Variability of Fruit Quality among 103 Acerola (Malpighia emarginata D. C.) Phenotypes from the Subtropical Region of Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Fruit Size, Weight, Color Parameters and Pulp Yield
2.3. Biochemical Parameters: Soluble Solids, pH, Titratable Acidity, Vitamin C and Polyphenols
2.4. Statistical Data Analysis
3. Results and Discussion
3.1. Morphological Characteristics of Acerola Fruits
3.2. Chemical Characteristics of Acerola Fruits
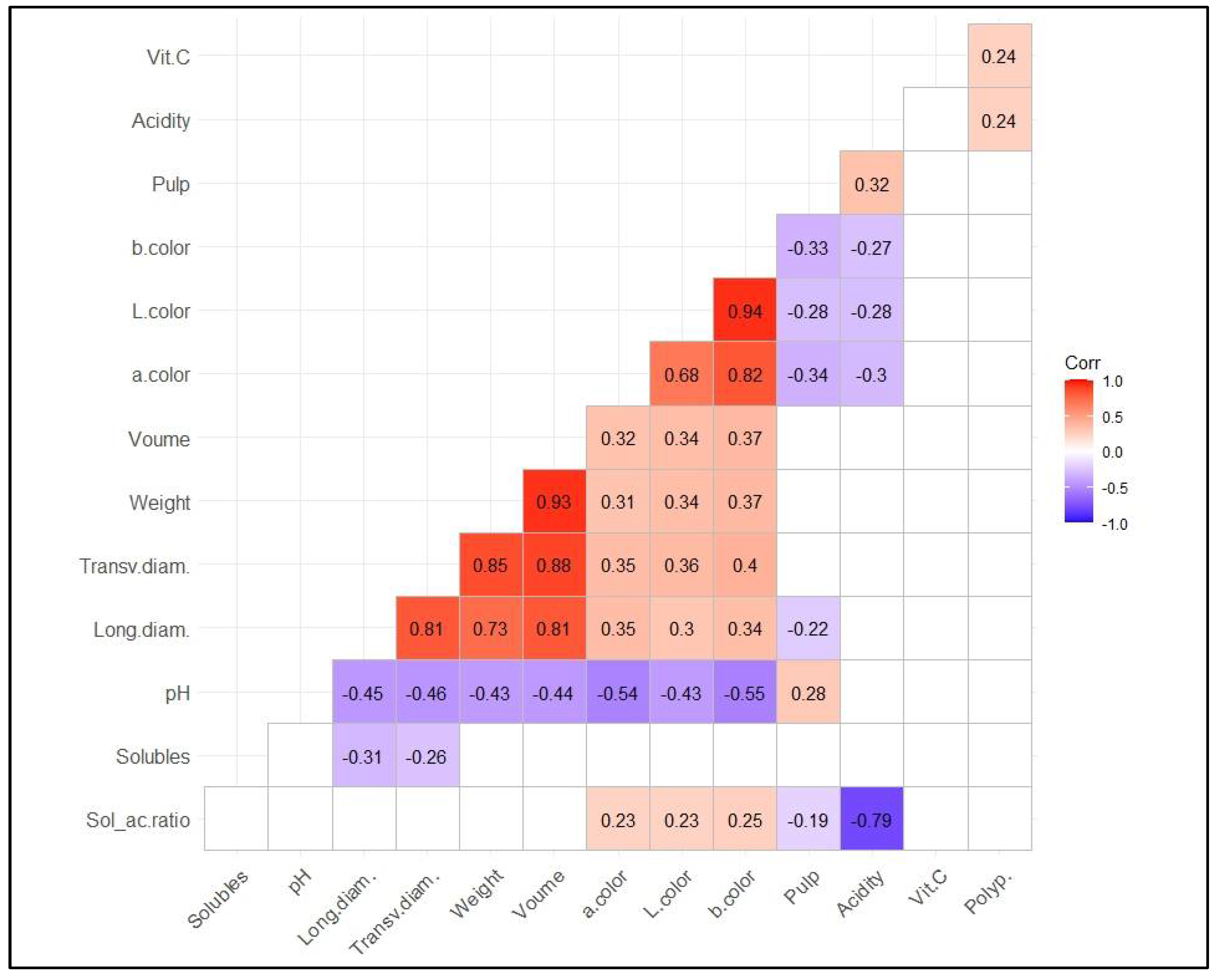
3.3. Correlations among Variables
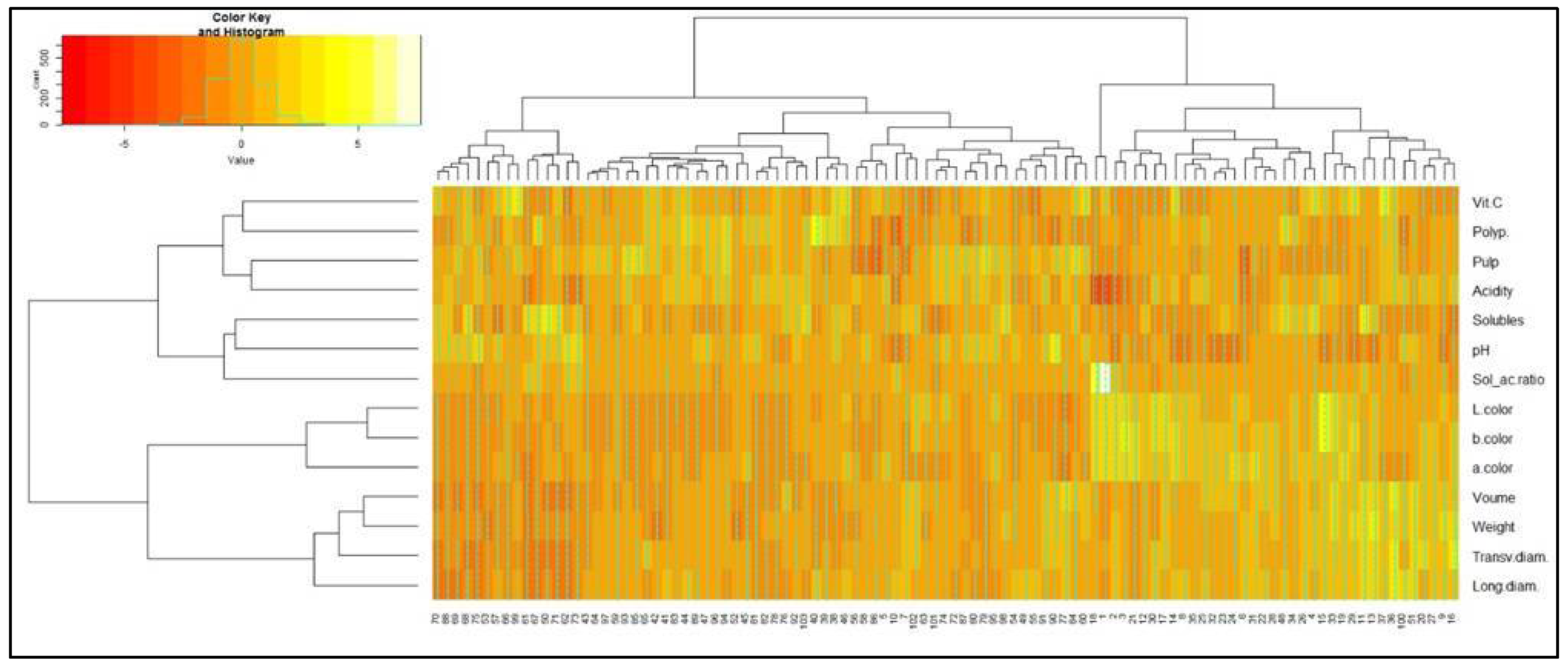
3.4. Classification of Acerola Phenotypes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Association of Official Agricultural Chemists. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Ritzinger, R.; Ritzinger, C.H.S.P.R. Acerola. Addit. Inf. 2011, 32, 17–25. [Google Scholar]
- Mezadri, T.; Villaño, D.; Fernández-Pachón, M.S.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant compounds and antioxidant activity in acerola (Malpighia emarginata D. C.) fruits and derivatives. J. Food Compost. Anal. 2008, 21, 282–290. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R. Acerola, an untapped functional superfruit: A review on latest frontiers. J. Food Sci. Technol. 2018, 55, 3373–3384. [Google Scholar] [CrossRef]
- Rekha, C.; Poornima, G.; Manasa, M.; Abhipsa, V.; Devi, J.P.; Kumar, H.T.V.; Kekuda, T.R.P. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem. Sci. Trans. 2012, 1, 303–310. [Google Scholar] [CrossRef]
- Ancos, B.; Fernández-Jalao, I.; Sánchez-Moreno, C. Compuestos funcionales en productos de Iv YV Gama. Rev. Iber. Tec. Post. 2016, 17, 130–148. [Google Scholar]
- Assis, S.A.; Fernandes, F.P.; Martins, A.B.G.; Oliveira, O.M.M.F. Acerola: Importance, culture conditions, production and biochemical aspects. Rev. Fruits 2008, 63, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Shen, C.; Zheng, H.; Xu, Y.; Xue, C.; Zhu, B.; Hu, J. Metabolomic analysis of acerola cherry (Malpighia emarginata D. C.) fruit during ripening development via UPLC-Q-TOF and contribution to the antioxidant activity. Food Res. Intern. 2020, 130, e108915. [Google Scholar] [CrossRef]
- Segtowick, E.C.D.S.; Brunelli, L.T.; Venturini Filho, W.G. Avaliação físico-química e sensorial de fermentado de acerola. Brazilian. J. Food Technol. 2013, 16, 147–154. [Google Scholar]
- Reis, E.F.; Pinto, J.F.N.; Assunção, H.F.; Silva, D.F.P. Diversidade genética de frutos de macaúba provenientes de 35 municípios de Goiás. Pesqui Agropecu Bras. 2017, 52, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Belwal, T.; Devkota, H.P.; Hassan, H.A.; Ahluwalia, S.; Ramadan, M.F.; Mocan, A.; Atanasov, A.G. Phytopharmacology of acerola (Malpighia spp.) and its potential as functional food. Trends Food Sci. Technol. 2018, 74, 99–106. [Google Scholar] [CrossRef]
- Gomes, J.E.; Perecin, D.; Martins, A.B.G.; Almeida, E.J.D. Variabilidade fenotípica em genótipos de acerola. Pesqui Agropecu Bras. 2000, 35, 2205–2221. [Google Scholar] [CrossRef] [Green Version]
- Semensato, L.R.; Pereira, A.S. Características de frutos de genótipos de aceroleira cultivados sob elevada altitude. Pesqui Agropecu Bras. 2000, 35, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Chitarra, M.I.F.; Chitarra, A.B. Pós-Colheita de Frutas E Hortaliças: Fisiologia e Manuseio, 3rd ed.; Editora UFLA: Lavras, Brazil, 2005; p. 785. [Google Scholar]
- Hanamura, T.; Uchida, E.; Aoki, H. Changes of the composition in acerola (Malpighia emarginata D. C.) fruit in relation to cultivar, growing region and maturity. J. Sci. Food Agric. 2008, 88, 1813–1820. [Google Scholar] [CrossRef]
- Maciel, M.I.S.; Mélo, E.; Lima, V.; Souza, K.A.; Silva, W. Caracterização físico-química de frutos de genótipos de aceroleira (Malpighia emarginata D. C.). Food Sci. Technol. 2010, 30, 865–869. [Google Scholar] [CrossRef] [Green Version]
- Mariano-Nasser, F.D.C.; Nasser, M.D.; Furlaneto, K.A.; Ramos, J.A.; Vieites, R.L.; Pagliarini, M.K. Bioactive compounds in different acerola fruit cultivares. Semina Ciências Agrárias 2017, 38, 2505–2514. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.S.; Freitas, S.T. Maturity stage at harvest and storage temperature to maintain postharvest quality of acerola fruit. Sci. Hortic. 2020, 260, e108901. [Google Scholar] [CrossRef]
- Santos, S.M.; Vasconcelos, A.M.; Oliveira, V.S.; Clemente, E.; Costa, J.M. Evaluation of physical and physicochemical characteristics of Malpighia emarginata D. C. from the state of Ceará. Int. J. Biochem. Res. Rev. 2012, 2, 152–163. [Google Scholar] [CrossRef]
- Ferreira, R.M.A.; Aroucha, E.M.M.; Souza, P.A.; Queiroz, R.F. Ponto de colheita da acerola visando à produção industrial de polpa. Rev. Verde Agro Des. Sust. 2009, 4, 5. [Google Scholar]
- Figueiredo Neto, A.; Reis, D.; Alves, E.; Gonçalves, E.; Anjos, F.D.; Ferreira, M. Determinação de vitamina e avaliação físico-química em três variedades de acerola cultivadas em Petrolina-PE. Nucleus 2014, 11, 83–92. [Google Scholar] [CrossRef]
- Furlaneto, F.P.B.; Nasser, M.D. Panorama da cultura da acerola no estado de São Paulo. Pesq. Tecn. 2015, 12, 1–6. [Google Scholar]
- Cavalcante, I.H.L.; Beckmann, M.Z.; Martins, A.B.G.; Campos, M.C.C. Preliminary selection of acerola genotypes in Brazil. Fruits 2007, 62, 27–34. [Google Scholar] [CrossRef]
- Souza, K.O.D.; Moura, C.F.H.; Brito, E.S.D.; Miranda, M.R.A.D. Antioxidant compounds and total antioxidant activity in fruits of acerola from cv. Flor Branca, Florida Sweet and BRS 366. Rev. Bras. Frut 2014, 36, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Zeitschrift 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Caviglione, J.H.; Kiihl, L.R.B.; Caramori, P.H.; Oliveira, D.; Galdino, J.; Borrozino, E.; Pugsley, L. Cartas Climáticas do Estado do Paraná; IAPAR: Londrina, Brazil, 2000. [Google Scholar]
- Ritzinger, R.; Ritzinger, C.H.S.P.; Fonseca, N.; Machado, C.F. Advances in the propagation of acerola. Rev. Bras. Frut. 2018, 40, e928. [Google Scholar] [CrossRef] [Green Version]
- Moura, C.F.H.; Alves, R.E.; Figueiredo, R.W.; Paiva, J.R. Avaliações físicas e físico-químicas de frutos de clones de aceroleira (Malpighia emarginata D. C.). Rev. Ciên Agron 2007, 38, 52–57. [Google Scholar]
- Oliveira, J.G.; Gomes Filho, A.; Pereira, M.G.; Viana, A.P.; Souza Filho, G.A.; Lopes, G.E.M. Diversidade genética de aceroleiras (Malphigia emarginata D. C.), utilizando marcadores moleculares RAPD e características morfoagronômicas. Rev. Bras. Frut 2009, 31, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Godoy, R.C.B.; Matos, E.L.S.; Amorim, T.D.S.; Sousa Neto, M.A.; Ritzinger, R.; Waszczynskyj, N. Avaliação de genótipos e variedades de acerola para consumo in natura e para elaboração de doces. Bol. CEPPA 2008, 26, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Musser, R.D.S.; Lemos, M.A.; Lima, V.L.A.G.D.; Mélo, E.D.A.; Lederman, I.E.; Santos, V.F.D. Características físico-químicas de acerola do banco ativo de germoplasma em Pernambuco. J. Food Sci. Technol. 2004, 24, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Gonzaga Neto, L.; Mattuz, B.; Santos, C.A.F. Caracterização agronômica de clones de aceroleira (Malpighia spp.) na Região do Submédio São Francisco. Rev. Bras. Frut 1999, 21, 110–115. [Google Scholar]
- Estevam, M.I.F.; Souza, P.A.; Maracajá, P.B.; Batista, E.M.; Reges, B.M. Physicochemical of acerola varieties in two stages of maturation. Rev. Verde Agro Des. Sust. 2018, 13, 459–465. [Google Scholar] [CrossRef]
- Freitas, R.V.S.; Souza, P.A.; Coelho, E.L.; Silva, M.S.; Batista, E.M.; Ferreira, E.D.O. Caracterização Físico-Química de Variedades De Acerolas Em Dois Estádios De Maturação. In Proceedings of the VII Congresso Norte Nordeste de Pesquisa e Inovação. Palmas—Tocantins (CONNEPI); 2012. Available online: https://pdfs.semanticscholar.org/9a4a/502022e7fc307e16a10133dff12a52dcce1a.pdf (accessed on 28 October 2021).
- Carpentieri-Pípolo, V.; Destro, D.; Prete, C.E.C.; Gonzales, M.G.N.; Popper, I.; Zanatta, S.; Silva, A. Seleção de genótipos parentais de acerola com base na divergência genética multivariada. Pesqui Agropecu Bras. 2000, 35, 1613–1619. [Google Scholar] [CrossRef]
- Moura, N.F.; Chaves, L.J.; Naves, R.V. Caracterização física de frutos de pequizeiro (Caryocar brasiliense Camb.) do cerrado. Rev. Árv. 2013, 37, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Almeida Júnior, E.B.; Chaves, L.J.; Soares, T.N. Genetic characterization of a germplasm collection of cagaiteira, a native species of the cerrado. Bragantia 2014, 73, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.A.; Machado, J.L.; Resende, J.C.; Oliveira, A.L.; Figueiredo, U.J.; Carvalho, G.R.; Ferrão, M.A.G.; Guimarães, R.J. Adaptability, stability, and genetic divergence of conilon coffee in Alto Suaçuí, Minas Gerais, Brazil. Crop. Breed. Appl. Biotechnol. 2017, 17, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Shimono, H.; Farquhar, G.; Brookhouse, M.; Busch, F.A.; O’Grady, A.; Tausz, M.; Pinkard, E.A. Prescreening in large populations as a tool for identifying elevated CO2-responsive genotypes in plants. Funct. Plant. Biol. 2018, 46, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bianco, R.L.; Panno, G.; Avellone, G. Characterization of Sicilian olive genotypes by multivariate analysis of leaf and fruit chemical and morphological properties. J. Agric. Sci. 2013, 5, 211–229. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, C.H.G.; Céron-Souza, I.; Arango, L.V. Agronomic evaluation of a Colombian passion fruit (Passiflora edulis Sims) germplasm collection. Agron 2018, 16, 1649–1659. [Google Scholar]
- Akça, Y.; Yuldaşulu, Y.B.; Murad, E.; Vahdati, K. Exploring of walnut genetic resources in Kazakhstan and evaluation of promising selections. Int. J. Hortic. Sci. Technol. 2020, 7, 93–102. [Google Scholar]
- Magalhães, D.S.; Rufini, J.C.M.; Albuquerque, A.S.; Viol, R.E.; Fagundes, M.C.P.; Menezes, T.P. Genetic diversity among accessions of acerola based on the quality of fruits. Comun. Scient 2018, 9, 133–141. [Google Scholar] [CrossRef]
- Brunini, M.A.; Macedo, N.B.; Coelho, C.V.; Siqueira, G.F.D. Caracterização física e química de acerolas provenientes de diferentes regiões de cultivo. Rev. Bras. Frut. 2004, 10, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, C.V.; Gouveia, A.M.S.; Martins, B.N.M.; Jorge, L.G.; Lanna, N.B.L.; Tavares, A.E.B.; Mendonça, V.Z.; Evangelista, R.M. Influence of ripening stages on physicochemical characteristics of acerola fruits. Rev. Ciênc. Agr. 2017, 40, 808–813. [Google Scholar] [CrossRef]
- Commission Internationale de l’Eclairage. Official Recommendations of the International Commission on Illumination; Publication CIE: Vienna, Austria, 1986. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Viúda-Martos, M.; Ruiz-Navajas, Y.; Zaldivar-Cruz, J.M.; Kuri, V.; Fernández-López, J.; Carbonell-Barrachina, A.A.; Pérez-Álvarez, J.A. Aroma profile and physico-chemical properties of artisanal honey from Tabasco Mexico. Int. J. Food Sci. 2010, 45, 1111–1118. [Google Scholar] [CrossRef]
- Mondragón-Cortez, P.; Ulloa, J.A.; Rosas-Ulloa, P.; Rodríguez-Rodríguez, R.; Vázquez, J.A.R. Physicochemical characterization of honey from the West region of México. CyTA J. Food 2013, 11, 7–13. [Google Scholar] [CrossRef]
- Ali, M.M.; Woods, M.; Caravan, P.; Opina, A.C.; Spiller, M.; Fettinger, J.C.; Sherry, A.D. Synthesis and relaxometric studies of a dendrimer-based pH-responsive MRI contrast agent. Chem. Eur. J. 2008, 14, 7250–7258. [Google Scholar] [CrossRef] [Green Version]
- Instituto Adolfo Lutz. Normas Analíticas do Instituto Adolfo Lutz. Métodos Químicos E Físicos Para Análise De Alimentos, 2nd ed.; IMESP: São Paulo, Brazil, 1985. [Google Scholar]
- Benassi, M.T.; Antunes, A.J. A comparison of metaphosphoric and oxalic acids as extractants solutions for the determination of Vitamin C in selected vegetables. Arq. Biol. Tecn. 1988, 31, 507–513. [Google Scholar]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Streit, M.; Gehlenborg, N. Points of view: Bar charts and box plots. Nat. Methods 2014, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Wise, B.M.; Gallagher, N.B. The process chemometrics approach to process monitoring and fault detection. J. Process. Control. 1996, 6, 329–348. [Google Scholar] [CrossRef]
- Granato, D.; Katayama, F.C.U.; Castro, I.A. Assessing the association between phenolic compounds and the antioxidant activity of Brazilian red wines using chemometrics. LBWTAP 2010, 43, 1542–1549. [Google Scholar] [CrossRef]
- Matta, L.B.D.; Tomé, L.G.O.; Salgado, C.C.; Cruz, C.D.; Silva, L.D.F. Hierarchical genetic clusters for phenotypic analysis. Acta Sci. Agron. 2015, 37, 447–456. [Google Scholar] [CrossRef]
- Portarena, S.; Leonardi, L.; Scartazza, A.; Lauteri, M.; Baldacchini, C.; Farinelli, D.; Famiani, F.; Ciolfi, M.; Brugnoli, E. Combining analysis of fatty acid composition and δ13C in extra-virgin olive oils as affected by harvest period and cultivar: Possible use in traceability studies. Food Control. 2019, 105, 151–158. [Google Scholar] [CrossRef]
- Lima, P.C.C.; Souza, B.S.; Souza, P.S.; Borges, S.D.S.; Assis, M.D.O.D. Caracterização e avaliação de frutos de aceroleira. Rev. Bras. Frut. 2014, 36, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Adriano, E.; Evangelista, R.M. Qualidade de fruto da aceroleira cv. Olivier em dois estádios de maturação. Rev. Bras. Frut. 2011, 33, 541–545. [Google Scholar] [CrossRef]
- Loápez, A.P. Relation of maturity to some fruit characters of the West Indian cherry. J. Agric. Univ. P. R. 1963, 47, 193–200. [Google Scholar]
- Alves, R.E.; Chitarra, A.B.; Chitarra, M.I.F. Postharvest physiology of acerola (Malpighia emarginata D. C.) fruits: Maturation changes, respiratory activity and refrigerated storage at ambient and modified atmospheres. Acta Hortic. 1995, 370, 223–230. [Google Scholar] [CrossRef]
- Brasil. Instrução Normativa n. 01, de 7 de janeiro de 2000. In Regulamento Técnico Geral Para Fixação Dos Padrões De Identidade E Qualidade Para Polpa De Fruta; Diário Oficial [da] República Federativa do BRASIL: Brasilia, Brazil, 2000; pp. 54–55. [Google Scholar]
- Nascimento, J.F.; Barroso, B.S.; Tostes, E.D.S.L.; Silva, A.D.S.S.; Silva Júnior, A.C.S. Análise físico-química de polpas de acerola (Malpighia glabra L.) artesanais e industriais congeladas. PubVet 2018, 12, 131. [Google Scholar] [CrossRef] [Green Version]
- Seymour, G.B.; Tucker, G.A. Avocado. In Biochemistry of Fruit Ripening; Springer: Dordrecht, The Netherlands, 1993; pp. 53–81. [Google Scholar]
- Instituto Brasileiro de Frutas. Soluções Fruta a Fruta: Acerola; IBRAF: São Paulo, Brazil, 1995. [Google Scholar]
- Nasser, M.D.; Zonta, A. Caracterização de frutos de genótipos de aceroleira em função de estádios de maturação. Tecn. Ciên. Agrop. 2014, 8, 76–78. [Google Scholar]
- Vendramini, A.L.; Trugo, L.C. Chemical composition of acerola fruit (Malpighia punicifolia L.) at three stages of maturity. Food Chem. 2000, 71, 195–198. [Google Scholar] [CrossRef]
- França, L.G.; Alves Filho, E.; Ribeiro, L.B.; Evangelista, J.S.B.; Silva, L.M.; Souza, P.A.; Aragão, F.A.S. Metabolomic profiling of acerola clones according to the ripening stage. J. Food Meas. Charact. 2020, 30, 1–9. [Google Scholar]
- Nakasone, H.Y.; Miyashita, R.K.; Yamane, G.M. Factors affecting ascorbic acid content of the acerola (Malpighia glabra L.). J. Am. Soc. Hortic. Sci. 1966, 89, 161–166. [Google Scholar]
- Oliveira, L.D.S.; Moura, C.F.H.; Brito, E.S.; Mamede, R.V.S.; Miranda, M.R.A. Antioxidant metabolism during fruit development of different acerola (Malpighia emarginata D. C.) clones. J. Agric. Food Chem. 2012, 60, 7957–7964. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The Effect of pH and Color Stability of Anthocyanin on Food Colorant. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Surakarta, Indonesia, 18–19 October 2016; Volume 193, p. 12047. [Google Scholar]
- Rotili, M.C.C.; Villa, F.; Braga, G.C.; França, D.L.B.; Rosanelli, S.; Laureth, J.C.U.; Silva, D.F. Bioactive compounds, antioxidant and physic-chemical characteristics of the dovyalis fruit. Acta Sci. Agric. 2018, 40, e35465. [Google Scholar] [CrossRef]
- Baldicchi, A.; Farinelli, D.; Micheli, M.; Di Vaio, C.; Moscatello, S.; Battistelli, A.; Walker, R.P.; Famiani, F. Analysis of seed growth, fruit growth and composition and PEPCK occurrence in apricot (Prunus armeniaca L.). Sci. Hort. 2015, 186, 38–46. [Google Scholar] [CrossRef]
- Famiani, F.; Bonghi, C.; Chen, Z.H.; Drincovich, M.F.; Farinelli, D.; Lara, M.V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R.P. Stone fruits: Growth and nitrogen and organic acid metabolism in the fruits and seeds—A review. Front. Plant. Sci. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J.; Fisher, D.R. Tests for hierarchical structure in random data sets. Syst. Biol. 1968, 17, 407–412. [Google Scholar] [CrossRef]
- Cruz, C.D.; Regazzi, A.J.; Carneiro, P.C.S. Modelos Biométricos Aplicados Ao Melhoramento Genético; UFV: Viçosa, Brazil, 2004; Volume 1, 480p. [Google Scholar]
- Bianchi, F.G.; Balbi, R.V.; Pio, R.; Bruzi, A.T.; Silva, D.F.D. Parent’s choice and genetic divergence between cambuci fruit tree accessions. Crop. Breed. Appl. Biotechnol. 2017, 17, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Mihaljevic, R.; Schön, C.C.; Utz, H.F.; Melchinger, A.E. Correlations and QTL correspondence between line per se and testcross performance for agronomic traits in four populations of European maize. Crop. Sci. 2005, 45, 114–122. [Google Scholar] [CrossRef]
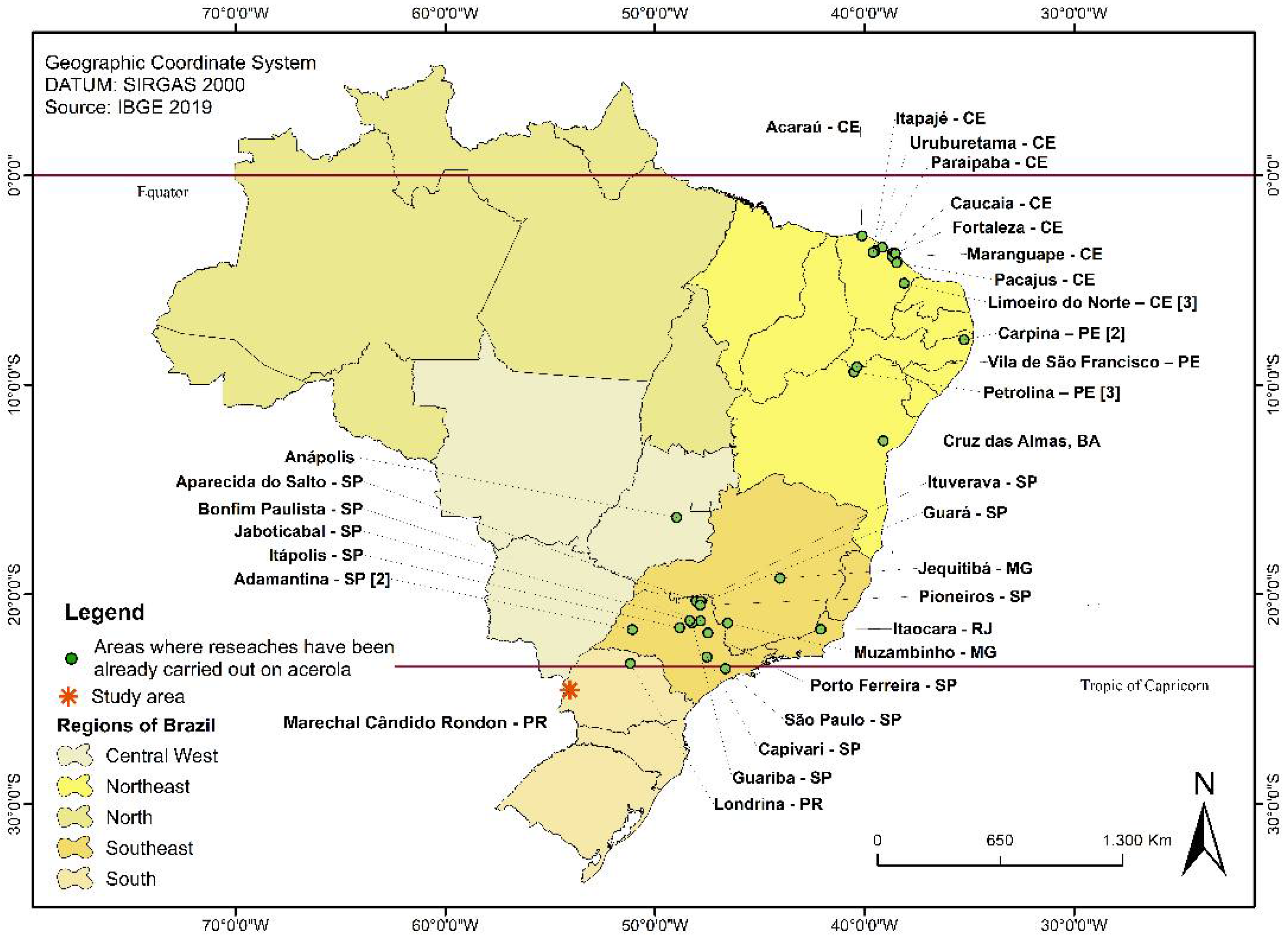
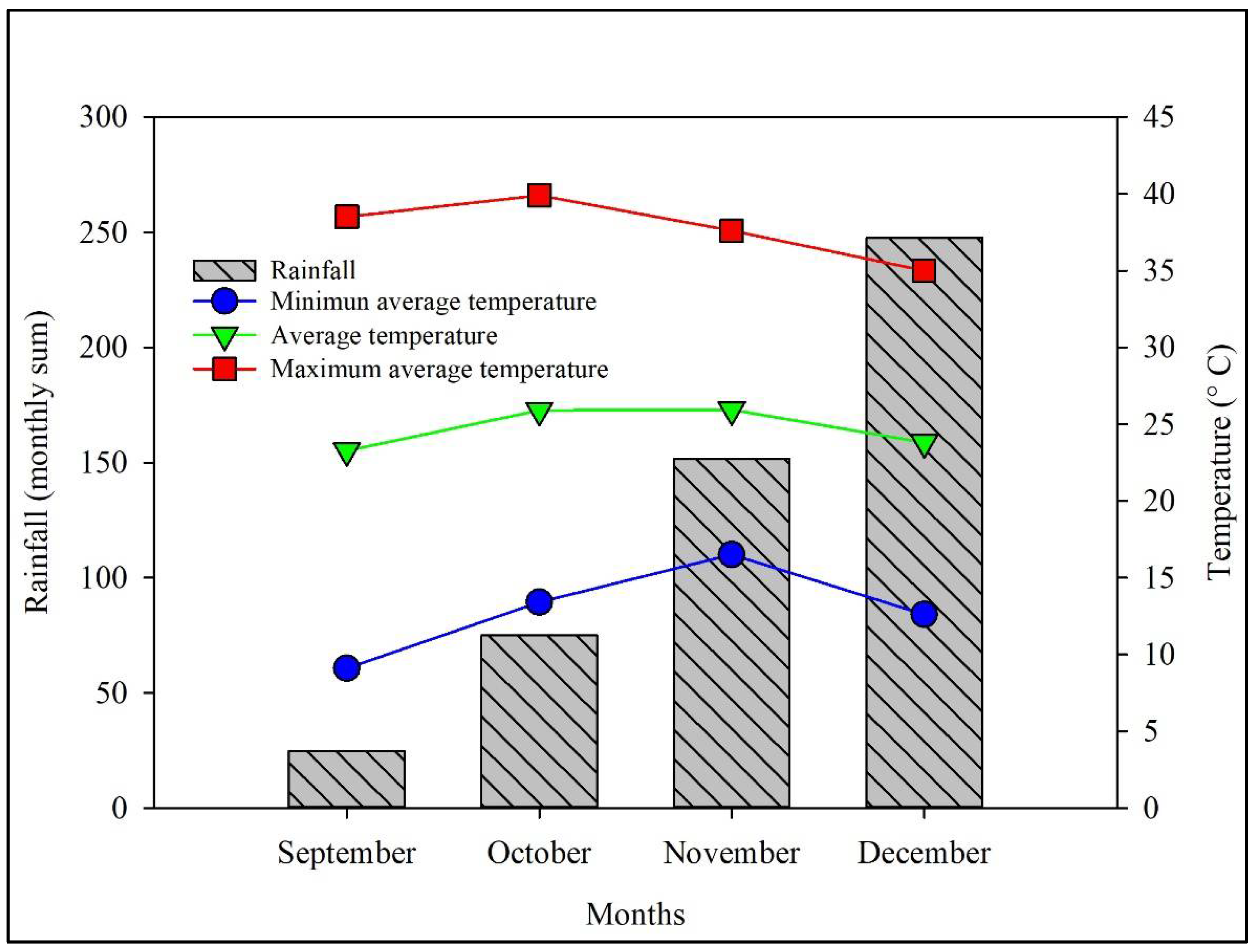


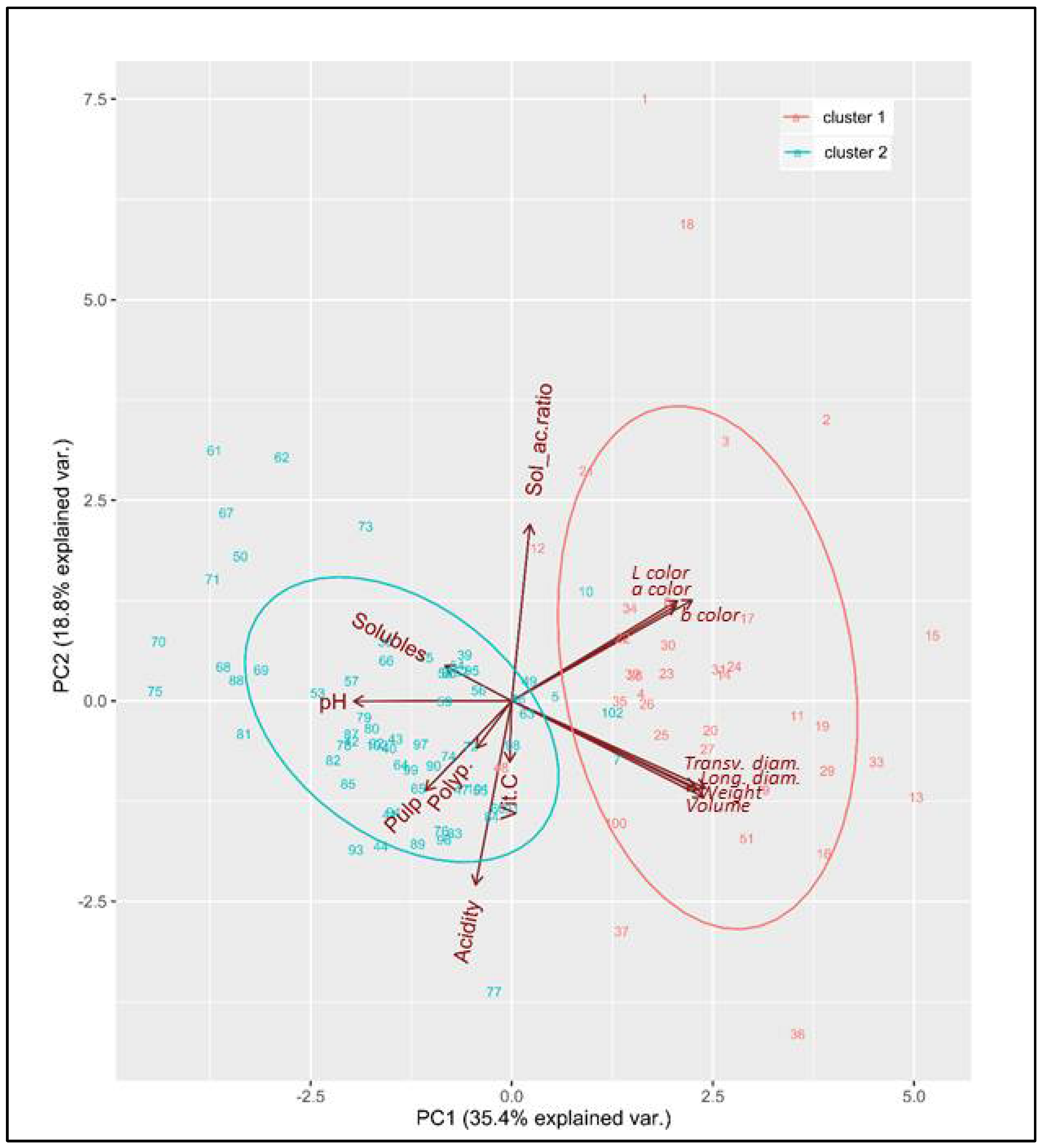
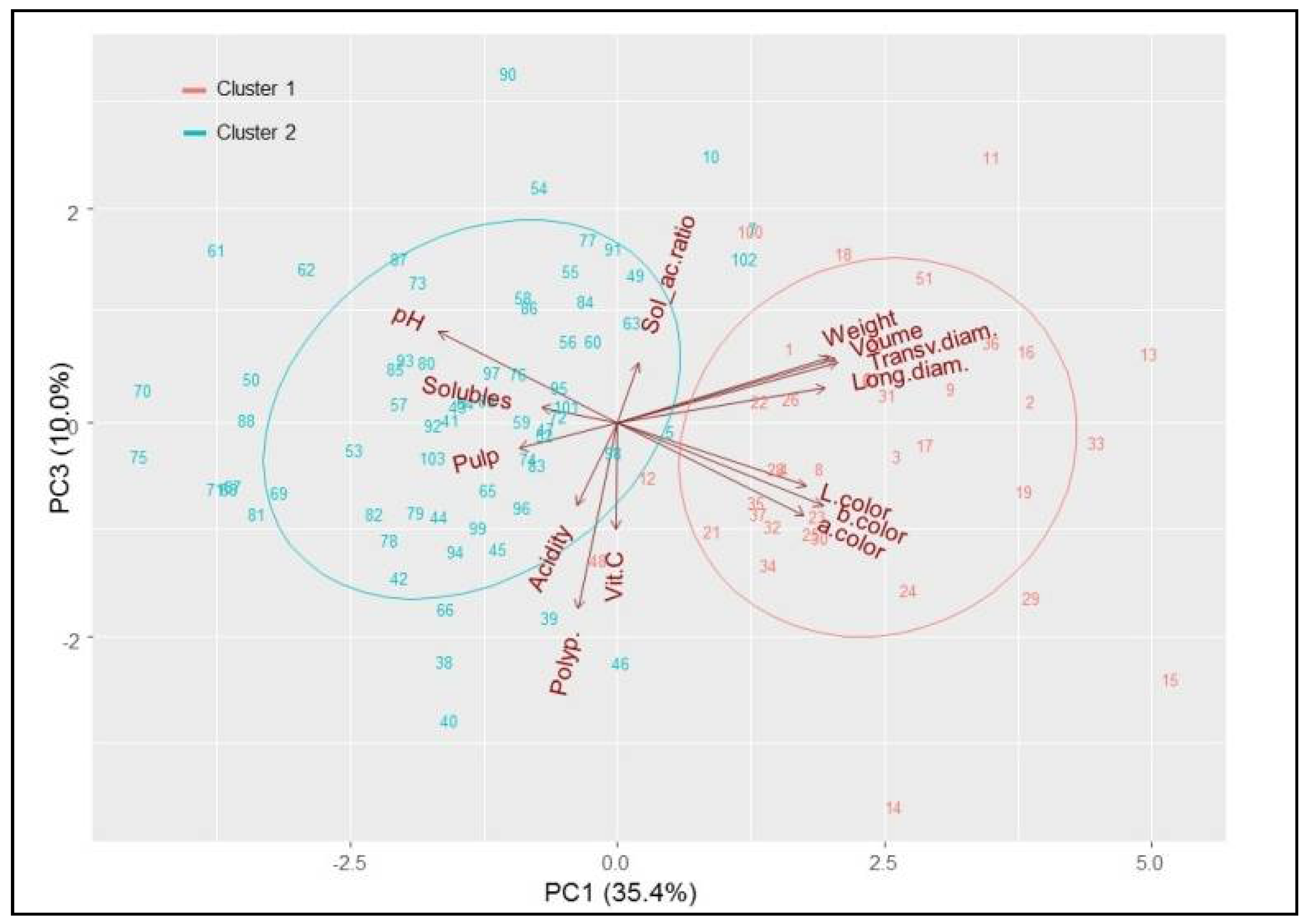

| Location | Geographic Coordinates | Cultivar | Vitamin C Content (mg/100 g Pulp) | Soluble Solids Content (° Brix) (SSC) | Titratable Acidity (%) (TA) | SSC/ TA Ratio | pH | Total Polyphenols (mg/kg Gallic Acid) | Source |
|---|---|---|---|---|---|---|---|---|---|
| Anápolis, Goiás Sate | 1000 m a.s.l. | 9 genotypes | 348–1503 | 5.4–8.27 | 0.68–1.68 | 0.40–0.95 | 2.34–3.15 | [12] | |
| Cruz das Almas, Bahia State | CMF 017, Rubra and Cabocla | 911–1192 | 7.88–8.84 | 0.83–1.35 | 5.84–10.71 | 3.29–3.60 | [30] | ||
| Carpina, Pernambuco State | 7°51′04″ S, 35°14′27″ W, 178 m a.s.l. | 12 genotypes | 1057–2032 | 6.2–10.3 | 1.09–1.89 | 4.4–6.4 | 2.98–3.37 | [31] | |
| Petrolina, Pernambuco State | 42 genotypes | 779–2444 | 5.3–9.2 | 0.79–1.90 | 3.11–3.70 | [32] | |||
| Petrolina, Pernambuco State | Flor Branca, | 1786–3597 | 8.43–12.7 | [20] | |||||
| Okinawa and Sertaneja | |||||||||
| São Francisco Valley, Pernambuco State | 09°09′ S, 40°22′ W, 365 m a.s.l. | Flor Branca and Junko | 2160–2770 | 7.7–8.6 | 1.52–1.88 | 3.0–8.31 | 2.97–3.75 | [17] | |
| Carpina, Pernambuco State | 7°51′04″ S, 35°14′27″ W, 178 m a.s.l. | 18 genotypes: PL26 to PL45 | 750–1678 | 6.66–11.46 | 0.96–1.97 | 3.79–7.06 | 2.9–3.5 | [15] | |
| Limoeiro do Norte, Ceará State | 5°20′ S, 38°5′ O | BV1, BV2, BV4, BV6, and C6P3 | 531–1087 | 6.32–7.48 | 0.97–1.02 | 6.49–7.63 | 3.21–3.59 | [33] | |
| Limoeiro do Norte, Ceará State | 38 clones and Sertaneja, Flor branca, Camta 40.2, Monami, Okinawa, Mineira and Barbados | 501–1855 | 5.7–11.1 | 0.53–1.52 | 4.32–11.94 | 3.31–3.91 | [27] | ||
| Limoeiro do Norte, Ceará State | BV1, BV2, BV3 and C6P3 | 732–1087 | 6.32–7.48 | 0.97–1.02 | 6.49–7.63 | 3.21–3.59 | [34] | ||
| Acaraú, Caucaia, Maranguape, Paraipaba, Uruburetama and Itapajé, Ceará State | A1 to A7 | 968–1349 | 4.7–5.3 | 0.86–0.99 | 5.33–5.74 | 3.39–3.59 | [18] | ||
| Pacajús, Ceará State | Flor Branca, BRS366 | 863–1364 | 6.63–9.46 | 0.61–1.06 | 5.98–15.42 | 3.18–3.68 | 1562–2631 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farinelli, D.; Portarena, S.; da Silva, D.F.; Traini, C.; da Silva, G.M.; da Silva, E.C.; da Veiga, J.F.; Pollegioni, P.; Villa, F. Variability of Fruit Quality among 103 Acerola (Malpighia emarginata D. C.) Phenotypes from the Subtropical Region of Brazil. Agriculture 2021, 11, 1078. https://doi.org/10.3390/agriculture11111078
Farinelli D, Portarena S, da Silva DF, Traini C, da Silva GM, da Silva EC, da Veiga JF, Pollegioni P, Villa F. Variability of Fruit Quality among 103 Acerola (Malpighia emarginata D. C.) Phenotypes from the Subtropical Region of Brazil. Agriculture. 2021; 11(11):1078. https://doi.org/10.3390/agriculture11111078
Chicago/Turabian StyleFarinelli, Daniela, Silvia Portarena, Daniel Fernandes da Silva, Chiara Traini, Giordana Menegazzo da Silva, Edvan Costa da Silva, Joice Ferreira da Veiga, Paola Pollegioni, and Fabíola Villa. 2021. "Variability of Fruit Quality among 103 Acerola (Malpighia emarginata D. C.) Phenotypes from the Subtropical Region of Brazil" Agriculture 11, no. 11: 1078. https://doi.org/10.3390/agriculture11111078
APA StyleFarinelli, D., Portarena, S., da Silva, D. F., Traini, C., da Silva, G. M., da Silva, E. C., da Veiga, J. F., Pollegioni, P., & Villa, F. (2021). Variability of Fruit Quality among 103 Acerola (Malpighia emarginata D. C.) Phenotypes from the Subtropical Region of Brazil. Agriculture, 11(11), 1078. https://doi.org/10.3390/agriculture11111078









