-related characteristics of vermicompost in substrate mixes.
1. Introduction
The use of renewable resources for nutrient management is an important aspect of sustainable agricultural and horticultural practices. Therefore, the development of sustainable plant production systems involves the search for different alternatives to chemical fertilizers [1]. One extremely promising direction in this respect is related to the application of vermicompost, which is a type of organic fertilizer produced by the concerted action of earthworms and their symbiotic microorganisms [2]. The application of vermicompost leads to increased soil sustainability, due to the enhancement of organic matter content and microbial diversity, as well as to the improvement of the physical properties of soil [3,4]. In addition, there are direct benefits for crop plants from the use of vermicompost. The physiological effects of vermicompost on plants has been recently reviewed and it was concluded that in conventional farming systems, vermicompost can substitute chemical fertilizers due to the significant concentration of plant-available mineral nutrients, while additional benefits have been associated with the presence of plant growth-stimulating substances and adaptogenic activity [5].
Traditionally, vermicomposts are produced from cattle manure with the addition of organic carbon-rich biomass, such as grass or straw [6], but it is possible to use different organic waste materials and byproducts for its production. The possibility for successful application of different types of vermicompost for the production of various crop species has been shown for vermicomposts produced from agricultural waste [7], byproducts of food production or food waste [8,9], urban organic waste [10], and composted sewage sludge [11].
Recently, several reviews and meta-analyses have summarized the beneficial effects of vermicompost-use in different farming systems, which also indicates directions for further studies of critical importance [5,12,13,14,15]. Organic agriculture and horticulture are especially important targets for vermicompost application, in a view of providing balanced nutrition for crop needs and soil sustainability through enhanced microbiological activity [16,17]. Another especially promising direction in horticulture is the inclusion of vermicompost as a major component in substrate mixes for the design of alternative plant-growing media to replace peat-based mixes [18,19]. While previous studies in this direction have produced promising results, physicochemical interactions between vermicompost and other substrate components, and their possible effect on the beneficial activity of vermicompost, have not been experimentally assessed.
Moldavian dragonhead or Moldavian balm (Dracocephalum moldavica L.) is a species of Lamiaceae family with a characteristic high content of essential oil in above-ground parts and fatty oil-producing seeds. The agrobiological characteristics of D. moldavica have been reviewed recently [20], and it was described as medicinal, spice, and nectar plant, with a high potential for the use of seeds in different food applications. Therefore, there is an increasing interest in the development of optimum cultivation technologies for this crop species.
The different aspects of D. moldavica fertilization in field conditions have been studied, including the effects of compost [21,22], compost and farmyard manure [23], forms of nitrogen [24], arbuscular mycorrhiza [25], and vermicompost [26], with a general aim to improve yield and quality of the plant while focusing on soil sustainability. Most importantly, the use of different organic fertilizers clearly promotes plant growth by providing additional plant-available nutrients to the soil and improving the essential oil content. It needs to be emphasized that the generalization of this type of information to establish best agrotechnical practices for the cultivation of D. moldavica is difficult because of the high variability of results between individual studies, due to differences in agroecological conditions [20]. Studies in controlled conditions greatly eliminate the undesirable effects of environmental heterogeneity, providing more comparable results with higher generalization ability. Recently, we examined a possibility for the organic production of D. moldavica in controlled conditions, using compost and vermicompost as soil amendments, and concluded that the use of vermicompost is superior to that of compost, even with the same amount of plant-available nutrients [27].
The nondestructive evaluation of the physiological status of intact growing plants often involves a measurement of leaf chlorophyll content by chlorophyll meters as well as a chlorophyll a fluorescence analysis, which characterizes the photochemical activity of photosynthesis [28,29]. The analysis of fluorescence transient has been most widely used as a tool to monitor environmental stress responses in plants [30]. However, it can also be used to characterize changes in the general physiological status of plants, as related to their performance and environmental adaptation in the case of wild plants [28] or growth/yield relationships of crop species due to different agricultural practices [27]. In particular, several studies have shown a relationship between the photochemistry of photosynthesis and the status of plant mineral nutrition [31,32,33,34], indicating that chlorophyll a fluorescence analysis can be also used to predict the physiological status of crops in studies aiming to assess the effects of organic fertilizers.
The aim of the present study is to compare the growth and physiological effects of vermicompost on D. moldavica plants in controlled conditions, using two types of commercially available substrates. The second aim is to assess if nondestructively measured photosynthesis-related parameters, leaf chlorophyll concentration, and chlorophyll a fluorescence-related indices, are useful for monitoring the physiological status of plants under different regimes of substrate amendment with the organic fertilizer, vermicompost.
2. Materials and Methods
2.1. Plant Material, Substrates, and Vermicompost
The study was performed with Dracocephalum moldavica L. plants grown from seeds purchased from Kurzemes Sēklas (Talsi, Latvia). Peat substrate KKS-1 (Laflora, Jelgava District, Latvia), commercial garden soil (Biolan, Eura, Finland), and vermicompost (Eko Zeme, Bauska District, Latvia) were purchased from local suppliers. The peat substrate contained fertilizer PG-Mix (15-10-20, 1 kg m−3), limestone (6 kg m−3), dolomite (1.8 kg m−3) and a wetting agent, Instant (0.3 L m−3). The garden soil contained mineral fertilizer (12-14-24, 1 kg m−3) and dolomite (4 kg m−3). Vermicompost was produced from composted cow manure and grass biomass.
The analysis of plant-available mineral nutrient concentration in the substrates and vermicompost was performed in a certified agrochemical laboratory (Laboratory of Plant Mineral Nutrition, Institute of Biology, University of Latvia). The results shown in Table 1 indicate that peat substrate had higher electrical conductivity (EC) in comparison to commercial garden soil, pointing to a higher concentration of soluble ions. Both substrates had a similar pH and concentration of plant-available K, Mg, Mn, Zn, and Cu. However, the N and P concentration was higher in peat substrate, but Ca, S, and Fe were higher in garden soil. In respect to the optimum element concentrations for cultivated plants, the peat substrate showed optimum or above-optimum levels, while a deficiency of N was evident for garden soil. Both substrates were extremely rich in Ca and Mg. Opposite to this, vermicompost was very good source of plant-available N, P, K, Mg, Mn, and Zn.

Table 1.
Concentration of plant-available mineral nutrients (mg L−1 dry substrate) in substrate and vermicompost samples used in the present study in comparison to approximate optimum concentrations in substrate for cultivated plants [35].
2.2. Plant Establishment, Treatments, and Cultivation Conditions
Seeds of D. moldavica were sown in heated (60 °C, 24 h) commercial garden soil (Biolan, Eura, UK) in 1 L plastic plant tissue culture containers, and closed and kept for two weeks in a growth cabinet (light/dark period of 16/8 h, photosynthetically active radiation with a photon flux density 100 μmol m−2 s−1, day/night temperature 15/20 °C). After that, the developed seedlings were individually transplanted to 200 mL plastic containers filled with commercial garden soil (Biolan, Eura, Finland). Containers with plants were placed in 48 L plastic boxes, closed with lids, and located in an experimental greenhouse with automatic control system (HortiMaX, Maasdijk, The Netherlands). Additional light was supplemented by Master SON-TPIA Green Power CG T 400 W (Philips, Amsterdam, Netherlands) and Powerstar HQI-BT 400 W/D PRO (Osram, Munich, Germany) lamps (380 μmol m−2 s−1 at the plant level) for a 16 h photoperiod, with day/night temperature 25/16 °C, and a relative air humidity of 60% to 70%. The plants were watered with deionized water. The boxes were periodically ventilated for the acclimation of the seedlings to greenhouse conditions. After two weeks, when the plants reached 5 to 10 cm height and developed four true leaves, they were used for the experiment.
The plants were transplanted to 1.2 L plastic containers filled with different substrate mixes. In total, eight treatments in five replicates were established, including two controls. Two commercial substrates, peat substrate KKS-1 (Laflora, Jelgava District, Latvia) and commercial compost-based garden soil (Biolan, Eura, Finland) were used as an alternative basis for the formation of substrate mixes. Peat substrates without the additives and garden soil without additives were used as the two controls. For two mineral fertilizer treatments, plants growing in peat substrate without additives or garden soil without additives received mineral fertilizer biweekly, starting from the third week after transplantation, using 0.075% Yara Tera Kristalon Blue fertilizer (19-6-20+MgO+micro; Yara International, Oslo, Norway), 200 mL per container. Four treatments with vermicompost were made by adding 20% or 30% (v/v) vermicompost to both peat substrate or garden soil. Individual containers were randomly arranged on the greenhouse bench and their location was changed biweekly. Conditions in the greenhouse were the same as described above. The substrate water content was monitored with a HH2 moisture meter equipped with WET-2 sensor (Delta-T Devices, Burwell, UK) and kept at 50% to 60% using deionized water. The plants were cultivated for eight weeks.
Substrate electrical conductivity (EC) and pH were measured 1 week and 8 weeks after the start of the experiment. For EC, the HH2 moisture meter equipped with a WET-2 sensor (Delta-T Devices, Burwell, UK) was used. Substrate pH was measured using a pH meter pH 3000 (STEP Systems, Nürnberg, Germany). For every container, four separate measurements on all sides of the container were performed for both measurements.
2.3. Measurement of Photosynthesis-Related Parameters
Measurements of photosynthesis-related parameters were performed weekly. The leaf chlorophyll concentration was measured using a chlorophyll meter CCM-300 (Opti-Sciences, Hudson, NH, USA). Four fully grown and actively photosynthesizing leaves from three randomly selected plants per treatment were measured. Chlorophyll a fluorescence was measured in four leaves dark-adapted for at least 20 min by the Handy PEA fluorometer (Hansatech Instruments, King’s Lynn, UK) on each of the three plants per treatment.
Fluorescence data analysis was performed by PEA Plus software (Hansatech Instruments, King’s Lynn, UK). A number of parameters derived from the fast fluorescence induction curve were used for the analysis [36,37]. Fv/Fm, calculated as (Fm − F0)/Fm, and represents the maximum quantum efficiency of photosystem II (PSII), indicating a probability that a trapped photon will perform a further photochemical energy transfer. Fv/F0, calculated as (Fm − F0)/F0, is considered to reflect an instant photochemical activity at the donor side of PSII. Performance Index (PI) represents the multiparametric and multitypal entity, used as relative indication of sample vitality, and can have different types of expression. Thus, PIinst combines three function-related (trapping of absorbed exciton, electron transport between the photosystems, and reduction of end-electron acceptors) parameters. PIabs indicates the functional activity of PSII related to the energy absorbed, as in addition to the three previous parameters, it also includes a structural parameter, the amount of chlorophyll per reaction center of chlorophyll. PItotal includes information on the status of both PSII and photosystem I (PSI), in addition to characterizing the electron flow between the two systems, which is also on an absorption basis.
2.4. Morphological Measurements
At the termination of the experiment, individual plants were cut, plant height was measured, the number of branches was counted, and the leaves were divided from the stems and weighed separately. Plant tissues were dried in an oven at 60 °C for 72 h and the dry mass was measured. The water content in plant leaves and stems was calculated in g H2O per g dry mass.
2.5. Data Analysis
The measurement results were analyzed and the graphs were made by KaleidaGraph (v. 4.1, Synergy Software, Reading, PA, USA). The statistical significance of the differences of individual parameters between treatments was evaluated by one-way ANOVA minimum significant difference tests using a Microsoft Excel spreadsheet (www.biostathandbook.com/anova.xls, accessed on 15 July 2021) [38].
3. Results
3.1. Effect of Substrate Type, Fertilizer, and Vermicompost on EC and pH
The substrate electrical conductivity (EC) was measured weekly throughout the experimental period to monitor changes in soluble salt concentration (Figure 1). A characteristic decrease of EC values during the cultivation of D. moldavica reflected the uptake of mineral nutrients by the plants. The initial EC in peat substrate was 8% higher than that in commercial garden soil, and the difference was statistically significant. Moreover, the addition of equal amounts of vermicompost resulted in a higher increase in substrate EC in the case of peat. Thus, EC increased by 268 and 411 mS m−1 for a 20% and 30% amendment, respectively, in peat substrate, and only by 242 and 324 mS m−1 for a 20% and 30% amendment, respectively, in commercial garden soil. Similarly, a decrease of EC during plant cultivation was more pronounced in the case of peat (356 and 444 mS m−1 for a 20% and 30% amendment, respectively) in comparison to that in soil (292 and 345 mS m−1 for 20% and 30% amendment, respectively). However, a decrease of substrate EC for control plants was identical in peat and soil (by 145 mS m−1).
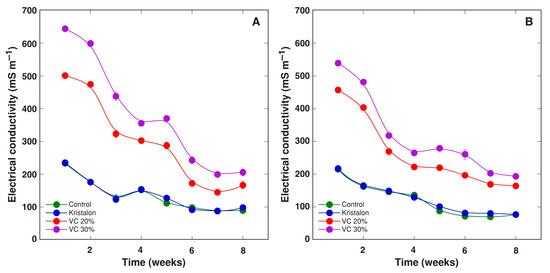
Figure 1.
Changes of substrate electrical conductivity as affected by mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate during cultivation of Dracocephalum moldavica plants in peat substrate (A) or commercial garden soil (B). Results are means ± SE from five replicates for each treatment, four measurements per replicate.
Initial pH in peat substrate was significantly higher than that in commercial garden soil (Figure 2A). An amendment with vermicompost significantly increased the pH in peat (by 4% and 6%, for a 20% and 30% amendment rate, respectively), but the increase in soil by the vermicompost amendment was more pronounced (by 16% and 17%, for a 20% and 30% amendment rate, respectively). The substrate pH further increased in all treatments during plant cultivation, but initial differences between peat and soil treatments remained significant (Figure 2B).
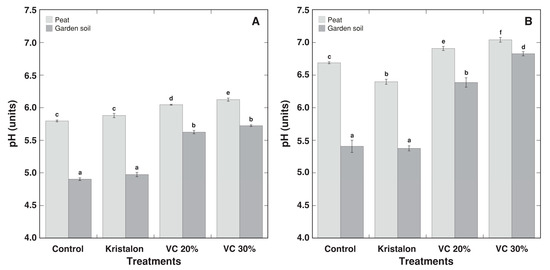
Figure 2.
Effect of mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate on substrate pH for Dracocephalum moldavica plants grown in peat substrate or commercial garden soil for 1 week (A) and 8 weeks (B) after the start of the experiment. Results are means ± SE from five replicates for each treatment, four measurements per replicate. Means with identical letters are not considered statistically significantly different (p < 0.05).
3.2. Effect of Substrate Type, Fertilizer, and Vermicompost on Morphological Parameters
As for morphological appearance, D. moldavica plants grown in vermicompost-amended substrate had denser and greener foliage, in comparison to the control and mineral fertilizer-treated plants (Figure 3). However, the growth of control plants in peat substrate or commercial garden soil was identical (Figure 4 and Figure 5), except that a fresh mass of stems was 16% lower in commercial garden soil in comparison to that in peat substrate (Figure 4B). The addition of mineral fertilizer increased both the fresh and dry mass of the leaves and stems, and this effect was significant for all parameters for peat-grown plants, but only for the fresh mass of leaves for soil-grown plants. The average growth increase by mineral fertilizer was 25% for D. moldavica plants grown in peat and 15% for plants grown in soil. The substrate amendment with 20% vermicompost resulted in a 114% average increase in biomass for plants grown in peat and a 98% average increase for plants grown in soil; however, for plants at a 30% amendment rate, the increase was 148% and 68% for peat and soil, respectively. Thus, for D. moldavica plants grown in peat, an amendment with 30% vermicompost resulted in an additional statistically significant increase of plant biomass, but that was not the case for plants grown in soil, with a significant decrease in the dry mass of both the leaves and stems in comparison to a 20% vermicompost amendment. As a result, at the highest vermicompost amendment rate, the plants grown in soil had a 47% and 30% lower fresh mass of the leaves and stems, as well as a 37% and 34% lower dry mass of the leaves and stems, in comparison to peat-grown plants.

Figure 3.
Morphology of the typical average Dracocephalum moldavica grown in different substrates for 8 weeks. P, peat substrate; K, mineral fertilizer Kristalon; VC20%, 20% (v/v) vermicompost; VC30%, 30% (v/v) vermicompost; S, commercial garden soil.
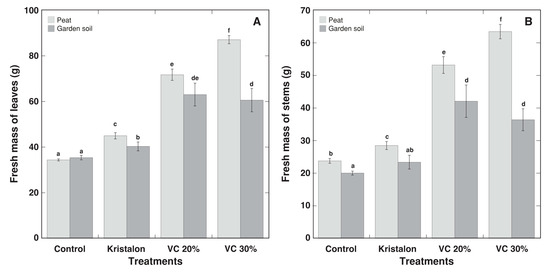
Figure 4.
Effect of mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate on fresh mass of leaves (A) and stems with inflorescences (B) of Dracocephalum moldavica plants grown in peat substrate or commercial garden soil for 8 weeks. Results are means ± SE from five replicates for each treatment. Means with identical letters are not considered statistically significantly different (p < 0.05).
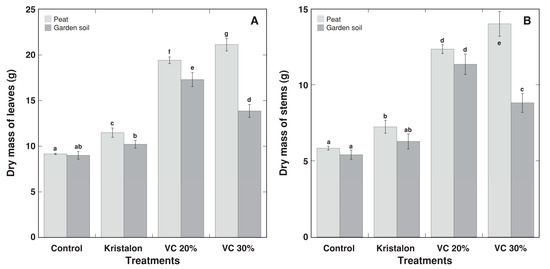
Figure 5.
Effect of mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate on dry mass of leaves (A) and stems with inflorescences (B) of Dracocephalum moldavica plants grown in peat substrate or commercial garden soil for 8 weeks. Results are means ± SE from five replicates for each treatment. Means with identical letters are not considered statistically significantly different (p < 0.05).
The water content of leaves showed only negligible differences between treatments (Figure 6A), but the water content of the stems tended to be lower for plants grown in soil (Figure 6B). Similarly, plant height tended to be lower for soil-grown plants in all treatments, with significant differences for both vermicompost amendment rates (Figure 7A). In addition, the number of branches increased in vermicompost-amended plants (Figure 7B).
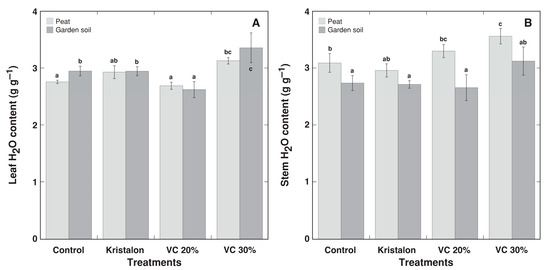
Figure 6.
Effect of mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate on H2O content in leaves (A) and stems with inflorescences (B) of Dracocephalum moldavica plants grown in peat substrate or commercial garden soil for 8 weeks. Results are means ± SE from five replicates for each treatment. Means with identical letters are not considered statistically significantly different (p < 0.05).
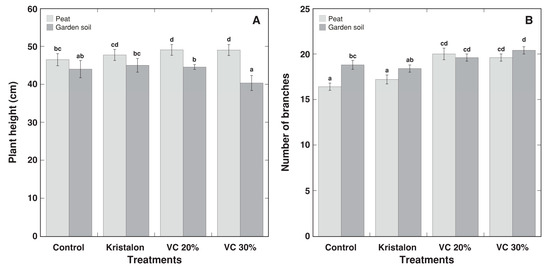
Figure 7.
Effect of mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate on plant height (A) and number of branches (B) of Dracocephalum moldavica plants grown in peat substrate or commercial garden soil for 8 weeks. Results are means ± SE from five replicates for each treatment. Means with identical letters are not considered statistically significantly different (p < 0.05).
3.3. Effect of Substrate Type, Fertilizer, and Vermicompost on Photosynthesis-Related Parameters
Leaf chlorophyll concentrations did not show any significant differences between treatments during four weeks of cultivation, where the concentration remained relatively stable (for plants in peat substrate) or showed an increasing trend (for plants in commercial garden soil) (Figure 8). Next, chlorophyll concentration decreased for plants in all treatments; however, the rate of decrease differed between the treatments, with more stable chlorophyll content for vermicompost-amended plants. As a result, there was no overall significant effect of vermicompost amendment on the chlorophyll concentration in both substrates (Table 2).
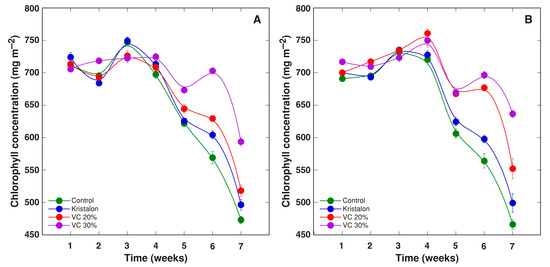
Figure 8.
Changes of leaf chlorophyll concentration as affected by mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate in Dracocephalum moldavica plants grown in peat substrate (A) or commercial garden soil (B). Results are means ± SE from five replicates for each treatment, four measurements per replicate.

Table 2.
Results of ANOVA analysis of physiological indices of Dracocephalum moldavica plants during cultivation in peat substrate and commercial garden soil with different amendments.
The chlorophyll a fluorescence fast induction curve in plant leaves was measured weekly during plant cultivation, and temporal changes of various parameters derived from the curve were compared in D. moldavica plants grown in different substrates (Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13). In general, the largest differences for Fv/Fm (Figure 9) and Fv/Fo (Figure 10) between treatments were evident at 4–6 weeks for plants grown in peat and at 5–6 weeks for plants grown in soil, with values for vermicompost-amended plants significantly higher than these for control or mineral-treated plants. Moreover, the statistically significant increase for plants amended with a 30% vermicompost in comparison to a 20% amendment was evident on week 6–7 for plants grown in peat (Figure 9A and Figure 10A) and on week 4–7 for plants grown in soil (Figure 9B and Figure 10B). According to the ANOVA analysis, the effect of treatment was significant for both Fv/Fm and Fv/F0, but the effect in the case of plants grown in soil was at a higher significance level than that of plants grown in peat (Table 2).
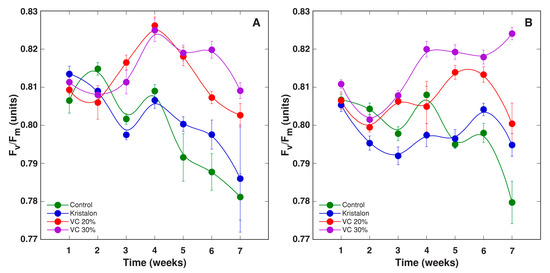
Figure 9.
Changes of chlorophyll a fluorescence parameter Fv/Fm as affected by mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate in Dracocephalum moldavica plants grown in peat substrate (A) or commercial garden soil (B). Results are means ± SE from five replicates for each treatment, three measurements per replicate.
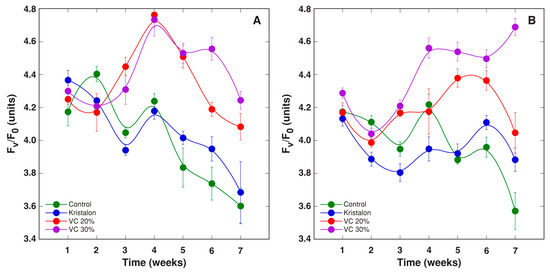
Figure 10.
Changes of chlorophyll a fluorescence parameter Fv/F0 as affected by mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate in Dracocephalum moldavica plants grown in peat substrate (A) or commercial garden soil (B). Results are means ± SE from five replicates for each treatment, three measurements per replicate.
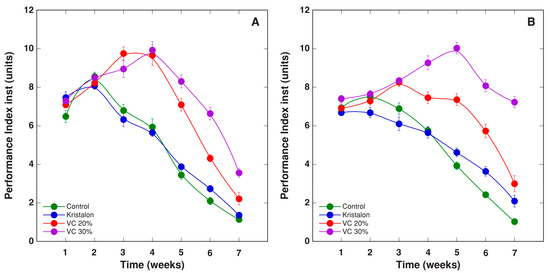
Figure 11.
Changes of chlorophyll a fluorescence parameter PIinst, as affected by mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate in Dracocephalum moldavica plants grown in peat substrate (A) or commercial garden soil (B). Results are means ± SE from five replicates for each treatment, three measurements per replicate.
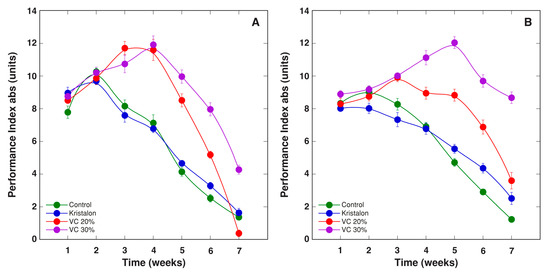
Figure 12.
Changes of chlorophyll a fluorescence parameter PIabs, as affected by mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate in Dracocephalum moldavica plants grown in peat substrate (A) or commercial garden soil (B). Results are means ± SE from five replicates for each treatment, three measurements per replicate.
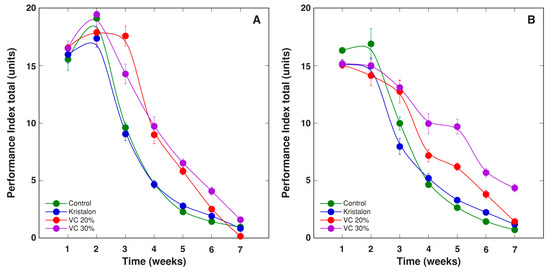
Figure 13.
Changes of chlorophyll a fluorescence parameter PItotal, as affected by mineral fertilizer (Kristalon) and amendment with vermicompost (VC) at a 20% and 30% (v/v) rate in Dracocephalum moldavica plants grown in peat substrate (A) or commercial garden soil (B). Results are means ± SE from five replicates for each treatment, three measurements per replicate.
Two other, more complex parameters of chlorophyll a fluorescence, PIinst and PIabs, showed a relatively similar trend during plant cultivation (Figure 11 and Figure 12). For vermicompost-amended plants grown in peat, both parameters increased up to week 4, and for amended plants at the highest rate in soil, up to week 5. For peat-grown plants, differences in PIinst and PIabs between the plants at two vermicompost amendment rates were less pronounced than these for soil-grown plants. As a result, the overall differences between treatments were not statistically significant for plants grown in peat, and significant for plants grown in soil (Table 2).
The chlorophyll a fluorescence parameter PItotal showed a pronounced decrease with plant age for all treatments, but some differences were evident between them (Figure 13). For plants grown in peat, the vermicompost amendment resulted in significantly higher PItotal during weeks 3–5, but there were no statistically significant differences between plants at two amendment rates (Figure 13A). For plants grown in soil, the vermicompost amendment resulted in significantly higher PItotal during weeks 3–6, and plants at a 30% amendment rate had significantly higher parameter values during weeks 4–7 (Figure 13B). However, the overall effect of treatments was not significant (Table 2).
To further analyze the possible contribution of chlorophyll concentration and a particular chlorophyll a fluorescence parameter to plant yield, as affected by different cultivation substrates, a correlation between the value of each physiological indication at each particular week of cultivation and each of four yield parameters (fresh and dry mass of leaves and stems) was calculated (Figure 14). The highest correlation between leaf chlorophyll concentration and yield was during weeks 5–6, evidently reflecting a delay of the senescence of plants grown in the vermicompost-amended substrate. In contrast, all fluorescence-derived parameters showed a high correlation with the yield at week 3, with pronounced differences between the parameters during the further cultivation period. Both PIinst and PIabs were the parameters with the highest degree of correlation during weeks 3–4; the correlation with Fv/Fm and Fv/F0 peaked at week 5, but the correlation with PItotal steadily decreased (Figure 14).
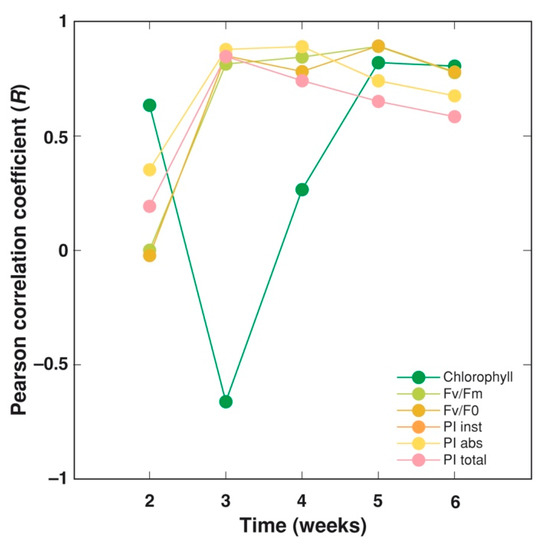
Figure 14.
Changes of average correlation between physiological indices and yield parameters (fresh and dry mass of leaves and stems) of Dracocephalum moldavica plants during cultivation in peat substrate and commercial garden soil with different amendments. PIinst has the same values as PIabs and cannot be seen.
4. Discussion
Both compost [21,22,23,27] and vermicompost [26,27] have been verified as promising types of organic fertilizers for the cultivation of D. moldavica. Most importantly, the application of compost and vermicompost increased the yield of the essential oil obtained from D. moldavica [21,23,26], but the use of vermicompost also resulted in an increase in soil microbial activity [26], indicating that not only yield and yield quality, but also soil sustainability, are positively affected by these organic fertilizers. The results of the present study provide additional support to these facts, but also indicate that special attention should be paid to the characteristics of substrates used for the preparation of vermicompost-containing mixes.
A direct positive effect of vermicompost for plants has been associated with the increased supply of plant-available mineral nutrients as well as the presence of plant growth-stimulating substances [5]. While it is reasonable to suggest that the addition of vermicompost to soil or any other substrate used for plant cultivation results in physicochemical or biological interactions between various components, it is not clear if these interactions could affect plants, as no studies so far have addressed the question of the influence of substrate type on the effect of vermicompost on plants. When in a previous study D. moldavica plants were cultivated in a relatively nutrient-poor soil, the plant shoot biomass linearly increased with an increasing vermicompost amendment rate up to 40%, mostly because of the additional supply of mineral nutrients [27]. In the present study, substrates with a relatively high content of plant-available mineral nutrients were used, with near-optimum concentrations of elements for peat substrate and only some shortage of N for commercial garden soil (Table 1). As a result, the growth of D. moldavica plants was relatively similar in pure substrates, with a 16% decrease of fresh mass of stems in the case of garden soil (Figure 4B) that was obviously only related to a lower tissue water content (Figure 6B). Moreover, the additional application of mineral fertilizer three times during the cultivation period increased plant biomass only by 25% in peat and 15% in soil (Figure 4 and Figure 5), indicating that the concentration of plant-available mineral nutrients in both substrates was close to optimum, initially, at least. Biomass increase by vermicompost amendment was much higher, at 114% and 98%, in comparison to control in the case of a 20% amendment rate, for peat- and soil-grown plants, respectively. However, for a 30% amendment rate, these values were 148% and 68% for peat- and soil-grown plants, respectively. Consequently, the addition of an identical amount of vermicompost resulted in a poorer growth response of plants in commercial garden soil as a substrate in comparison to peat, but an increase of the amendment rate from 20% to 30% resulted in some growth inhibition for these plants.
Poor crop growth in substrates at high rates of amendment with organic fertilizers has been associated with a too high amount of soluble salts, possibly leading to osmotic or ionic stress [39,40]. Root exudates of some plant species (i.e., Thymus vulgaris) can facilitate the mineralization of organic matter, further enhancing the content of soluble salts in organic fertilizer-amended soil [27,41]. However, the growth suppression of D. moldavica plants in garden soil, amended with 30% vermicompost, could not be due to excessive soluble salt concentration, as the initial EC in cultivation substrate in the case of the vermicompost-amended peat substrate was even higher than that for the amended soil (Figure 1). In addition, no metabolic toxicity could be proposed, as plants grown in soil amended with 30% vermicompost had values of Fv/Fm, which is an indicator of plant stress, around 0.82, corresponding to a highly optimal physiological state of photochemistry [42]. It seems that some other characteristics, apart from the soluble salt content, in the two substrate systems could account for the differences in the growth responses of D. moldavica plants in those substrates amended with an equal amount of vermicompost. One possible target characteristic could be related to microbial activity, which is evidently significantly higher in commercial garden soil in comparison to that in peat substrate. Vermicompost itself is a very rich source of bacterial and fungal diversity, and the total number of cultivable filamentous fungi shows a significant impact on the growth-stimulating activity of vermicompost extracts [43]. It seems that some type of unidentified active factor from commercial garden soil changed some characteristic of vermicompost important for plant growth stimulation, through an inactivation of hormone-like substances or production of growth inhibitors.
The stimulation of water accumulation in plant shoots is one of the main effects of the application of humic substances for plants, besides stimulation of root growth [44], and this effect has been also found for vermicompost application [27]. In the present study, the water content of leaves significantly increased only at a 30% vermicompost amendment rate for both substrates (Figure 6A); however, for the stems there was a significant effect only in the case of a 30% substitution in peat substrate (Figure 6B). Most likely, a higher degree of water accumulation in plant tissues reflects an increase in mineral nutrient availability and is related to the efficient decrease of ionic strength and/or the osmotic potential in cells [45]. Similarly, the increased tissue water content of halophytic species under high soil salinity is probably associated with extensive vacuolar development because of ion accumulation [46].
The chlorophyll a fluorescence measurements provide complex information on the physiological status of PSII, both reaction centers and antenna, and the components of electron transfer chain at both donor and acceptor sides, as well as the effect of light-independent reactions [30]. Among them, PI is an essence of photochemical reactions, incorporating information on energy fluxes at the most crucial steps of energy transfer during the photochemical reactions of photosynthesis. It needs to be stressed that the absolute values of PI measurement cannot be used for the characterization of any sample, but only the changes in these parameters in the sample adds meaning to these measurements [47].
In respect to the usefulness of physiological indices for monitoring the status of plants and predicting plant yield, it needs to be stressed that changes in chlorophyll concentration reflect important metabolic changes due to changed conditions, rather than acting as a cause of these changes. In contrast, the variation of activity in the photochemical processes of photosynthesis because of changed conditions can directly lead to a decrease in photosynthesis rate and a reduced plant growth and yield. Chlorophyll concentration increased in field-grown D. moldavica plants due to compost application, but there was no relationship with the compost dose applied [21]. Similar results were obtained in another field study with D. moldavica [23]. In the present experiments, the chlorophyll concentration was positively affected by a vermicompost amendment in a concentration-dependent manner, but this effect appeared relatively late during a cultivation period, when the general decreasing trend of changes in chlorophyll content was evident (Figure 8). Therefore, it is most likely that the increased chlorophyll concentration in the leaves of D. moldavica plants grown in vermicompost-amended substrate reflected delayed senescence of these plants, due to a prolonged supply of mineral nutrients.
In the present study, large differences were found between three groups of fluorescence-derived parameters: (i) Fv/Fm and Fv/F0, (ii) PIinst and PIabs, and (iii) PItotal (Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13). In addition, these groups showed a variable level of predictability, with respect to differences in plant yield due to a pronounced variation, in correlation with time (Figure 14). While both PIabs and PItotal are calculated from temporal changes in fluorescence emission on an absorption basis, PItotal also involves changes in energy fluxes as related to the reduction of PSI end electron acceptors [47]. Thus, the extreme decrease of PItotal with plant age seems to be related to the diminished efficiency of electron transfer at the PSI side. Similarly, PItotal has been shown to represent a sensitive indicator of leaf senescence, especially in comparison to PIabs [48]. As Fv/F0 values during the second half of the vegetation period had a good predictability of yield of D. moldavica, it seems that mostly photochemical reactions at the donor side of PSII, including the activity of the water-splitting complex, were important as yield determinants during that particular period of plant development.
In conclusion, the incorporation of vermicompost for the cultivation of D. moldavica, even in substrate mixes with a relatively high and balanced composition of plant-available nutrients, benefits plant growth, physiological status, and biomass yield. It appears that organic fertilizers and especially earthworm-produced vermicomposts are useful substrate components for the sustainable cultivation of D. moldavica. The nondestructive chlorophyll fluorescence analysis can be successfully used to predict biomass accumulation of D. moldavica plants grown in different substrates. It appears that, to gain maximal benefits from vermicompost application in a form of increased plant yield and quality, it is necessary to explore interactions between vermicompost and other substrates leading to possible changes in the quality-related characteristics of vermicompost in substrate mixes. Most importantly, a detailed analysis of microbial processes and their effect on the quality characteristics of vermicompost needs to be investigated.
Author Contributions
A.O. and G.I. conceived and designed the study; A.O., U.A.-O. and G.I. performed experiments and gathered data; A.O. and G.I. analyzed and interpreted the data; A.O. and G.I. wrote the manuscript. All authors have approved the manuscript for publication. G.I. takes responsibility for the integrity of the work as a whole. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data reported here is available from the authors upon request.
Acknowledgments
We thank Jānis Freibergs (Eko Zeme, Latvia) for providing the vermicompost sample used in the present study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cakmak, I. Plant nutrition research: Priorities to meet human needs for food in sustainable ways. Plant Soil 2002, 247, 3–24. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Domínguez, J. Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 2008, 72, 1013–1019. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Sunil, E.; Thomas, G.V. Amplification of plant beneficial microbial communities during conversion of coconut leaf substrate to vermicompost by Eudrilus sp. Curr. Microbiol. 2009, 59, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, J.; Vig, A.P.; Dhaliwal, S.S.; Rup, P.J. Cocomposting with and without Eisenia fetida for conversion of toxic paper mill sludge to a soil conditioner. Bioresour. Technol. 2010, 101, 8192–8198. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G. Review on physiological effects of vermicomposts on plants. In Biology of Compost; Soil Biology Volume 58; Meghvansi, M.K., Varma, A., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 63–86. [Google Scholar]
- Aslam, Z.; Bashir, S.; Hassan, W.; Bellitürk, K.; Ahmad, N.; Niazi, N.K.; Khan, A.; Khan, M.I.; Chen, Z.; Maitah, M. Unveiling the efficiency of vermicompost derived from different biowastes on wheat (Triticum aestivum L.) plant growth and soil health. Agronomy 2019, 9, 791. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.; Prakash, D.; Gupta, S.; Nazareno, M.A. Role of vermicomposting in agricultural waste management. In Sustainable Green Technologies for Environmental Management; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Springer Nature: Singapore, 2019; pp. 283–295. [Google Scholar]
- Arancon, N.Q.; Edwards, C.A.; Atiyeh, R.; Metzger, J.D. Effects of vermicompost produced from food waste on the growth and yields of greenhouse peppers. Bioresour. Technol. 2004, 93, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Pączka, G.; Mazur- Pączka, A.; Garczyńska, M.; Hajduk, E.; Kostecka, J.; Bartkowska, I.; Butt, K.R. Use of vermicompost from sugar beet pulp in cultivation of peas (Pisum sativum L.). Agriculture 2021, 11, 919. [Google Scholar] [CrossRef]
- Schröder, C.; Häfner, F.; Larsen, O.C.; Krause, A. Urban organic waste for urban farming: Growing lettuce using vermicompost and thermophilic compost. Agronomy 2021, 11, 1175. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A.; Andersone-Ozola, U.; Ievinsh, G. Vermicompost from municipal sewage sludge affects growth and mineral nutrition of winter rye (Secale cereale) plants. J. Plant Nutr. 2016, 39, 765–780. [Google Scholar] [CrossRef]
- Hussain, N.; Abbasi, S.A. Efficacy of the vermicomposts of different organic wastes as “clean” fertilizers: State-of-the-art. Sustainability 2018, 10, 1205. [Google Scholar] [CrossRef] [Green Version]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Pierre-Louis, R.; Kader, M.A.; Desai, N.M.; John, E.H. Potentiality of vermicomposting in the South Pacific island countries: A review. Agriculture 2021, 11, 876. [Google Scholar] [CrossRef]
- Vuković, A.; Velki, M.; Ečimović, S.; Vuković, R.; Čamagajevac, I.Š.; Lončarić, Z. Vermicomposting–facts, benefits and knowledge gaps. Agronomy 2021, 11, 1952. [Google Scholar] [CrossRef]
- Soni, R.; Sharma, A. Vermiculture technology: A novel approach in organic farming. Indian Hortic. J. 2016, 6, 150–154. [Google Scholar]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef]
- Warman, P.R.; AngLopez, M.J. Vermicompost derived from different feedstocks as a plant growth medium. Bioresour. Technol. 2010, 101, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; La Placa, G.; Saiano, F.; Mammano, M.M.; Orlando, S.; Laudicina, V.A. Effects of vermicompost, compost and digestate as commercial alternative peat-based substrates on qualitative parameters of Salvia officinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Aćimović, M.; Sikora, V.; Brdar-Jokanović, M.; Kiprovski, B.; Popović, V.; Koren, A.; Puvača, N. Dracocephalum moldavica: Cultivation, chemical composition and biological activity. J. Agron. Technol. Eng. Manag. 2019, 2, 152–167. [Google Scholar]
- Hussein, M.S.; El-Sherbeny, S.E.; Khalil, M.Y.; Nagib, N.Y.; Aly, S.M. Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Sci. Hortic. 2006, 1108, 322–331. [Google Scholar] [CrossRef]
- Mostafa, H.S. Complementary effect between compost rate and ascorbic acid concentration on enhancing dragonhead (Dracocephalum moldavica) plant on growth and productivity. Middle East J. Agric. 2018, 7, 1811–1818. [Google Scholar]
- Badawy, E.S.M.; El-Sherbeny, S.; Hussein, M.S.; Swaefy, H.M.F.; Amer, H.M. Effect of organic manure on growth, production and active ingredients in dragonhead (Dracocephalum moldavica L.). Medic. Aromat. Plant Sci. Biotechnol. 2009, 3, 9–13. [Google Scholar]
- Nejatzadeh-Barandozi, F.; Shahvaladi, E.H.; Gholami-Borujeni, F. Nitrogen fertilization and microelements influences growth index and yield in Dracocephalum moldavica L. Bulg. J. Agric. Sci. 2015, 21, 266–269. [Google Scholar]
- Nasiri, Y. Crop productivity and chemical compositions of dragonhead (Dracocephalum moldavica L.) essential oil under different cropping patterns and fertilization. Industr. Crops Prod. 2021, 171, 113920. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Amirnia, R.; Chiyaneh, S.F.; Maggi, F.; Barin, M.; Razavi, B.S. Improvement of dragonhead (Dracocephalum moldavica L.) yield quality through a coupled intercropping system and vermicompost application along with maintenance of soil microbial activity. Land Degrad. Dev. 2021, 32, 2833–2848. [Google Scholar] [CrossRef]
- Ievinsh, G.; Andersone-Ozola, U.; Zeipiņa, S. Comparison of the effects of compost and vermicompost soil amendments in organic production of four herb species. Biol. Agric. Hortic. 2020, 36, 267–282. [Google Scholar] [CrossRef]
- Andersone, U.; Druva-Lūsīte, I.; Ieviņa, B.; Karlsons, A.; Ņečajeva, J.; Samsone, I.; Ievinsh, G. The use of nondestructive methods to assess a physiological status and conservation perspectives of Eryngium maritimum L. J. Coast. Conserv. 2011, 15, 509–522. [Google Scholar] [CrossRef]
- Matisons, R.; Krišāns, O.; Jansons, Ā.; Kondratovičs, T.; Elferts, D.; Ievinsh, G. Norway spruce seedlings from an Eastern Baltic provenance show tolerance to simulated drought. Forests 2021, 12, 82. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Strand, M. Effect of mineral nutrient content on oxygen exchange and chlorophyll a fluorescence in needles of Norway spruce. Tree Physiol. 1997, 17, 221–230. [Google Scholar] [CrossRef]
- Kastori, R.; Plesnicar, M.; Arsenijevic-Maksimovic, I.; Petrovic, N.; Pankovic, D.; Sakac, Z. Photosynthesis, chlorophyll fluorescence, and water relations in young sugar beet plants as affected by sulfur supply. J. Plant Nutr. 2000, 23, 1037–1049. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.L.; Jin, J.Y. Effects of zinc and soil moisture on photosynthetic rate and chlorophyll fluorescence parameters of maize. Biol. Plant. 2009, 53, 191–194. [Google Scholar] [CrossRef]
- Horaczek, T.; Dąbrowski, P.; Kalaji, H.M.; Baczewska-Dąbrowska, A.H.; Pietkiewicz, S.; Stępień, W.; Gozdowski, D. JIP-test as a tool for early detection of the macronutrients deficiency in Miscanthus plants. Phtosynthetica 2020, 58, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Osvalde, A. Optimization of plant mineral nutrition revisited: The roles of plant requirements, nutrient interactions, and soil properties in fertilization management. Environ. Exp. Biol. 2011, 9, 1–8. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterise and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practicioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014; p. 299. [Google Scholar]
- Tiquia, S.M. Reduction of compost phytotoxicity during the process of decomposition. Chemosphere 2010, 79, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Jakubus, M. A comparative study of composts prepared from various organic wastes based on biological and chemical parameters. Agronomy 2020, 10, 869. [Google Scholar] [CrossRef]
- Kramer, C.; Gleixner, G. Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol. Biochem. 2006, 38, 3267–3278. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Grantina-Ievina, L.; Andersone, U.; Berkolde-Pīre, D.; Nikolajeva, V.; Ievinsh, G. Critical tests for determination of microbiological quality and biological activity in commercial vermicompost samples of different origins. Appl. Microbiol. Biotechnol. 2013, 97, 10541–10554. [Google Scholar] [CrossRef]
- Piccolo, A.; Celano, G.; Pietramellara, G. Effects of fractions of coal-derived humic substances on seed germination and growth of seedlings (Lactuga sativa and Lycopersicon esculentum). Biol. Fertil. Soils 1993, 16, 11–15. [Google Scholar] [CrossRef]
- Ievinsh, G.; Andersone-Ozola, U.; Sieriņa, A. Development and physiological performance of hydroponically-grown ornamental indoor plants in respect to their potential use in botanical biofilters: Effect of mineral nutrient availability. Proc. Latvian Acad. Sci. B 2021, in press. [Google Scholar]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. In Advances in Botanical Research; Kader, J.-C., Delseny, M., Eds.; Academic Press: Burlington, MA, USA, 2010; Volume 55, pp. 179–225. [Google Scholar]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approcimations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Vuletić, M.V.; Španić, V. Characterization of photosynthetic performance during natural leaf senescence in winter wheat: Multivariate analysis as a tool for phenotypic characterization. Photosynthetica 2020, 58, 301–313. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).