Priming with Humic Acid to Reverse Ageing Damage in Soybean [Glycine max (L.) Merrill.] Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Planting Material
2.2. Seed Priming
2.3. Experimental Design, Treatment and Germination Test
2.4. Experiment Design and Treatment for Greenhouse Seedling Emergence Test
2.5. Seedling Emergence in the Greenhouse
2.6. Root Characteristics
2.7. Seedling Characteristics
2.8. Electrical Conductivity
2.9. Antioxidant Enzyme Analysis
2.10. Malondialdehyde (MDA) Assay
2.11. Statistical Analysis
3. Results
3.1. Effects of Priming on Standard Germination under Laboratory Conditions
3.1.1. Germination Traits
3.1.2. Seedling Quality Traits
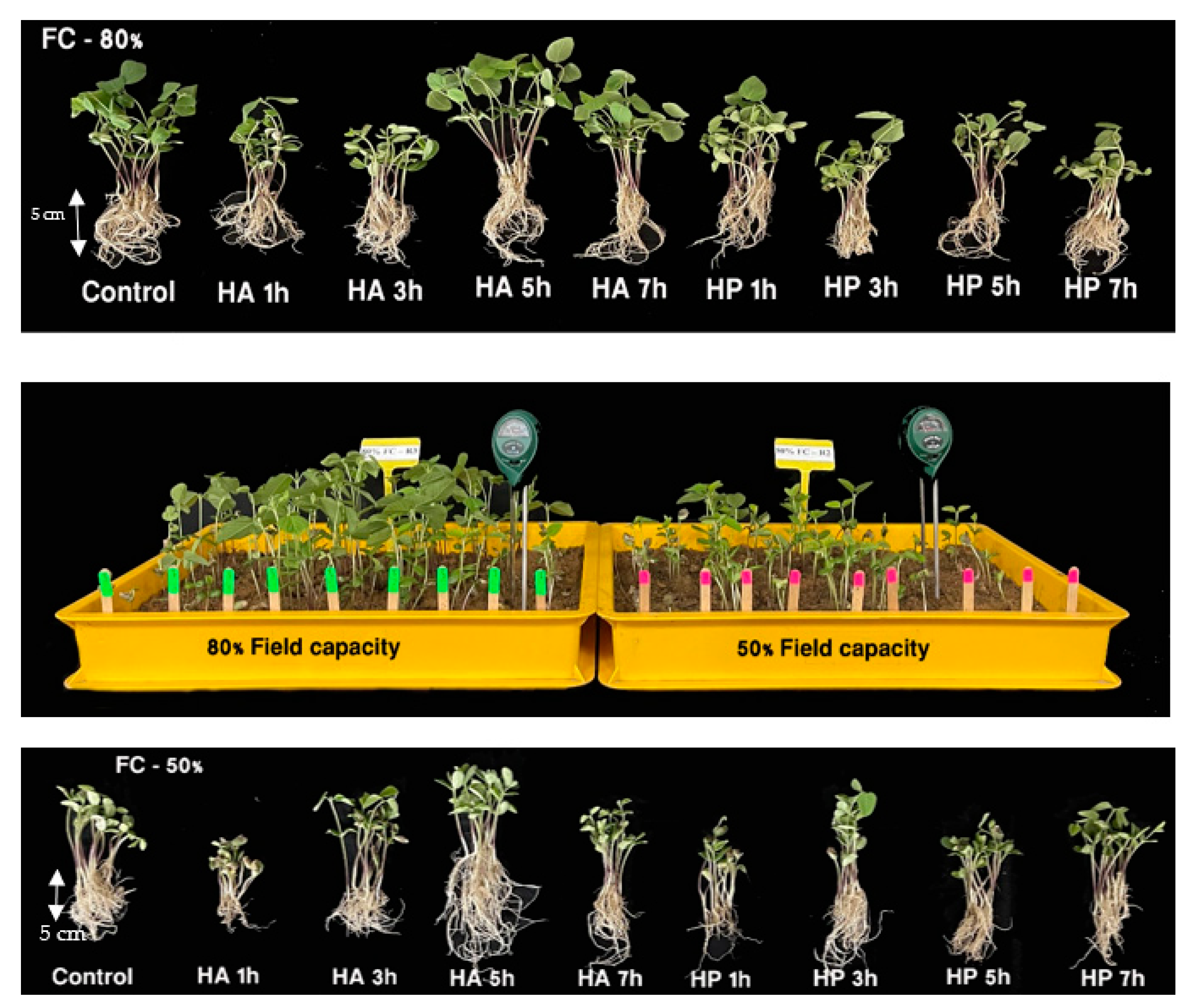
3.2. Effects of Priming and Soil Moisture Level on Seedling Emergence under Green House Conditions
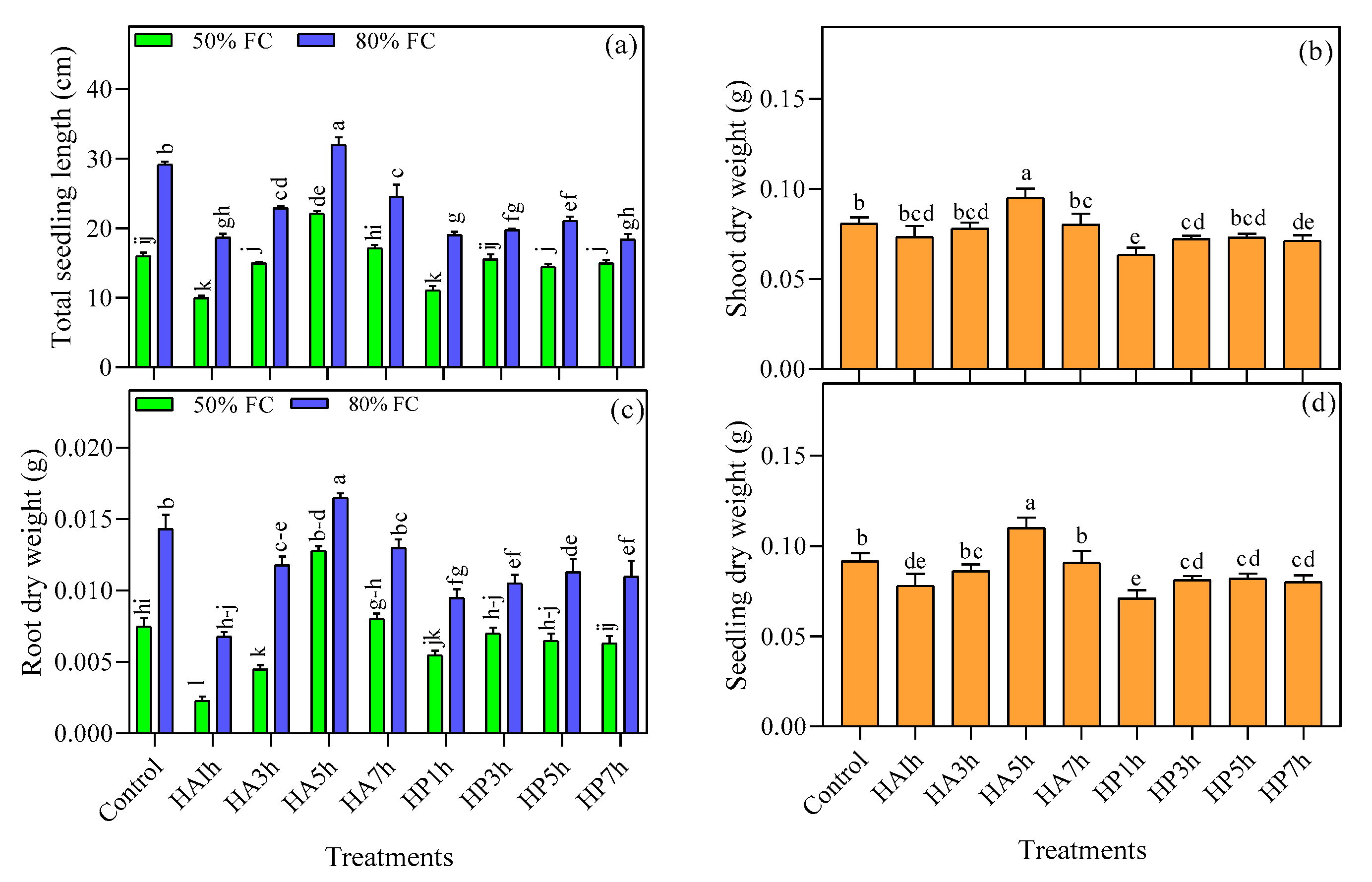
3.2.1. Seedling Emergence Traits
3.2.2. Seedling Quality Traits
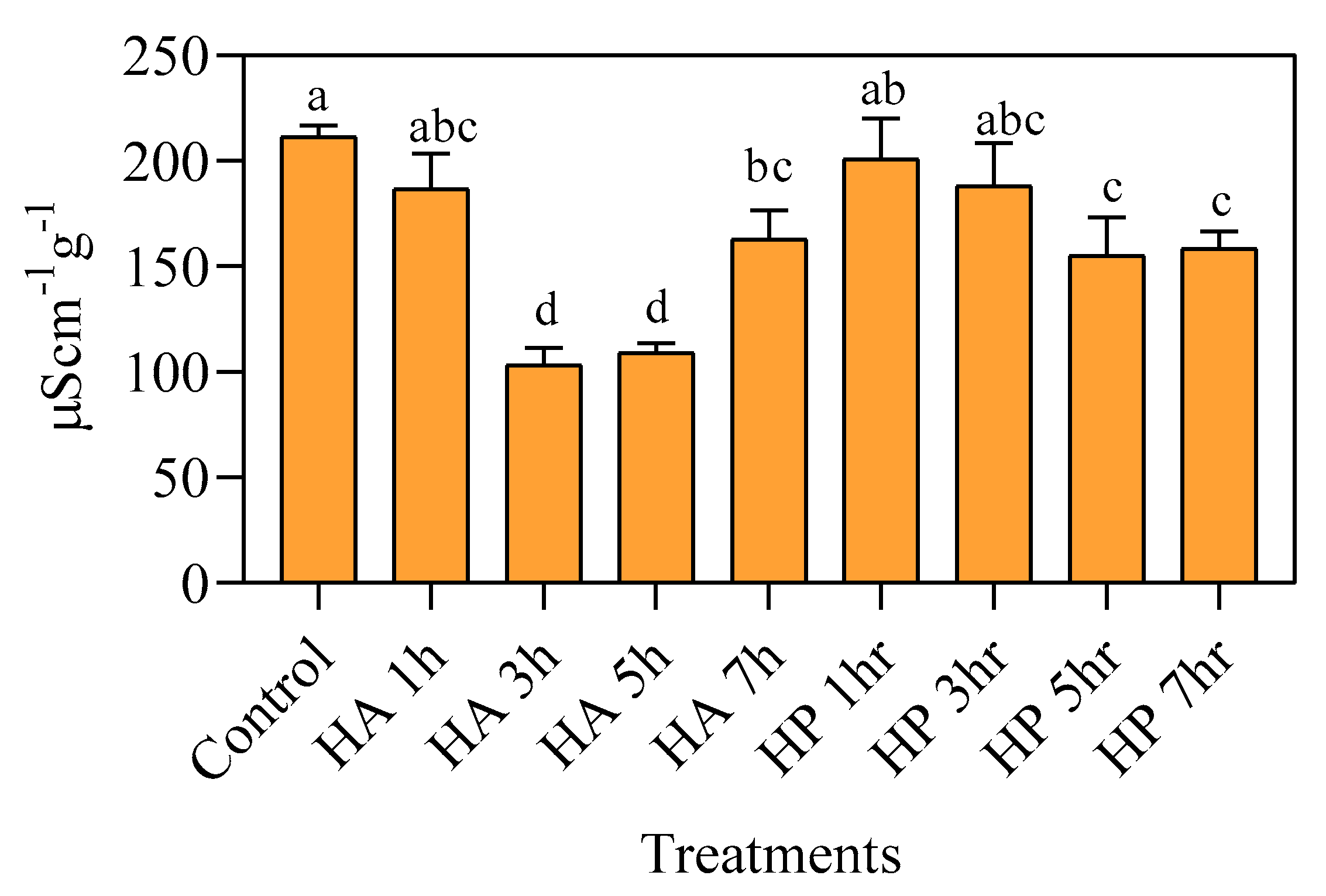
3.3. Effects of Priming on Electrical Conductivity
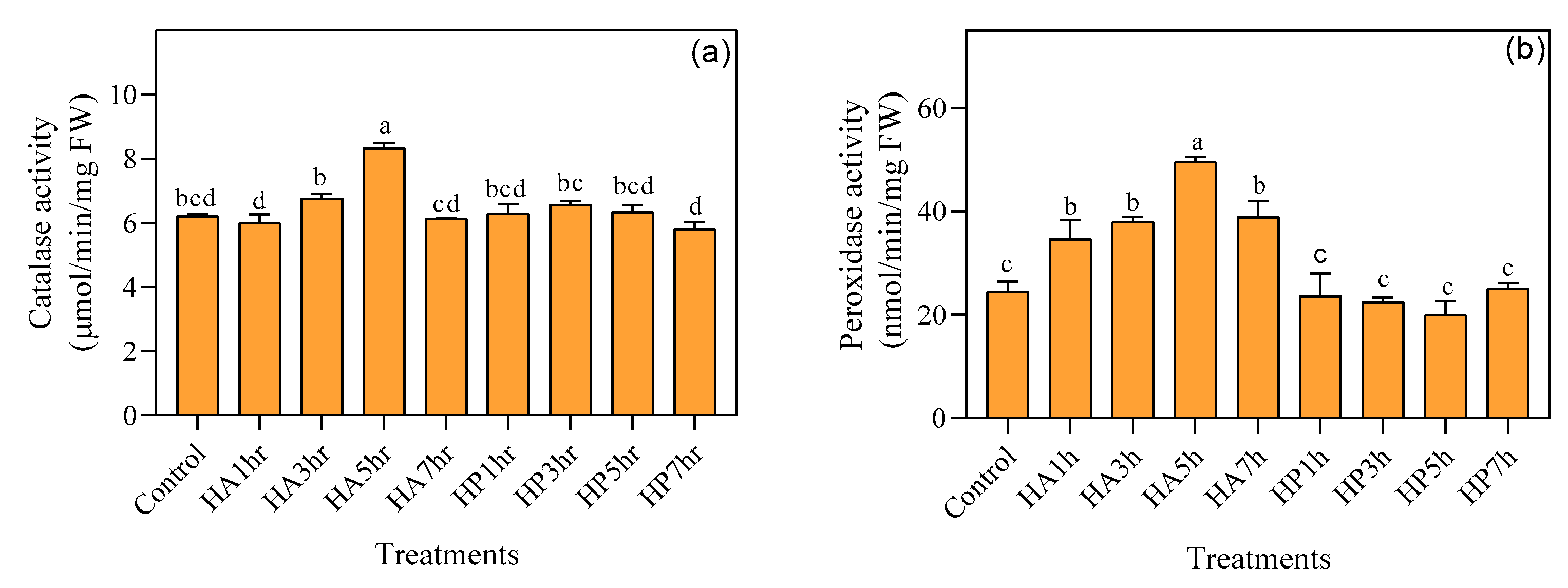
3.4. Effects of Priming on Antioxidant Enzyme Activity
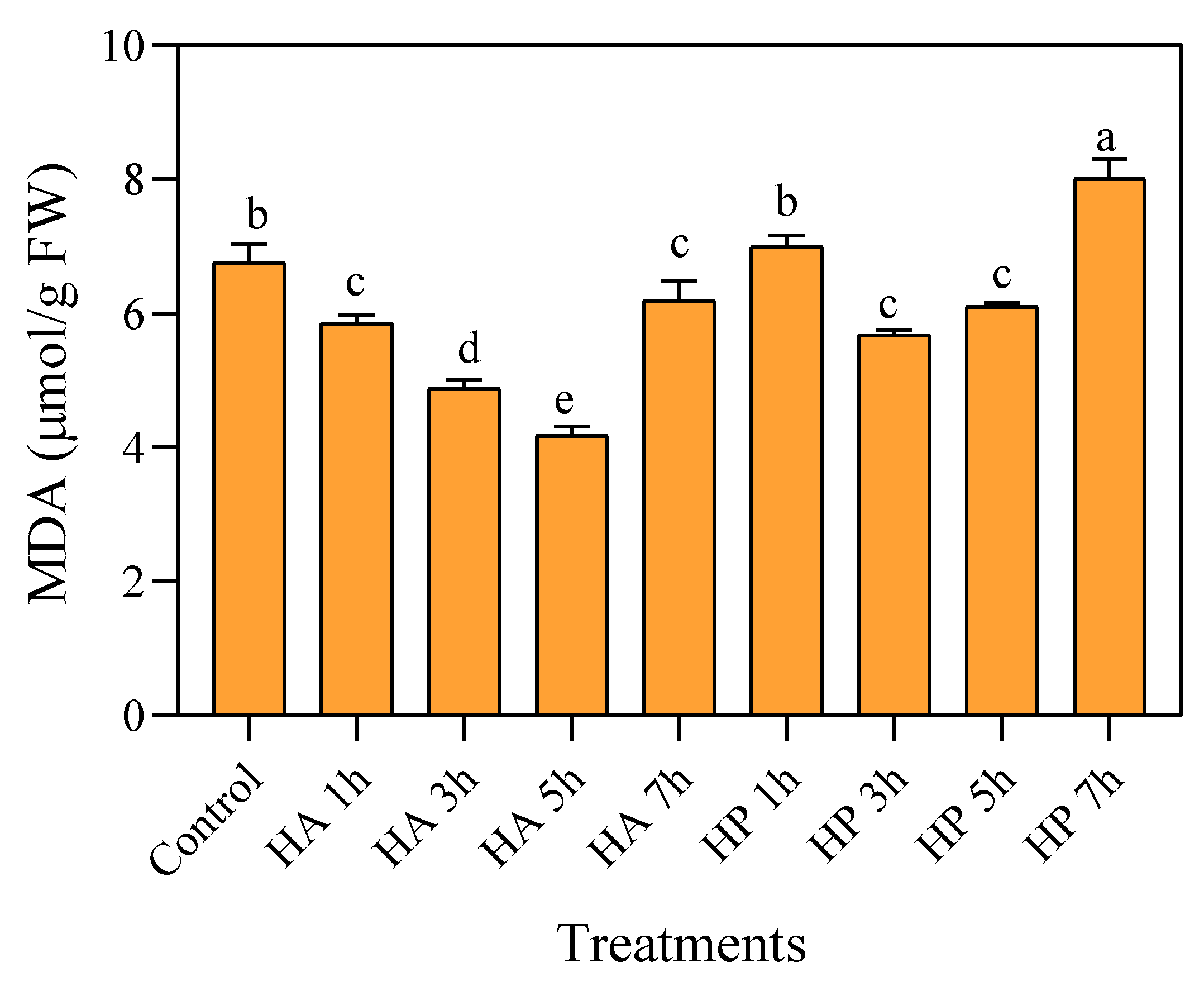
3.5. Effects of Priming on Malondialdehyde Content
3.6. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed Storage: Maintaining Seed Viability and Vigor for Restoration Use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Weerasekara, I.; Sinniah, U.R.; Namasivayam, P.; Nazli, M.H.; Abdurahman, S.A.; Ghazali, M.N. The Influence of Seed Production Environment on Seed Development and Quality of Soybean (Glycine max (L.) Merrill). Agronomy 2021, 11, 1430. [Google Scholar] [CrossRef]
- Abbasi, A.S.; Sharifzadeh, F.; Afshari, R.T. Effect of Drying Conditions and Harvest Time on Soybean Seed Viability and Deterioration under Different Storage Temperature. Afr. J. Agric. Res. 2012, 7, 5118–5127. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chowdhury, R.; Mandal, A.K. Seed Invigoration Treatments for Improved Germinability and Field Performance of Soybean [Glycine max (L.) Merill]. Indian J. Agric. Res. 2015, 49, 32–38. [Google Scholar] [CrossRef]
- Shelar, V.R.; Shaikh, R.S.; Nikam, A.S. Soybean Seed Quality during Storage: A Review. Agric. Rev. 2008, 29, 125–131. [Google Scholar]
- Biradar, R.; Jirali, D.I. Impact of the Seed Treatment, Packing Materials and Storage Temperature on Viability of Soybean. Plant Arch. 2017, 17, 1078–1084. [Google Scholar]
- Akter, N.; Haque, M.; Islam, M.; Alam, K. Seed Quality of Stored Soybean (Glycine max L.) as Influenced by Storage Containers and Storage Periods. Agriculturists 2014, 12, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Chandra, J.; Varghese, B.; Keshavkant, S. The Potential of ROS Inhibitors and Hydrated Storage in Improving the Storability of Recalcitrant Madhuca Latifolia Seeds. Seed Sci. Technol. 2019, 47, 33–45. [Google Scholar] [CrossRef]
- Ludwig, V.; Berghetti, M.R.P.; Rossato, F.P.; Wendt, L.M.; Schultz, E.E.; Both, V.; Brackmann, A. Impact of Controlled Atmosphere Storage on Physiological Quality of Soybean Seed. J. Stored Prod. Res. 2021, 90, 101749. [Google Scholar] [CrossRef]
- Komala, N.T.; Arya, B.R.B.; Sumalatha, G.M.; Gurumurthy, R.; Surendra, P. Seed Quality Enhancement Techniques. J. Pharmacogn. Phytochem. 2018, SP1, 3124–3128. [Google Scholar]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef]
- Copeland, L.O.; McDonald, M.B. Principles of Seed Science and Technology; Kluwer Academic Publishers: London, UK, 2001. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, H.M.; Zaki, A.M.; El-Bagoury, O.H.; Younis, R.A.A. Biological Changes Occured in Soybean Seed during Exposing to Several Types of Seed Priming. Arab Univ. J. Agric. Sci. 2018, 26, 1841–1856. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, H.; Khazaei, F.; Yari, L.; Sheidaei, S. Effect of Seed Osmopriming on Seed Germination Behavior and Vigor of Soybean (Glycine max L.). ARPN J. Agric. Biol. Sci. 2011, 6, 39–43. [Google Scholar] [CrossRef]
- Thant, P.S.; Puteh, A.B.; Sinniah, U.R.; Firdaus, M.; Ismail, B. Effect of Priming on Physiological and Chromosomal Changes of Aged Soybean Seeds. In Proceedings of the 6th International Conference on Chemical, Agricultural, Biological and Environmental Sciences (CAFES-17), Manila, Philippines, 18–19 September 2017; pp. 17–20. [Google Scholar]
- Nazari, R.; Parsa, S.; Tavakkol Afshari, R.; Mahmoodi, S.; Seyyedi, S.M. Salicylic Acid Priming before and after Accelerated Aging Process Increases Seedling Vigor in Aged Soybean Seed. J. Crop Improv. 2020, 34, 218–237. [Google Scholar] [CrossRef]
- Miladinov, Z.; Maksimovic, I.; Balesević-Tubic, S.; Djukic, V.; Canak, P.; Miladinovic, J.; Djordjevic, V. Priming Seed Mitigates the Effects of Saline Stress in Soybean Seedlings. Legum. Res. 2020, 43, 263–267. [Google Scholar] [CrossRef]
- Ananthi, M.; Selvaraju, P.; Sundaralingam, K. Standardization of Suitable Concentration and Duration of Seed Biopriming with Humic Acid in Chilli. Int. J. Chem. Stud. 2017, 5, 636–639. [Google Scholar]
- Souguiri, M.; Hannachi, C. Respons of Sesame Seedling to Different Eoncentration of Humic Acids or Calcium Nitrate at Germination and Early Growth. Cercet. Agron. Mold. 2017, 50, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Patil, R.B.; Mokle, S.S.; Wadje, S.S. Effect of Potassium Humate on Seed Germination, Seedling Growth and Vegetative Characters of Triticum aestivum (L.) Cv. Lokvan. Int. J. Pharma Bio Sci. 2010, 1, 148–151. [Google Scholar]
- Nazi, F.; Reza, H.; Rahman, R. Effect of Humic Fertilizer on Germination of Wheat Seeds under Drought Stress. Adv. Biores. 2014, 5, 98–102. [Google Scholar] [CrossRef]
- Gawlik, A.; Gołębiowska, D.; Bejger, R.; Kulpa, D.; Smolik, B. The Influence of Humic Acids on Seed Swelling Process of Pisum Sativum L. Cv Ramrod. Polish J. Soil Sci. 2013, 46, 107–114. [Google Scholar] [CrossRef]
- Asgharipour, M.R.; Rafiei, M. The Effect of Different Concentrations Ofhumic Acidonseed Germination Behavior and Vigor of Barley. Aust. J. Basic Appl. Sci. 2011, 5, 610–613. [Google Scholar]
- Prakash, P.; Alien, M.R.M.; Sai Nandhini, R.; Masilamani Selvam, M.; Thirugnanasambandam, R.; Stanley, A.L. Effect of Humic Acid on Seed Germination of Raphanus Sativus L. Int. J. Chem Tech Res. 2014, 6, 4180–4185. [Google Scholar]
- ISTA. International Rules for Seed Testing 2016; The International Seed Testing Association: Wallisellen, Switzerland, 2016. [Google Scholar]
- Kader, M.A.; Jutzi, S.C. Effects of Thermal and Salt Treatments during Imbibition on Germination and Seedling Growth of Sorghum at 42/19 °C. J. Agron. Crop Sci. 2004, 190, 35–38. [Google Scholar] [CrossRef]
- Al-Ansari, F.; Ksiksi, T. A Quantitative Assessment of Germination Parameters: The Case of Crotalaria Persica and Tephrosia Apolllinea. Open Ecol. J. 2016, 9, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria 1. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Schuab, S.R.P.; Braccini, A.L.; Scapim, C.A.; Franca-Neto, J.B.; Meschede, D.K.; Avila, M.R. Germination Test under Water Stress to Evaluate Soybean Seed Vigour. Seed Sci. Technol. 2007, 35, 187–199. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The Quantification of Ageing and Survival in Orthodox Seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Mangure, J.D. Speed of Germination: Aid in Selection and Evaluation for Seedling Emergence and Vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar]
- Furbeck, S.M.; Bourland, F.M.; Watson, C.E. Relationships of Seed and Germination Measurements with Resistance to Seed Weathering in Cotton. Seed Sci. Technol. 1993, 21, 505–512. [Google Scholar]
- Dutra, A.S.; Vieira, R.D. Electrical Conductivity as Vigour Test for Squash Seeds. Braz. J. Seeds 2006, 28, 117–122. [Google Scholar] [CrossRef]
- Aebi, H. Formation or Removal of Oxygem Radicals. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Maehly, C. Plant Peroxidases. Methods Enzymol. 1955, 2, 801–813. [Google Scholar]
- Stewart, R.R.C.; Bewley, J.D. Lipid Peroxidation Associated with Accelerated Aging of Soybean Axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.N.; Sani, M.N.H.; Uddain, J.; Azad, M.O.K.; Kabir, M.S.; Rahman, M.S.; Choi, K.Y.; Naznin, M.T. Stimulatory Effect of Seed Priming as Pretreatment Factors on Germination and Yield Performance of Yard Long Bean (Vigna Unguiculata). Horticulturae 2020, 6, 104. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J.T. Influence of Different Seed Priming Techniques on Oxidative and Antioxidative Responses during the Germination of Oryza Sativa Varieties. Physiol. Mol. Biol. Plants 2020, 26, 551–565. [Google Scholar] [CrossRef]
- Ali, I.; Nulit, R.; Ibrahim, M.H.; Uddin, M.K. Deterioration of Quality Soybean Seeds (Glycine max (L.) Merr. AGS 190) at Harvest Stages, Seed Moisture Content and Storage Temperature in Malaysia. Int. J. Biosci. 2017, 10, 372–381. [Google Scholar] [CrossRef]
- Bussotti, F.; Ferrini, F.; Pollastrini, M.; Fini, A. The Challenge of Mediterranean Sclerophyllous Vegetation under Climate Change: From Acclimation to Adaptation. Environ. Exp. Bot. 2014, 103, 80–98. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, J.; Johnston, C.R.; Wang, Y.; Zhu, K.; Lu, F.; Zhang, Z.; Zou, J. Seed Priming with Polyethylene Glycol Induces Physiological Changes in Sorghum (Sorghum Bicolor L. Moench) Seedlings under Suboptimal Soil Moisture Environments. PLoS ONE 2015, 10, e0140620. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.S.; Rajendra, P.; Kumar, M.; Singh, C.; AK, S.; Pathak, A. Seed Quality Markers: A Review. J. Bot. Sci. 2016, 5, 24–28. [Google Scholar]
- Arif, M.; Jan, M.T.; Marwat, K.B.; Khan, M.A. Seed Priming Improves Emergence and Yield of Soybean. Pakistan J. Bot. 2008, 40, 1169–1177. [Google Scholar]
- Ebrahimi, M.; Miri, E. Effect of Humic Acid on Seed Germination and Seedling Growth of Borago Officinalis and Cichorium Intybus. Ecopersia 2016, 4, 1239–1249. [Google Scholar] [CrossRef]
- Du, B.; Luo, H.; He, L.; Zhang, L.; Liu, Y.; Mo, Z.; Pan, S.; Tian, H.; Duan, M.; Tang, X. Rice Seed Priming with Sodium Selenate: Effects on Germination, Seedling Growth, and Biochemical Attributes. Sci. Rep. 2019, 9, 4311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Farooq, M.; Lee, D.J. Evaluating the Role of Seed Priming in Improving Drought Tolerance of Pigmented and Non-Pigmented Rice. J. Agron. Crop Sci. 2017, 203, 269–276. [Google Scholar] [CrossRef]
- Vieira, J.H.; Kledson, L.; De Oliveira, L.C.; Braga, J.; Maria, L.; Rosa, T.; Botero, W.G. Evaluation of Germination of Chilli Pepper Using Humic Substances and Humic Acids. IOSR J. Environ. Sci. Toxicol. Food Technol. 2018, 12, 33–39. [Google Scholar] [CrossRef]



| Treatments ID | Priming Agent/Duration |
|---|---|
| Control | non-primed seeds |
| HA-1 h | 0.2 g/L HA (1 h) |
| HA-3 h | 0.2 g/L HA (3 h) |
| HA-5 h | 0.2 g/L HA (5 h) |
| HA-7 h | 0.2 g/L HA (7 h) |
| HP-1 h | distill water (1 h) |
| HP-3 h | distill water (3 h) |
| HP-5 h | distill water (5 h) |
| HP-7 h | distill water (7 h) |
| Parameters | Formula | References |
|---|---|---|
| Final germination % (FG%) | [25] | |
| Germination index (GI) | Where n1, n2, and … are the number of germinated seeds on the first day, second day, and other day and numbers 7, 6, and … are respectively weight imposed on the number of seeds germinated on the first day, second day and other days. | [26] |
| Germination rate index (GRI) | Where G1 is the germination percentage on day 1, G2 is the germination percentage on day 2, and so on. | [27] |
| Mean germination time (MGT) | Where “f” is the number of seeds germinated on day x. | [27] |
| Coefficient velocity of germination (CVG) | Where N is the number of seeds germinated every day and T is the number of days from seeding corresponding to N. | [27] |
| Seedling vigour index (SVI) | [28] |
| Parameters | Formula | References |
|---|---|---|
| Seedling emergence percentage (SEP) | [29] | |
| Mean emergence time (MET) | Where “f” is the number of seeds emerged on day x. | [30] |
| Speed of emergence index (SEI) | Where E1 is the number of emerged seeds on day 1, E2 is the number of emerged seeds on day 2, and so on. | [31] |
| Speed of emergence Coefficient velocity of germination (CVG) | Where E is the number of seeds emerged every day and T is the number of days from seeding corresponding to E. | [32] |
| Treat. | FG% | MGT (day) | GI | GRI | CVG (%/day) |
|---|---|---|---|---|---|
| Control | 62 ± 1.2 b | 4.3 ± 0.2 a | 232 ± 12.8 c | 15.6 ± 0.7 b | 23.6 ± 0.9 d |
| HA 1 h | 58 ± 2.9 b | 3.8 ± 0.2 bc | 244 ± 11.2 bc | 16.2 ± 0.6 b | 26.6 ± 1.1 abc |
| HA 3 h | 63 ± 1.0 b | 3.7 ± 0.1 c | 268± 2.8 b | 17.8 ± 0.2 b | 26.8 ± 0.6 abc |
| HA 5 h | 71 ± 1.0 a | 3.5 ± 0.1 c | 320 ± 6.5 a | 21.1 ± 0.4 a | 28.7 ± 0.9 a |
| HA 7 h | 60 ± 3.7 b | 4.1 ± 0.1 ab | 234 ± 12.5 bc | 16.0 ± 0.9 b | 24.6 ± 0.9 cd |
| HP 1 h | 42 ± 2.6 c | 4.1 ± 0.1 ab | 164± 8.3 d | 10.9 ± 0.6 c | 24.5 ± 0.3 cd |
| HP 3 h | 59 ± 3.0 b | 3.5 ± 0.1 c | 264 ± 16.1 bc | 17.4 ± 1.0 b | 28.3 ± 0.4 ab |
| HP 5 h | 56 ± 3.7 b | 3.8 ± 0.1 bc | 234 ± 15.5 bc | 15.5 ± 1.0 b | 26.3 ± 0.8 bc |
| HP 7 h | 41 ± 3.4 c | 3.7 ± 0.1 c | 175 ± 16.8 d | 11.6 ± 1.1 c | 26.8 ± 1.0 abc |
| LSD | 7.7 | 0.3 | 35.5 | 2.3 | 2.4 |
| CV % | 9.4 | 6.1 | 10.3 | 10.0 | 6.2 |
| Treat. | SHL (cm) | SL (cm) | RODW (g Seedling−1) | SH:RO (Ratio) | SVI (Nos) |
|---|---|---|---|---|---|
| Control | 10.1 ± 0.5 cd | 15.6 ± 0.8 d | 0.006 ± 0.000 e | 11.6 ± 0.6 a | 967.0 ± 44.5 de |
| HA1 h | 9.1 ± 0.7 d | 13.5 ± 0.7 d | 0.009 ± 0.002 cde | 8.0 ± 1.8 bcd | 789.5 ± 73.5 ef |
| HA3 h | 14.1 ± 0.8 ab | 21.0 ± 0.8 b | 0.01 ± 0.000 c | 7.1 ± 0.6 cd | 1323.9 ± 51.5 b |
| HA5 h | 15.2 ± 0.6 a | 23.6 ± 0.7 a | 0.016 ± 0.001 a | 5.2 ± 0.7 d | 1677.4 ± 65.2 a |
| HA7 h | 13.1 ± 0.5 ab | 19.9 ± 0.5 bc | 0.009 ± 0.000 cd | 5.7 ± 0.4 cd | 1197.5 ± 88.7 bc |
| HP1 h | 9.5 ± 0.7 d | 15.2 ± 0.5 d | 0.007 ± 0.000 de | 10.7 ± 1.6 ab | 634.6 ± 34.0 f |
| HP3 h | 12.4 ± 0.6 b | 18.8 ± 0.7 bc | 0.014 ± 0.000 ab | 5.2 ± 0.6 d | 1111.7 ± 82.0 cd |
| HP5 h | 12.0 ± 1.1 bc | 18.4 ± 1.0 c | 0.011 ± 0.001 bc | 6.7 ± 1.1 cd | 1025.7 ± 72.9 cd |
| HP7 h | 9.0 ± 1.0 d | 15.7 ± 1.2 d | 0.009 ± 0.002 cde | 8.8 ± 1.7 abc | 645.1 ± 67.4 f |
| LSD | 2.2 | 2.3 | 0.003 | 3.3 | 193.2 |
| CV | 12.8 | 8.7 | 21 | 29.3 | 12.8 |
| Treat. | RL (cm) | TRL (cm) | ADI (mm) | TRV (cm3) | NRT (nos) |
|---|---|---|---|---|---|
| Control | 5.5 ± 0.5 d | 30.2 ± 1.9 f | 0.62 ± 0.05 bc | 0.08 ± 0.00 e | 29.6 ± 3.6 f |
| HA1 h | 4.4 ± 0.2 e | 33.3 ± 5.2 ef | 0.63 ± 0.02 bc | 0.1 ± 0.02 de | 41.3 ± 8.2 def |
| HA3 h | 6.9 ± 0.5 b | 51.8 ± 2.5 bc | 0.65 ± 0.02 abc | 0.17 ± 0.00 bc | 63.0 ± 6.7 bc |
| HA5 h | 8.5 ± 0.2 a | 69.0 ± 3.5 a | 0.71 ± 0.04 a | 0.28 ± 0.03 a | 83.3 ± 2.2 a |
| HA7 h | 6.8 ± 0.3 b | 57.7 ± 3.4 b | 0.59 ± 0.02 bc | 0.16 ± 0.02 bc | 72.9 ± 6.6 ab |
| HP1 h | 5.7 ± 0.3 cd | 31.6 ± 1.0 f | 0.57 ± 0.03 c | 0.08 ± 0.01 e | 36.3 ± 1.5 ef |
| HP3 h | 6.4 ± 0.3 bcd | 59.6 ± 3.1 ab | 0.67 ± 0.03 ab | 0.21 ± 0.02 b | 75.2 ± 6.6 ab |
| HP5 h | 6.4 ± 0.5 bcd | 46.5 ± 4.2 cd | 0.67 ± 0.01 ab | 0.16 ± 0.02 c | 56.0 ± 3.9 ab |
| HP7 h | 6.7 ± 0.3 bc | 41.7 ± 2.8 de | 0.66 ± 0.01 ab | 0.14 ± 0.01 cd | 47.9 ± 1.2 de |
| LSD | 1.0 | 9.6 | 0.08 | 0.04 | 14.9 |
| CV | 11.3 | 14.1 | 8.4 | 20.2 | 18.3 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| (1) FG | 1.00 | ||||||||
| (2) MGT | −0.15 ns | 1.00 | |||||||
| (3) GI | 0.92 ** | −0.52 ** | 1.00 | ||||||
| (4) GRI | 0.94 ** | −0.47 ** | 1.00 ** | 1.00 | |||||
| (5) CVG | 0.17 ns | −1.00 ** | 0.53 ** | 0.48 ** | 1.00 | ||||
| (6) SVI | 0.79 ** | −0.30 ns | 0.81 ** | 0.82 ** | 0.31 ns | 1.00 | |||
| (7) EC | −0.34 * | 0.39 * | −0.45 ** | −0.45 ** | −0.38 * | −0.43 ** | 1.00 | ||
| (8) MDA | −0.73 ** | 0.42 ** | −0.80 ** | −0.80 ** | −0.42 ** | −0.63 ** | 0.54 ** | 1.00 | |
| (9) POD | 0.54 ** | −0.18 ns | 0.55 ** | 0.56 ** | 0.20 ns | 0.54 ** | −0.46 ** | −0.60 ** | 1.00 |
| (10) CAT | 0.55 ** | −0.48 ** | 0.68 ** | 0.67 ** | 0.50 ** | 0.61 ** | −0.51 ** | −0.69 ** | 0.48 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerasekara, I.; Sinniah, U.R.; Namasivayam, P.; Nazli, M.H.; Abdurahman, S.A.; Ghazali, M.N. Priming with Humic Acid to Reverse Ageing Damage in Soybean [Glycine max (L.) Merrill.] Seeds. Agriculture 2021, 11, 966. https://doi.org/10.3390/agriculture11100966
Weerasekara I, Sinniah UR, Namasivayam P, Nazli MH, Abdurahman SA, Ghazali MN. Priming with Humic Acid to Reverse Ageing Damage in Soybean [Glycine max (L.) Merrill.] Seeds. Agriculture. 2021; 11(10):966. https://doi.org/10.3390/agriculture11100966
Chicago/Turabian StyleWeerasekara, Indika, Uma Rani Sinniah, Parameswari Namasivayam, Muhamad Hazim Nazli, Sharif Azmi Abdurahman, and Mohd Norsazwan Ghazali. 2021. "Priming with Humic Acid to Reverse Ageing Damage in Soybean [Glycine max (L.) Merrill.] Seeds" Agriculture 11, no. 10: 966. https://doi.org/10.3390/agriculture11100966
APA StyleWeerasekara, I., Sinniah, U. R., Namasivayam, P., Nazli, M. H., Abdurahman, S. A., & Ghazali, M. N. (2021). Priming with Humic Acid to Reverse Ageing Damage in Soybean [Glycine max (L.) Merrill.] Seeds. Agriculture, 11(10), 966. https://doi.org/10.3390/agriculture11100966







