Evaluation of Chestnut Susceptibility to Cryphonectria parasitica: Screening under Controlled Conditions
Abstract
:1. Introduction
2. Materials and Methods
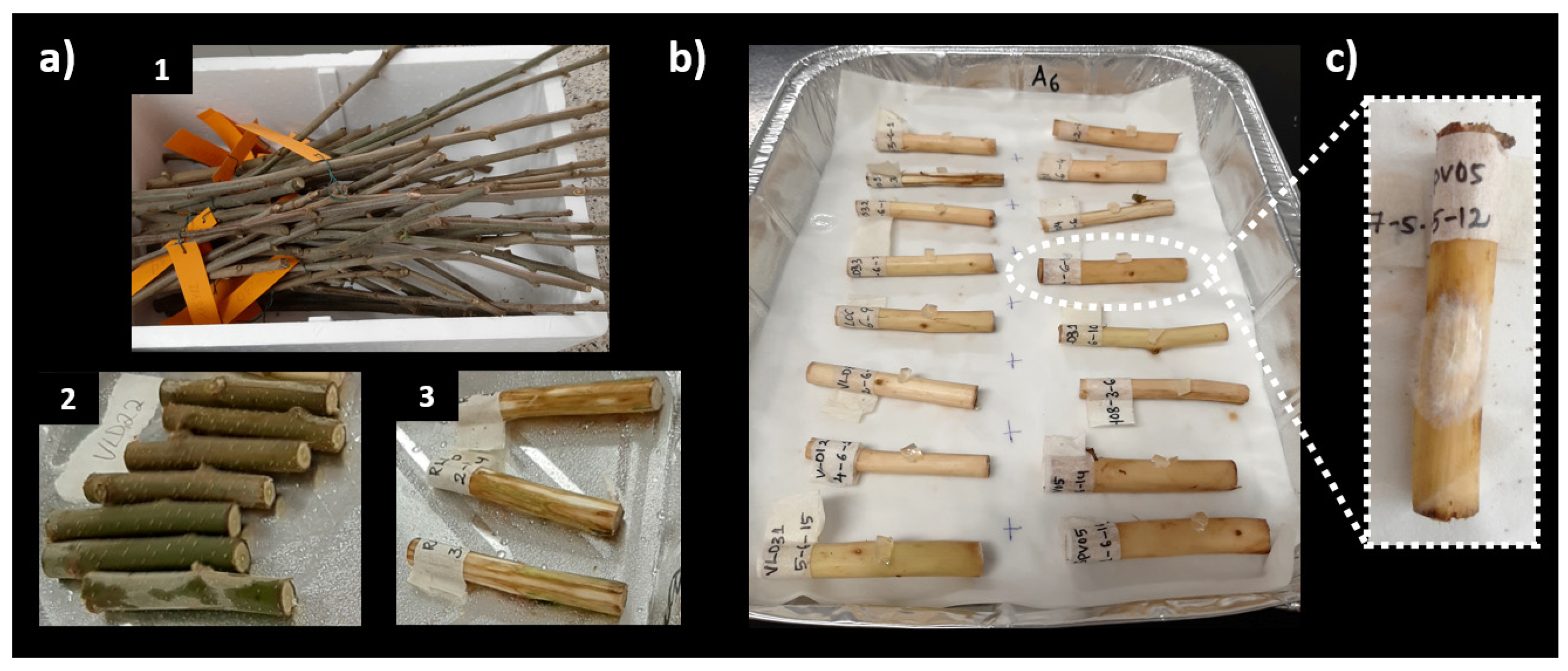
2.1. In Vitro Inoculation: Budstick Assay
2.1.1. Assay A
2.1.2. Assay B
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robin, C.; Heiniger, U. Chestnut blight in Europe: Diversity of Cryphonectria parasitica, hypovirulence and biocontrol. For. Snow Landsc. Res. 2001, 76, 361–367. [Google Scholar]
- Anagnostakis, S.L. Chestnut Blight: The Classical Problem of an Introduced Pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Heiniger, U.; Rigling, D. Biological control of chestnut blight in Europe. Annu. Rev. Phytopathol. 1994, 32, 581–599. [Google Scholar] [CrossRef]
- Krstin, L.; Novak-Agbaba, S.; Rigling, D.; Krajačić, M.; Ćurković Perica, M. Chestnut blight fungus in Croatia: Diversity of vegetative compatibility types, mating types and genetic variability of associated Cryphonectria hypovirus 1. Plant Pathol. 2008, 57, 1086–1096. [Google Scholar] [CrossRef]
- EPPO. Cryphonectria parasitica. Available online: https://gd.eppo.int/taxon/ENDOPA (accessed on 10 October 2021).
- Milgroom, M.G.; Sotirovski, K.; Spica, D.; Davis, J.E.; Brewer, M.T.; Milev, M.; Cortesi, P. Clonal population structure of the chestnut blight fungus in expanding ranges in southeastern Europe. Mol. Ecol. 2008, 17, 4446–4458. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Aguayo, J.; Fourrier-Jeandel, C.; Husson, C.; Loos, R. Assessment of Passive Traps Combined with High-Throughput. Appl. Environ. Microbiol. 2018, 84, e02637-17. [Google Scholar] [CrossRef] [Green Version]
- West, J.S.; Kimber, R.B.E. Innovations in air sampling to detect plant pathogens. Ann. Appl. Biol. 2015, 166, 4–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lione, G.; Giordano, L.; Turina, M.; Gonthier, P. Hail-induced infections of the chestnut blight pathogen Cryphonectria parasitica depend on wound size and may lead to severe diebacks. Phytopathology 2020, 110, 1280–1293. [Google Scholar] [CrossRef]
- Bryner, S.F.; Prospero, S.; Rigling, D. Dynamics of Cryphonectria hypovirus infection in chestnut blight cankers. Phytopathology 2014, 104, 918–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pažitný, J.; Bolvanský, M.; Adamčíková, K. Screening for resistance of progenies derived from Castanea sativa × C. crenata and C. crenata to Cryphonectria parasitica. For. Pathol. 2018, 48, e12439. [Google Scholar] [CrossRef]
- Cipollini, M.L.; Moss, J.P.; Walker, W.; Bailey, N.; Foster, C.; Reece, H.; Jennings, C. Evaluation of an Alternative Small Stem Assay for Blight Resistance in American, Chinese, and Hybrid Chestnuts (Castanea spp.). Plant Dis. 2021, 105, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Aguín, O.; Sainz, M.; Montenegro, D.; Mansilla, J.P. Biodiversidad e hipovirulencia de Cryphonectria parasitica en Europa: Implicaciones para el control biológico del cancro del castaño. Recur. Rurais 2011, 7, 35–47. [Google Scholar] [CrossRef]
- Aghayeva, D.N.; Rigling, D.; Prospero, S. Low genetic diversity but frequent sexual reproduction of the chestnut blight fungus Cryphonectria parasitica in Azerbaijan. For. Pathol. 2017, 47, 1–7. [Google Scholar] [CrossRef]
- Cortesi, P.; Milgroom, M.G. Genetics of vegetative incompatibility in Cryphonectria parasitica. Appl. Environ. Microbiol. 1998, 64, 2988–2994. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Adalia, E.J.; Fernández, M.M.; Diez, J.J. The use of mycoviruses in the control of forest diseases. Biocontrol Sci. Technol. 2016, 26, 577–604. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnagnostakis, S.L. Chestnut Breeding in the United States for Disease and Insect Resistance. Plant Dis. 2012, 96, 1392–1403. [Google Scholar] [CrossRef]
- Clark, S.L.; Schlarbaum, S.E.; Saxton, A.M.; Baird, R. Eight-year blight (Cryphonectria parasitica) resistance of backcross-generation American chestnuts (Castanea dentata) planted in the southeastern United States. For. Ecol. Manag. 2019, 433, 153–161. [Google Scholar] [CrossRef]
- Griffin, G.; Hebard, F.; Wendt, R.; Elkins, J. Survival of American Chestnut Trees: Evaluation of Blight Resistance and virulence of Endothia parasitica. Phytopathology 1983, 73, 1084–1092. [Google Scholar] [CrossRef]
- Guàrdia, M.; Charrier, G.; Vilanova, A.; Savé, R.; Ameglio, T.; Aletà, N. Genetics of frost hardiness in Juglans regia L. and relationship with growth and phenology. Tree Genet. Genomes 2016, 12, 83. [Google Scholar] [CrossRef]
- Fernández-López, F.; Díaz-Vázquez, R.; Vázquez-Ruiz, R.; Pereira-Lorenzo, S. Evaluation of resistance of Castanea sp. clones to Phytophthora sp. using excised chestnut shoots. For. Snow Landsc. Res. 2001, 76, 451–454. [Google Scholar]
- Rodríguez, J.; Colinas, C. Resistance test for chestnut against Cryphonectria (Endothia) parasitica. In Proceedings of the 2nd International Symposium on Chestnut, Bordeaux, France, 19–23 October 1998; p. 494. [Google Scholar]
- R Rcore Team. R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Anderson, D. Model Based Inference in the Life Sciences: A Primer on Evidence; Springer: New York, NY, USA, 2008. [Google Scholar]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c), R Package Version 2.2-1; 2019. Available online: https://rdrr.io/cran/AICcmodavg/ (accessed on 11 October 2021).
- Lee, J.K.; Tattar, T.A.; Berman, P.M.; Mount, M.S. A Rapid Method for Testing the Virulence of Cryphonectria parasitica Using Excised Bark and Wood of American Chestnut. Phytopathology 1992, 82, 1454–1456. [Google Scholar] [CrossRef]
- Dennert, F.; Rigling, D.; Meyer, J.B.; Schefer, C.; Augustiny, E.; Prospero, S. Testing the Pathogenic Potential of Cryphonectria parasitica and Related Species on Three Common European Fagaceae. Front. For. Glob. Chang. 2020, 3, 52. [Google Scholar] [CrossRef]
- Peever, T.L.; Liu, Y.C.; Cortesi, P.; Milgroom, M.G. Variation in tolerance and virulence in the chestnut blight fungus-hypovirus interaction. Appl. Environ. Microbiol. 2000, 66, 4863–4869. [Google Scholar] [CrossRef] [Green Version]
- Colinas González, C.; Rojo Sanz, M.; Argemí Relat, J.; Heras Dolander, J.; Castaño Soler, C.; Rotllan Puig, X.; Gómez Gallego, M.; Gilarte Cayuela, S.; Ustrell Juan, E.; Sarr Torras, H. El control biológico del chancro del castaño en Cataluña. In Proceedings of the 5th Congreso Forestal Español, Montes y Sociedad: Saber qué Hacer, Avila, Spain, 21–25 September 2009. [Google Scholar]
- González, M.; Cuenca, B.; López, M.; Prado, M.; Rey, M. Molecular characterization of chestnut plants selected for putative resistance to Phytophtora cinnamomi using SSR markers. Sci. Hortic. 2011, 130, 459–467. [Google Scholar] [CrossRef]
- Cuenca-Valera, B.; González-González, M.; López-Rodríguez, M.; Ferradás-Rial, Y.; González- Simón, L.; Rey-Fraile, M. Hablan japonés los castaños gallegos? Introgresión genética en el castaño del país. In Proceedings of the 6th Congreso Forestal Español, Montes: Servicios y Desarrollo Rural, Vitoria-Gasteiz, Spain, 10–14 June 2013. [Google Scholar]
- Fernández-López, J.; Fernández-Cruz, J.; Míguez-Soto, B. The demographic history of Castanea sativa Mill. in southwest Europe: A natural population structure modified by translocations. Mol. Ecol. 2021, 30, 3930–3947. [Google Scholar] [CrossRef]
- Mattioni, C.; Martin, M.A.; Chiocchini, F.; Cherubini, M.; Gaudet, M.; Pollegioni, P.; Velichkov, I.; Jarman, R.; Chambers, F.M.; Paule, L.; et al. Landscape genetics structure of European sweet chestnut (Castanea sativa Mill): Indications for conservation priorities. Tree Genet. Genomes 2017, 13, 39. [Google Scholar] [CrossRef]

| Species | Assay | Genotype/Progeny | N Genotypes | n | Cutting Diameter (cm) | G (mm/Day) |
|---|---|---|---|---|---|---|
| Castanea mollissima | A/B | M2 | 1 | 8/4 | 0.84 ± 0.06/0.79 ± 0.14 | 3.9 ± 1.2/5.8 ± 2.5 |
| M5M | 1 | 8/4 | 1.14 ± 0.05/0.60 ± 0.06 | 4.6 ± 1.4/4.6 ± 2.7 | ||
| Castanea sativa | A | BRL02 | 1 | 8 | 1.41 ± 0.08 | 5.6 ± 1.2 |
| A | BRL03 | 1 | 8 | 0.90 ± 0.03 | 4.4 ± 1.7 | |
| A | BRL06 | 1 | 8 | 0.98 ± 0.13 | 4.9 ± 1.4 | |
| A | CNV01 | 1 | 8 | 0.91 ± 0.07 | 5.8 ± 1.2 | |
| A | FGM01 | 1 | 8 | 1.21 ± 0.08 | 5.4 ± 1.5 | |
| A | FGM04 | 1 | 8 | 1.43 ± 0.15 | 5.4 ± 1.8 | |
| A | FGM11 | 1 | 8 | 0.93 ± 0.10 | 6 ± 1.2 | |
| A | MSY03 | 1 | 8 | 0.95 ± 0.06 | 5.8 ± 1.7 | |
| A | MSY04 | 1 | 8 | 0.89 ± 0.09 | 5.4 ± 0.9 | |
| A | MSY08 | 1 | 8 | 0.91 ± 0.04 | 5.6 ± 2.2 | |
| A | MSY09 | 1 | 8 | 0.93 ± 0.08 | 5.3 ± 1.8 | |
| A | MSY10 | 1 | 8 | 1.06 ± 0.08 | 3.9 ± 1.6 | |
| A | MSY11 | 1 | 8 | 1.08 ± 0.04 | 5.5 ± 0.7 | |
| A | MSY12 | 1 | 8 | 1.16 ± 0.38 | 5.9 ± 0.8 | |
| A | MSY14 | 1 | 8 | 1.24 ± 0.12 | 4.3 ± 2.0 | |
| A | PO11 | 1 | 8 | 1.13 ± 0.13 | 4.5 ± 1.3 | |
| A | RLL05 | 1 | 8 | 1.03 ± 0.12 | 4.5 ± 1.1 | |
| A | RLL10 | 1 | 8 | 0.96 ± 0.20 | 6.4 ± 1.1 | |
| A | SPV05 | 1 | 8 | 1.35 ± 0.06 | 5.9 ± 1.8 | |
| A | VLD12 | 1 | 8 | 1.01 ± 0.08 | 5.7 ± 1.1 | |
| A | VLD14 | 1 | 8 | 1.23 ± 0.10 | 5.4 ± 1.3 | |
| A | VLD22 | 1 | 8 | 1.14 ± 0.05 | 5 ± 0.9 | |
| A | VLD29 | 1 | 8 | 1.11 ± 0.04 | 5.4 ± 1.0 | |
| A | VLD30 | 1 | 8 | 1.19 ± 0.10 | 4.4 ± 1.7 | |
| A | VLD31 | 1 | 8 | 1.00 ± 0.11 | 6 ± 1.5 | |
| A | VLD32 | 1 | 8 | 1.02 ± 0.04 | 5.3 ± 1.6 | |
| A | VLD33 | 1 | 8 | 1.11 ± 0.06 | 6.1 ± 1.3 | |
| A | VLD34 | 1 | 8 | 1.21 ± 0.11 | 5.5 ± 1.9 | |
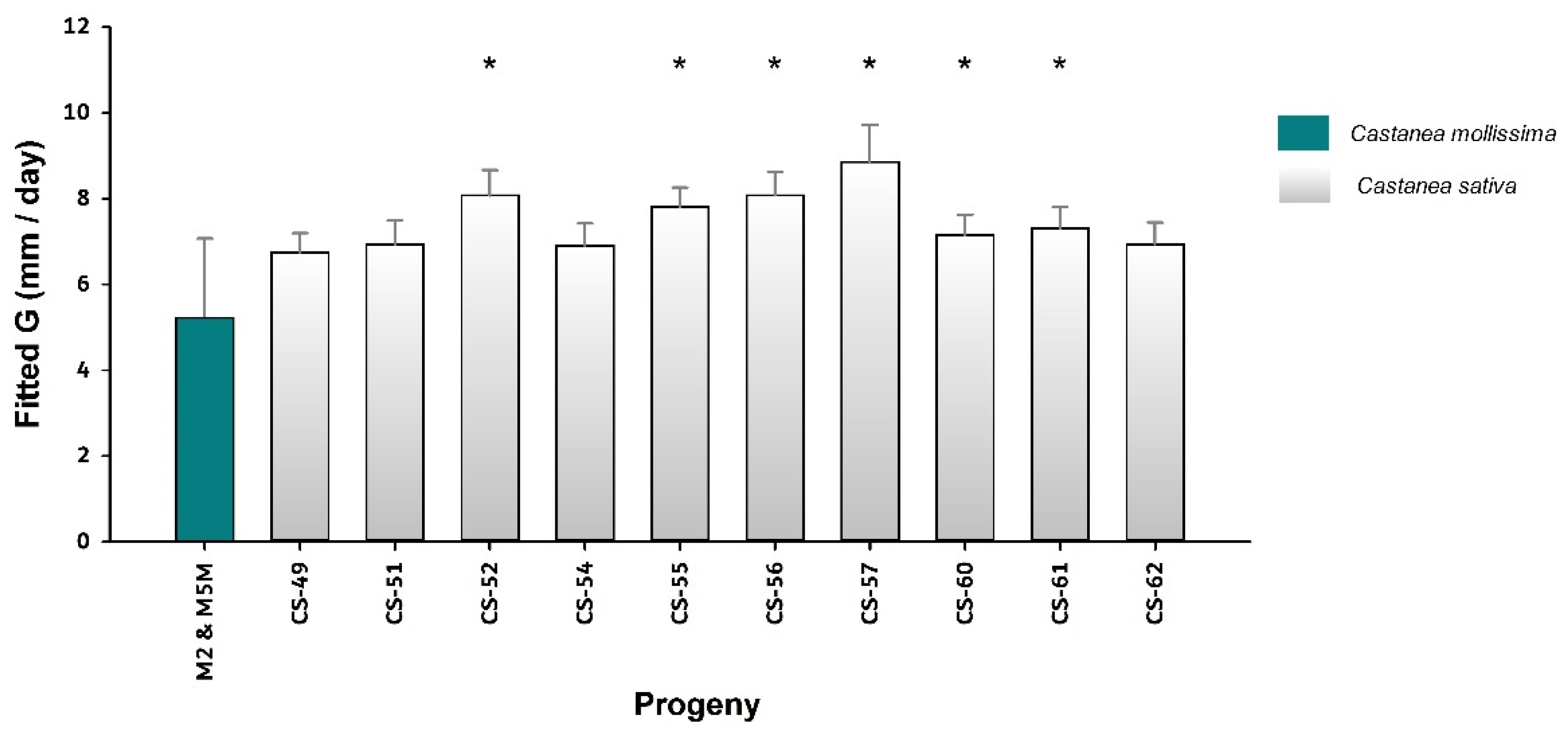
| B | CS-49 | 23 | 4 | 0.71 ± 0.13 | 6.7 ± 1.7 | |
| B | CS-51 | 14 | 4 | 0.69 ± 0.11 | 6.9 ± 1.8 | |
| B | CS-52 | 12 | 4 | 0.69 ± 0.11 | 8 ± 1.4 | |
| B | CS-54 | 17 | 4 | 0.71 ± 0.09 | 6.9 ± 1.5 | |
| B | CS-55 | 24 | 4 | 0.69 ± 0.11 | 7.7 ± 2.1 | |
| B | CS-56 | 14 | 4 | 0.69 ± 0.12 | 8.1 ± 2 | |
| B | CS-57 | 5 | 4 | 0.72 ± 0.14 | 8.8 ± 1.3 | |
| B | CS-60 | 20 | 4 | 0.68 ± 0.11 | 7.1 ± 2.1 | |
| B | CS-61 | 18 | 4 | 0.66 ± 0.12 | 7.2 ± 1.8 | |
| B | CS-62 | 18 | 4 | 0.69 ± 0.09 | 6.9 ± 2 |
| Assay | Model | n | Description | Df | logLik | Deviance | AIC | ΔAIC |
|---|---|---|---|---|---|---|---|---|
| A | Ma0 | 232 | G~genotype | 30 | −403.73 | 807.46 | 867.46 | 0 |
| Ma1 | G~genotype + (d) | 31 | −403.73 | 807.46 | 869.46 | 2 | ||
| B | Mbp0 | 664 | G~progeny | 12 | −1355 | 2710 | 2734 | 0 |
| Mbp1 | G~progeny + (d) | 13 | −1351.80 | 2703.70 | 2729.70 | −4.30 | ||
| Mbg0 | G~seedling-genotype | 167 | −1161.40 | 2322.70 | 2656.70 | 0 | ||
| Mbg1 | G~seedling-genotype + (d) | 168 | −1161.40 | 2322.70 | 2658.70 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Adalia, E.J.; Meijer, A.; Abel, J.; Colinas, C.; Aletà, N.; Guàrdia, M. Evaluation of Chestnut Susceptibility to Cryphonectria parasitica: Screening under Controlled Conditions. Agriculture 2021, 11, 1158. https://doi.org/10.3390/agriculture11111158
Muñoz-Adalia EJ, Meijer A, Abel J, Colinas C, Aletà N, Guàrdia M. Evaluation of Chestnut Susceptibility to Cryphonectria parasitica: Screening under Controlled Conditions. Agriculture. 2021; 11(11):1158. https://doi.org/10.3390/agriculture11111158
Chicago/Turabian StyleMuñoz-Adalia, Emigdio Jordán, Andreu Meijer, Joan Abel, Carlos Colinas, Neus Aletà, and Mercè Guàrdia. 2021. "Evaluation of Chestnut Susceptibility to Cryphonectria parasitica: Screening under Controlled Conditions" Agriculture 11, no. 11: 1158. https://doi.org/10.3390/agriculture11111158
APA StyleMuñoz-Adalia, E. J., Meijer, A., Abel, J., Colinas, C., Aletà, N., & Guàrdia, M. (2021). Evaluation of Chestnut Susceptibility to Cryphonectria parasitica: Screening under Controlled Conditions. Agriculture, 11(11), 1158. https://doi.org/10.3390/agriculture11111158






