Coordinated Effect of Ascorbate Biosynthesis and Recycling in Maize Seed Germination and Seedling Establishment under Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Germination Assay
2.2. Measurement of Chlorophyll Content
2.3. Quantification of H2O2 Content
2.4. Quantification of Superoxide Content
2.5. Measurement of Nonenzymatic Antioxidants Content
2.6. Measurement of Activities of Antioxidant Enzymes
2.7. RNA Extraction and Expression Analysis
2.8. Statistical Methods
3. Results
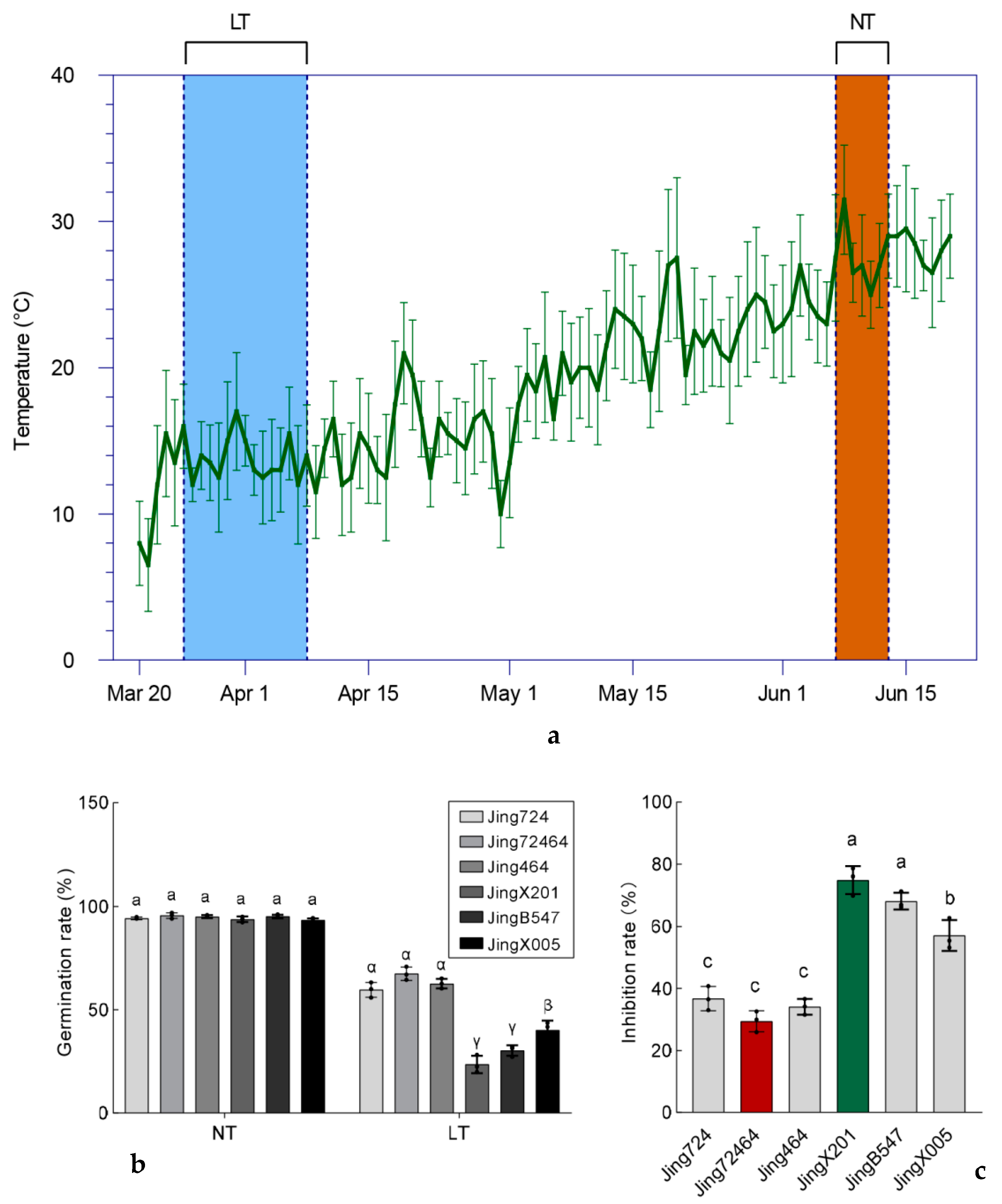
3.1. Germination Rate of Different Elite Maize Inbred Accessions under Low Temperature
3.2. Variation in Seedling Establishment among Maize Accessions under Low Temperature
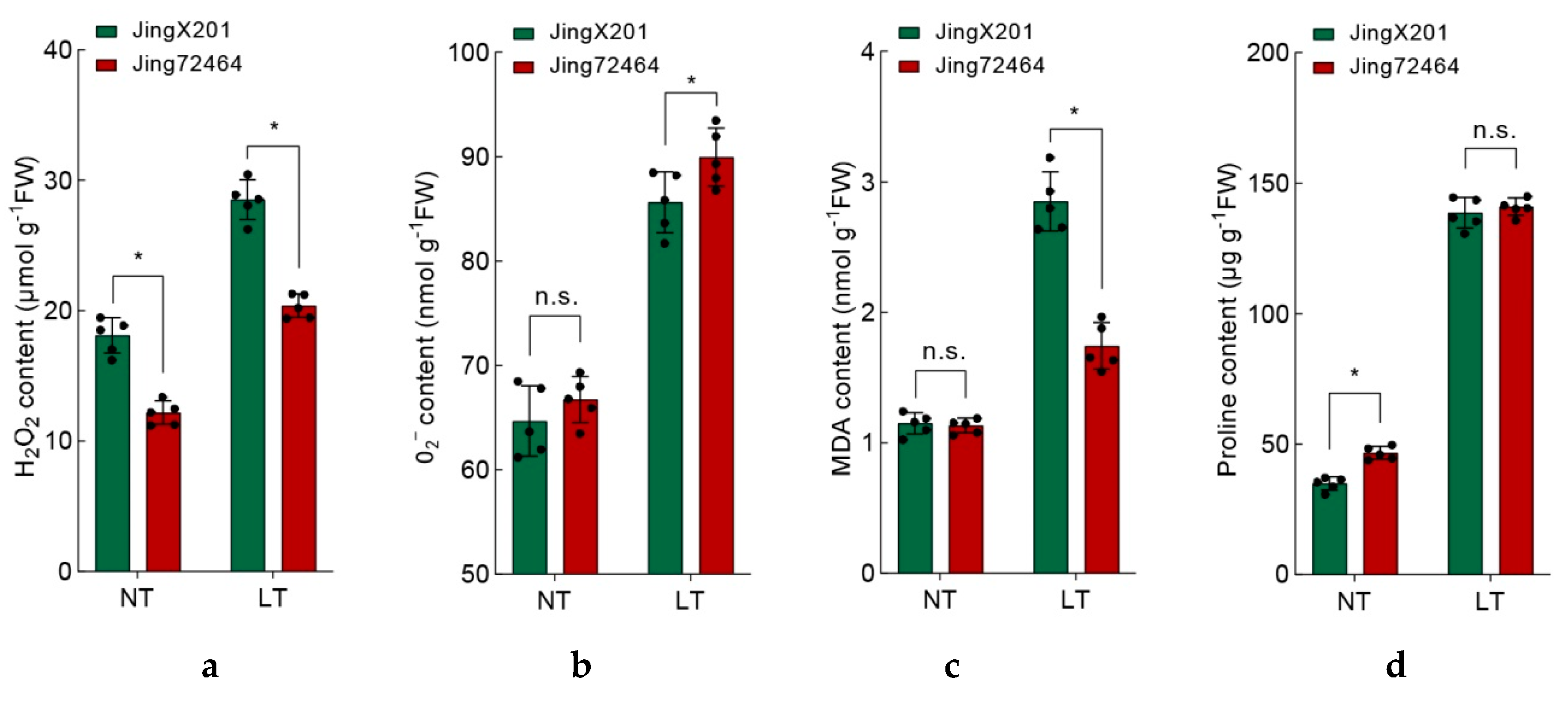
3.3. Ameliorated Oxidative Stress in LT-Tolerant Accession
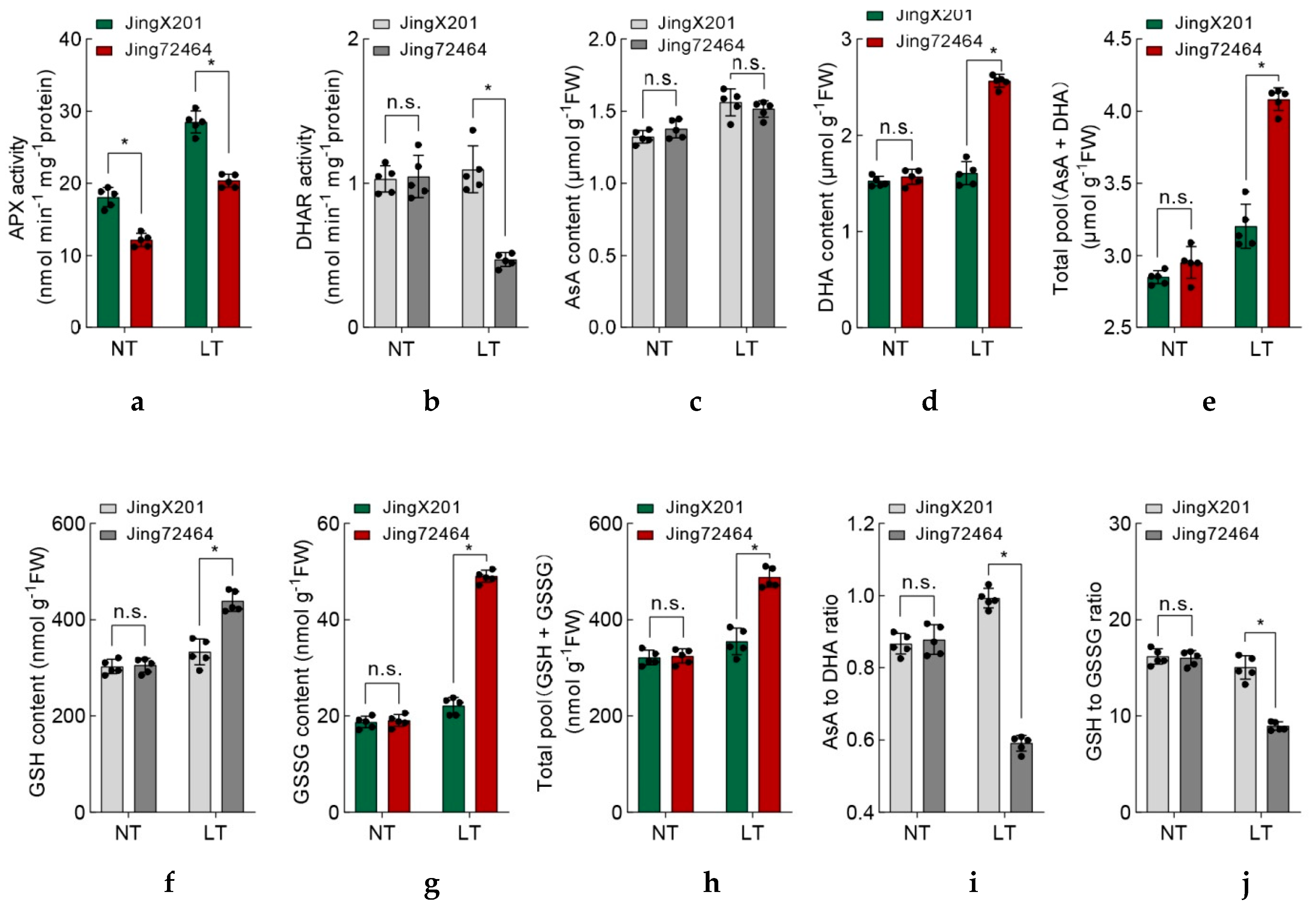
3.4. Coordinated Antioxidant Defense Systems in LT-Tolerant Accession
3.5. Transcriptional Profile Analysis of AsA-GSH Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- yyGreaves, J.A. Improving suboptimal temperature tolerance in maize—the search for variation. J. Exp. Bot. 1996, 47, 307–323. [Google Scholar] [CrossRef]
- Xue, X.; Du, S.; Jiao, F.; Xi, M.; Wang, A.; Xu, H.; Jiao, Q.; Zhang, X.; Jiang, H.; Chen, J.; et al. The regulatory network behind maize seed germination: Effects of temperature, water, phytohormones, and nutrients. Crop. J. 2021, 9, 718–724. [Google Scholar] [CrossRef]
- Verheul, M.J.; Picatto, C.; Stamp, P. Growth and development of maize (Zea mays L.) seedlings under chilling conditions in the field. Eur. J. Agron. 1996, 5, 31–43. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Fu, J.; Li, L.; Jia, G.; Ren, L.; Lubberstedt, T.; Wang, G.; Wang, J.; Gu, R. QTL Mapping in Three Connected Populations Reveals a Set of Consensus Genomic Regions for Low Temperature Germination Ability in Zea mays L. Front. Plant Sci. 2018, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, A.; Dietz, K.J. Reactive Oxygen Species and the Redox-Regulatory Network in Cold Stress Acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H. Redox homeostasis: Opening up ascorbate transport. Nat. Plants 2015, 1, 14012. [Google Scholar] [CrossRef]
- Debolt, S.; Melino, V.; Ford, C.M. Ascorbate as a biosynthetic precursor in plants. Ann. Bot. 2007, 99, 3–8. [Google Scholar] [CrossRef]
- Cheng, F.; Lu, J.; Gao, M.; Shi, K.; Kong, Q.; Huang, Y.; Bie, Z. Redox Signaling and CBF-Responsive Pathway are Involved in Salicylic Acid-Improved Photosynthesis and Growth under Chilling Stress in Watermelon. Front. Plant Sci. 2016, 7, 1519. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S.; et al. Effects of Melatonin on Anti-oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Yin, H.; Fan, Z.; Li, X.; Ni, S.; He, L.; Li, J. Overexpression of CaAPX Induces Orchestrated Reactive Oxygen Scavenging and Enhances Cold and Heat Tolerances in Tobacco. BioMed Res. Int. 2017, 2017, 4049534. [Google Scholar]
- Kwon, S.Y.; Choi, S.M.; Ahn, Y.O.; Lee, H.S.; Lee, H.B.; Park, Y.M.; Kwak, S.S. Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J. Plant Physiol. 2003, 160, 347–353. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Constructing a Standard Curve for Real-Time Polymerase Chain Reaction (PCR) Experiments. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095026. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, W.; Luo, Y.; Gao, S.; Zhao, J. The HuangZaoSi Maize Genome Provides Insights into Genomic Variation and Improvement History of Maize. Mol. Plant 2019, 12, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raju, S.K.K.; Barnes, A.C.; Schnable, J.C.; Roston, R.L. Low-temperature tolerance in land plants: Are transcript and membrane responses conserved? Plant Sci. Int. J. Exp. Plant Biol. 2018, 276, 73–86. [Google Scholar]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Maruta, T.; Yoshimura, K.; Smirnoff, N. Biosynthesis and Regulation of Ascorbic Acid in Plants. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 163–179. [Google Scholar]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Broad, R.C.; Bonneau, J.P.; Hellens, R.P.; Johnson, A.A.T. Manipulation of Ascorbate Biosynthetic, Recycling, and Regulatory Pathways for Improved Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 1790. [Google Scholar] [CrossRef] [Green Version]
- Eastmond, P.J. MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell 2007, 19, 1376–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Tamaoki, M.; Shikano, T.; Nakajima, N.; Ogawa, D.; Ioki, M.; Aono, M.; Kubo, A.; Kamada, H.; Inoue, Y.; et al. Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, S.; Qazi, H.A.; Dar, Z.A.; John, R. Low temperature elicits differential biochemical and antioxidant responses in maize (Zea mays) genotypes with different susceptibility to low temperature stress. Physiol. Mol. Biol. Plants 2021, 27, 1395–1412. [Google Scholar] [CrossRef]
- Yoshimura, K.; Nakane, T.; Kume, S.; Shiomi, Y.; Maruta, T.; Ishikawa, T.; Shigeoka, S. Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Biosci. Biotechnol. Biochem. 2014, 78, 60–66. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Xu, T.; Wang, Y.; Xing, J.; Wang, R.; Su, A.; Wang, S.; Song, W.; Zhao, J. Coordinated Effect of Ascorbate Biosynthesis and Recycling in Maize Seed Germination and Seedling Establishment under Low Temperature. Agriculture 2021, 11, 1160. https://doi.org/10.3390/agriculture11111160
Xiao S, Xu T, Wang Y, Xing J, Wang R, Su A, Wang S, Song W, Zhao J. Coordinated Effect of Ascorbate Biosynthesis and Recycling in Maize Seed Germination and Seedling Establishment under Low Temperature. Agriculture. 2021; 11(11):1160. https://doi.org/10.3390/agriculture11111160
Chicago/Turabian StyleXiao, Senlin, Tianjun Xu, Yuandong Wang, Jinfeng Xing, Ronghuan Wang, Aiguo Su, Shuaishuai Wang, Wei Song, and Jiuran Zhao. 2021. "Coordinated Effect of Ascorbate Biosynthesis and Recycling in Maize Seed Germination and Seedling Establishment under Low Temperature" Agriculture 11, no. 11: 1160. https://doi.org/10.3390/agriculture11111160
APA StyleXiao, S., Xu, T., Wang, Y., Xing, J., Wang, R., Su, A., Wang, S., Song, W., & Zhao, J. (2021). Coordinated Effect of Ascorbate Biosynthesis and Recycling in Maize Seed Germination and Seedling Establishment under Low Temperature. Agriculture, 11(11), 1160. https://doi.org/10.3390/agriculture11111160




