Phone App to Perform Quality Control of Pesticide Spray Applications in Field Crops
Abstract
1. Introduction
2. Spray Coverage
3. Possible Consequences of Low and Inconsistent Pesticide Spray Coverages
4. Water-Sensitive Spray Cards to Quantify Spray Coverage
5. Pixel Classification to Quantify Pesticide Spray Coverages
- Blue channel/sum of three color channels
- Green channel/sum of three color channels
- Red channel/sum of three color channels
- Red channel/green channel
6. Classification Performance of Spray Coverage
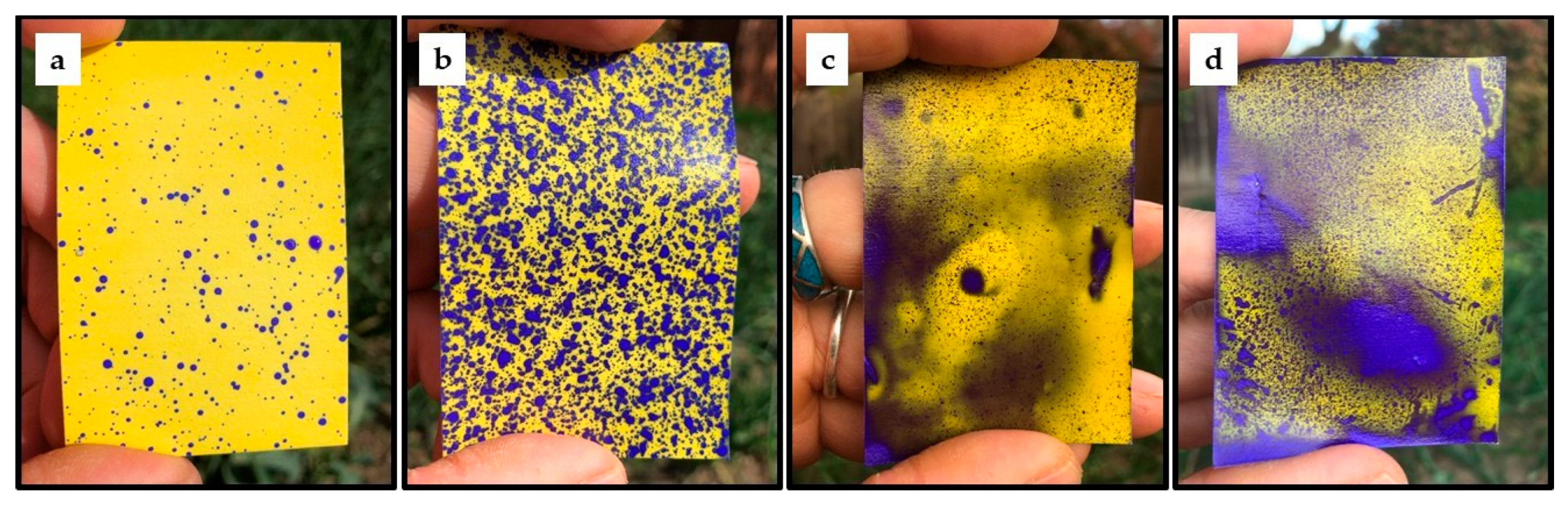
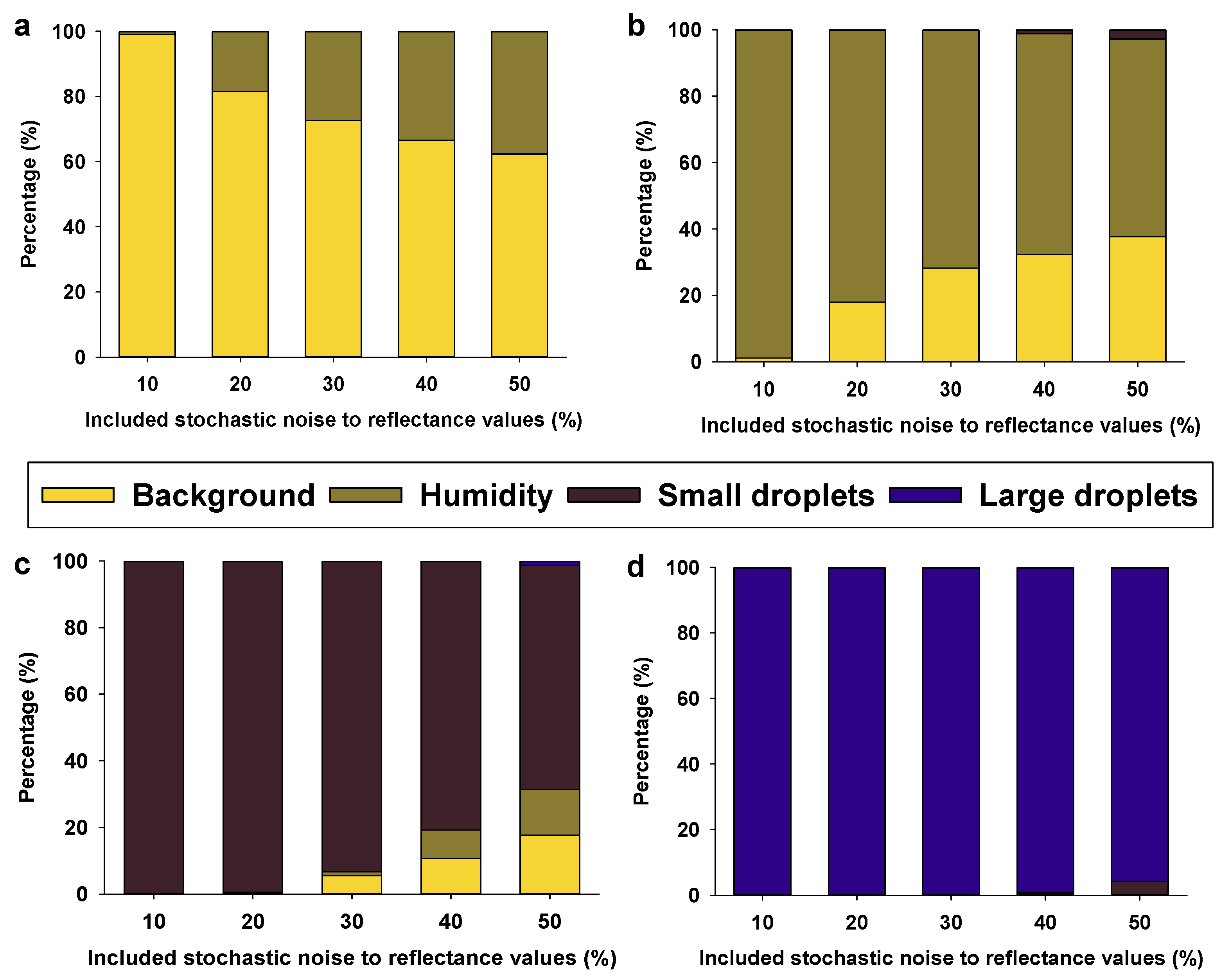
7. Effect of Light Conditions on Spray Coverage Estimates
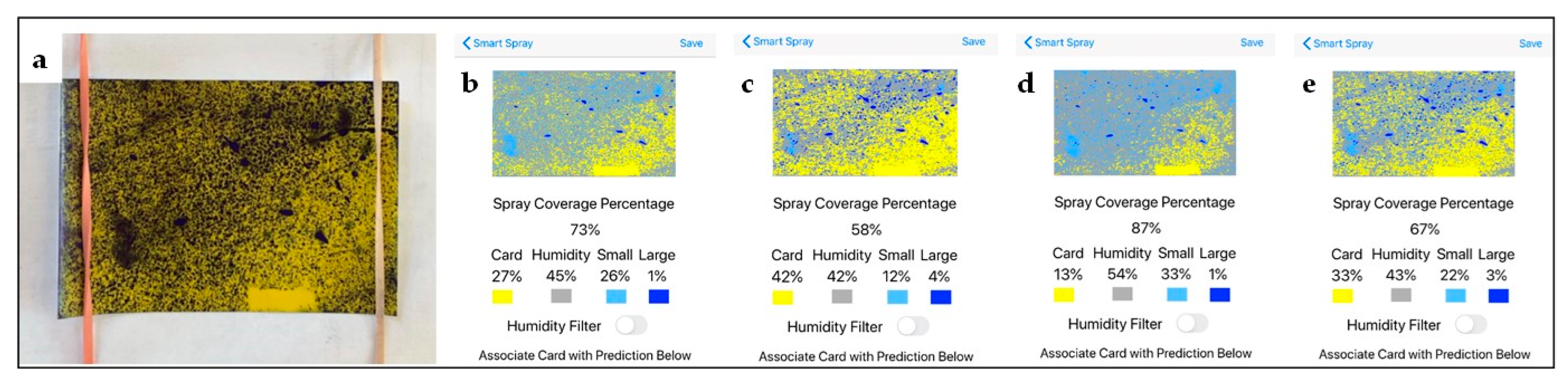
8. Use and Practical Recommendations Regarding Smart Spray
9. Final Comments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, S.-H.; Kim, Y.-K.; Jun, H.-J.; Choi, I.S.; Woo, J.-K.; Kim, Y.-H.; Yun, Y.-T.; Choi, Y.; Alidoost, R.; Lee, J. Evaluation of spray characteristics of pesticide injection system in agricultural drones. J. Biosyst. Eng. 2020, 45, 272–280. [Google Scholar] [CrossRef]
- Li, X.; Giles, D.K.; Niederholzer, F.J.; Andaloro, J.T.; Lang, E.B.; Watson, L.J. Evaluation of an unmanned aerial vehicle as a new method of pesticide application for almond crop protection. Pest Manag. Sci. 2021, 77, 527–537. [Google Scholar] [CrossRef]
- He, X. Rapid development of unmanned aerial vehicles (UAV) for plant protection and application technology in China. Outlooks Pest Manag. 2018, 29, 162–167. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Chechetto, R.G.; O’Donnell, C.C.; Fritz, B.K.; Hoffmann, W.C.; Coleman, C.E.; Chauhan, B.S.; Adkins, S.W.; Kruger, G.R.; Hewitt, A.J. Assessing a novel smartphone application—SnapCard, compared to five imaging systems to quantify droplet deposition on artificial collectors. Comput. Electron. Agric. 2016, 128, 193–198. [Google Scholar] [CrossRef]
- Nansen, C.; Ferguson, J.C.; Moore, J.; Groves, L.; Emery, R.; Garel, N.; Hewitt, A. Optimizing pesticide spray coverage using a novel web and smartphone tool, SnapCard. Agron. Sustain. Dev. 2015, 35, 1075–1085. [Google Scholar] [CrossRef]
- Google App Store. Available online: https://play.google.com/store/apps/details?id=com.dafwa.snapcard&hl=en_US (accessed on 23 September 2021).
- Smart Spray. Available online: http://chrnansen.wixsite.com/nansen2/smartspray (accessed on 23 September 2021).
- Hall, F.; Reed, J.P.; Reichard, D.L.; Riedel, R.M.; Lehtinen, J. Pesticide Delivery Systems: Spray Distribution and Partitioning in Plant Canopies Pesticide Formulations and Application Systems; Bode, L., Hazen, J., Chasin, D., Eds.; ASTM International: West Conshohocken, PA, USA, 1990; Volume 10, pp. 184–203. [Google Scholar]
- Hostetler, M.E.; Brenner, R.J. Behavioral and physiological resistance to insecticides in the German cockroach (Dictyoptera: Blattellidae): An experimental reevaluation. J. Econ. Entomol. 1994, 87, 885–893. [Google Scholar] [CrossRef]
- Lockwood, J.A.; Byford, R.L.; Story, R.N.; Sparks, T.C.; Quisenberry, S.S. Behavioral resistance to the pyrethroids in the Horn Fly, Haematobia irritans (Diptera: Muscidae). Environ. Entomol. 1985, 14, 873–880. [Google Scholar] [CrossRef]
- Nansen, C.; Baissac, O.; Nansen, M.; Powis, K.; Baker, G. Behavioral avoidance—Will physiological insecticide resistance level of insect strains affect their oviposition and movement responses? PLoS ONE 2016, 11, e0149994. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Scharf, M.E.; Bennett, G.W. Behavioral and physiological resistance of the German cockroach to gel baits (Blattodea: Blattellidae). J. Econ. Entomol. 2004, 97, 2067–2072. [Google Scholar] [CrossRef]
- Sarfraz, M.; Dosdall, L.M.; Keddie, B.A. Resistance of some cultivated Brassicaceae to infestations by Plutella xylostella (L.) (Lepidoptera: Plutellidae). J. Econ. Entomol. 2007, 100, 215–224. [Google Scholar] [CrossRef]
- Sarfraz, M.; Dosdall, L.M.; Keddie, B.A. Evidence for behavioural resistance by the diamondback moth, Plutella xylostella (L.). J. Appl. Entomol. 2005, 129, 340–341. [Google Scholar] [CrossRef]
- Jallow, M.F.A.; Hoy, C.W. Indirect selection for increased susceptibility to permethrin in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2007, 100, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Mechanisms. Available online: http://www.irac-online.org/about/resistance/mechanisms/ (accessed on 12 June 2021).
- Renton, M.; Busi, R.; Neve, P.; Thornby, D.; Vila-Aiub, M. Herbicide resistance modelling: Past, present and future. Pest Manag. Sci. 2014, 70, 1394–1404. [Google Scholar] [CrossRef]
- Renton, M.; Diggle, A.; Sudheesh, M.; Powles, S. Does cutting herbicide rates threaten the sustainability of weed management in cropping systems? J. Theor. Biol. 2011, 283, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Mortensen, D.A.; Egan, J.F.; Maxwell, B.D.; Ryan, M.R.; Smith, R.G. Navigating a critical juncture for sustainable weed management. BioScience 2012, 62, 75–84. [Google Scholar] [CrossRef]
- Khodaverdi, H.; Fowles, T.; Bick, E.; Nansen, C. Does drought increase the risk of insects developing behavioral resistance to systemic insecticides? J. Econ. Entomol. 2016, 109, 2027–2031. [Google Scholar] [CrossRef]
- Hill, B.D.; Inaba, D.J. Use of water-sensitive paper to monitor the deposition of aerially applied insecticides. J. Econ. Entomol. 1989, 82, 974–980. [Google Scholar] [CrossRef]
- Turner, C.R.; Huntington, K.A. The use of a water sensitive dye for the detection and assessment of small spray droplets. J. Agric. Eng. Res. 1970, 15, 385–387. [Google Scholar] [CrossRef]
- Syngenta. Water-Sensitive Paper for Monitoring Spray Distributions; Syngenta Crop Protection: Basel, Switzerland, 2002. [Google Scholar]
- Sama, M.P.; Evans, J.T.; Turner, A.P.; Dasika, S.S. As-applied estimation of volumetric flow rate from a single sprayer nozzle series using water-sensitive spray cards. Trans. ASABE 2016, 59, 861–869. [Google Scholar] [CrossRef]
- Sama, M.P.; Weiss, A.M.; Benedict, E.K. Validating spray coverage rate using liquid mass on a spray card. Trans. ASABE 2018, 61, 887–895. [Google Scholar] [CrossRef]
- Fox, R.D.; Salyani, M.; Cooper, J.A.; Brazee, R.D. Spot size comparisons on oil- and water-sensitive paper. Appl. Eng. Agric. 2001, 17, 131. [Google Scholar]
- Cunha, J.P.A.R.; Farnese, A.C.; Olivet, J.J. Computer programs for analysis of droplets sprayed on water sensitive papers. Planta Daninha 2013, 31, 715–720. [Google Scholar] [CrossRef]
- Marçal, A.R.S.; Cunha, M. Image processing of artificial targets for automatic evaluation of spray quality. Trans. ASABE 2008, 51, 811–821. [Google Scholar] [CrossRef]
- Zhu, H.; Salyani, M.; Fox, R.D. A portable scanning system for evaluation of spray deposit distribution. Comput. Electron. Agric. 2011, 76, 38–43. [Google Scholar] [CrossRef]
- Cunha, M.; Carvalho, C.; Marcal, A.R.S. Assessing the ability of image processing software to analyse spray quality on water-sensitive papers used as artificial targets. Biosyst. Eng. 2012, 111, 11–23. [Google Scholar] [CrossRef]
- Degre, A.; Mostade, O.; Huyghebaert, B.; Tissot, S.; Debouche, C. Comparison by image processing of target supports of spray droplets. Trans. ASAE 2001, 44, 217–222. [Google Scholar] [CrossRef]
- Garcia, L.C.; Ramos, H.H.; Justino, A. Evaluation of software for analysis of spraying parameters carried over water-sensitive papers (Availação de softwares para análise de parâmetros da pulverização realizada sobre papéis hidrossensíveis). Rev. Bras. Da Agrocomputação 2004, 2, 19–28. [Google Scholar]
- Özlüoymak, Ö.B.; Bolat, A. Development and assessment of a novel imaging software for optimizing the spray parameters on water-sensitive papers. Comput. Electron. Agric. 2020, 168, 105104. [Google Scholar] [CrossRef]
- Hoffman, W.C.; Hewitt, A.J. Comparison of three imaging systems for water-sensitive papers. Appl. Eng. Agric. 2005, 21, 961–964. [Google Scholar] [CrossRef]
- Wolf, R.E. Assessing the ability of DropletScan to analyze spray droplets from a ground operated sprayer. Appl. Eng. Agric. 2003, 19, 525–530. [Google Scholar]
- Latheef, M.A.; Carlton, J.B.; Kirk, I.W.; Hoffmann, W.C. Aerial electrostatic-charged sprays for deposition and efficacy against sweet potato whitefly (Bemisia tabaci) on cotton. Pest Manag. Sci. 2008, 65, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Latheef, M.A.; Kirk, I.W.; Bouse, L.F.; Carlton, J.B.; Hoffmann, W.C. Evaluation of aerial delivery systems for spray deposition and efficacy against sweet potato whitefly on cotton. Appl. Eng. Agric. 2008, 24, 415–422. [Google Scholar] [CrossRef]
- Nansen, C.; Vaughn, K.; Xue, Y.; Rush, C.; Workneh, F.; Goolsby, J.; Troxclair, N.; Anciso, J.; Gregory, A.; Holman, D.; et al. A decision-support tool to predict spray deposition of insecticides in commercial potato fields and its implications for their performance. J. Econ. Entomol. 2011, 104, 1138–1145. [Google Scholar] [CrossRef]
- Hunter, J.E.; Gannon, T.W.; Richardson, R.J.; Yelverton, F.H.; Leon, R.G. Coverage and drift potential associated with nozzle and speed selection for herbicide applications using an unmanned aerial sprayer. Weed Technol. 2020, 34, 235–240. [Google Scholar] [CrossRef]
- Fink, C.; Banuelos, J.; Rossi, L.; Barker, M.; Edsall, M.; Olivier, D.; Lin, J. An evaluation of spray rig designs for California strawberries using water-sensitive paper. Int. J. Fruit Sci. 2020, 20, 997–1004. [Google Scholar] [CrossRef]
- Bock, C.H.; Hotchkiss, M.W.; Cottrell, T.E.; Wood, B.W. The effect of sample height on spray coverage in mature pecan trees. Plant Dis. 2015, 99, 916–925. [Google Scholar] [CrossRef]
- Bock, C.; Hotchkiss, M.W. A comparison of ground-based air-blast sprayer and aircraft application of fungicides to manage scab in tall pecan trees. Plant Dis. 2020, 104, 1675–1684. [Google Scholar] [CrossRef]
- Siegel, J.P.; Strmiska, M.M.; Niederholzer, F.J.A.; Giles, D.K.; Walse, S.S. Evaluating insecticide coverage in almond and pistachio for control of navel orangeworm (Amyelois transitella) (Lepidoptera: Pyralidae). Pest Manag. Sci. 2019, 75, 1435–1442. [Google Scholar] [CrossRef]
- Abbott, C.P.; Beckerman, J.L. Incorporating adjuvants with captan to manage common apple diseases. Plant Dis. 2018, 102, 231–236. [Google Scholar] [CrossRef]
- Teejet. Available online: www.teejet.com (accessed on 23 September 2021).
- Rapid Tables. Available online: https://www.rapidtables.com/web/color/RGB_Color.html (accessed on 23 September 2021).
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Syngenta Chess Label. Available online: https://www.syngenta.co.za/product/crop-protection/chess (accessed on 23 September 2021).

| Average Droplet Diameter (µm) | |||||||
|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 300 | 400 | 500 | 600 | |
| Volume of single droplet (µm3) | 6.55 × 104 | 5.24 × 105 | 4.19 × 106 | 1.40 × 107 | 3.35 × 107 | 6.54 × 107 | 1.13 × 108 |
| Volume of single droplet (mL) | 6.55 × 10−8 | 5.24 × 10−7 | 4.19 × 10−6 | 1.40 × 10−5 | 3.35 × 10−5 | 6.54 × 10−5 | 1.13 × 10−4 |
| Droplets in 20 mL | 3.06 × 108 | 3.82 × 107 | 4.77 × 106 | 1.43 × 106 | 5.97 × 105 | 3.06 × 105 | 1.77 × 105 |
| Area of single droplet (m2) | 1.96 × 10−9 | 7.85 × 10−9 | 3.14 × 10−8 | 7.07 × 10−8 | 1.26 × 10−7 | 1.96 × 10−7 | 2.83 × 10−7 |
| Total droplet area (m2) | 0.599 | 0.300 | 0.150 | 0.101 | 0.075 | 0.060 | 0.050 |
| Spray coverage (%) | 59.9 | 30.0 | 15.0 | 10.1 | 7.5 | 6.0 | 5.0 |
| Color Channel | ||||
|---|---|---|---|---|
| Main Class | Red | Green | Blue | Code |
| Background | 229 | 196 | 44 | E5C42C |
| Background | 243 | 225 | 97 | F3E161 |
| Small droplet | 52 | 33 | 29 | 34211D |
| Small droplet | 64 | 42 | 61 | 402A3D |
| Large droplet | 37 | 8 | 89 | 250859 |
| Large droplet | 46 | 4 | 172 | 2E04AC |
| Humidity | 109 | 94 | 35 | 6D5E23 |
| Humidity | 158 | 151 | 82 | 9E9752 |
| Device | Condition 1 | Condition 2 | Background | Small | Large | Humidity | Average |
|---|---|---|---|---|---|---|---|
| Android | In_shade | In_light | 0.987 | 0.984 | 0.955 | 0.897 | 0.975 |
| Android | In_shade | Out_shade | 0.974 | 0.995 | 0.986 | 0.880 | 0.985 |
| Android | In_light | Out_shade | 0.992 | 0.993 | 0.959 | 0.968 | 0.981 |
| Android | In_shade | Out_light | 0.990 | 0.988 | 0.985 | 0.909 | 0.988 |
| Android | In_light | Out_light | 0.991 | 0.996 | 0.970 | 0.970 | 0.986 |
| Android | Out_shade | Out_light | 0.982 | 0.994 | 0.986 | 0.962 | 0.987 |
| iOS | In_shade | In_light | 0.990 | 0.962 | 0.990 | 0.986 | 0.981 |
| iOS | In_shade | Out_shade | 0.939 | 0.964 | 0.995 | 0.802 | 0.966 |
| iOS | In_light | Out_shade | 0.962 | 0.985 | 0.998 | 0.782 | 0.982 |
| iOS | In_shade | Out_light | 0.991 | 0.986 | 0.987 | 0.969 | 0.988 |
| iOS | In_light | Out_light | 0.986 | 0.982 | 0.999 | 0.985 | 0.989 |
| iOS | Out_shade | Out_light | 0.927 | 0.972 | 0.996 | 0.729 | 0.965 |
| Android | 0.986 | 0.992 | 0.974 | 0.931 | 0.984 | ||
| iOS | 0.966 | 0.975 | 0.994 | 0.876 | 0.978 | ||
| Total | 0.976 | 0.983 | 0.984 | 0.903 | 0.981 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nansen, C.; Villar, G.D.; Recalde, A.; Alvarado, E.; Chennapragada, K. Phone App to Perform Quality Control of Pesticide Spray Applications in Field Crops. Agriculture 2021, 11, 916. https://doi.org/10.3390/agriculture11100916
Nansen C, Villar GD, Recalde A, Alvarado E, Chennapragada K. Phone App to Perform Quality Control of Pesticide Spray Applications in Field Crops. Agriculture. 2021; 11(10):916. https://doi.org/10.3390/agriculture11100916
Chicago/Turabian StyleNansen, Christian, Gabriel Del Villar, Alexander Recalde, Elvis Alvarado, and Krishna Chennapragada. 2021. "Phone App to Perform Quality Control of Pesticide Spray Applications in Field Crops" Agriculture 11, no. 10: 916. https://doi.org/10.3390/agriculture11100916
APA StyleNansen, C., Villar, G. D., Recalde, A., Alvarado, E., & Chennapragada, K. (2021). Phone App to Perform Quality Control of Pesticide Spray Applications in Field Crops. Agriculture, 11(10), 916. https://doi.org/10.3390/agriculture11100916







