Persistence of Human Pathogens in Manure-Amended Australian Soils Used for Production of Leafy Vegetables
Abstract
1. Introduction
2. Materials and Methods
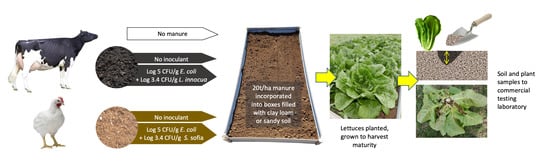
2.1. Trial Site Preparation
2.2. Bacterial Cultures
2.3. Manure Inoculation and Incorporation
- Unamended control;
- Poultry litter;
- Dairy cow manure;
- Poultry litter + log 5.0 CFU/g E. coli + log 3.4 CFU/g S. sofia;
- Dairy cow manure + log 5.0 CFU/g E. coli + log 3.4 CFU/g L. innocua.
2.4. Plant Materials
2.5. Sampling
2.6. Microbial Testing
2.6.1. E. coli
2.6.2. Salmonella spp.
- Where Salmonella was detected, but the population was recorded as “<3”, a value of “2” (log 0.3 CFU/g) was substituted.
- Where Salmonella was not detected by enrichment, a value of “log 0 CFU/g” was substituted.
- As the maximum calculable value with MPN was “>1100”, a value of “1200” (log 3.1 CFU/g) was substituted.
2.6.3. Listeria spp.
2.7. Statistical Analysis
2.7.1. E. coli Data Modelling
2.7.2. Salmonella spp. Data Modelling
3. Results
3.1. Initial Populations of Human Pathogens in Manures
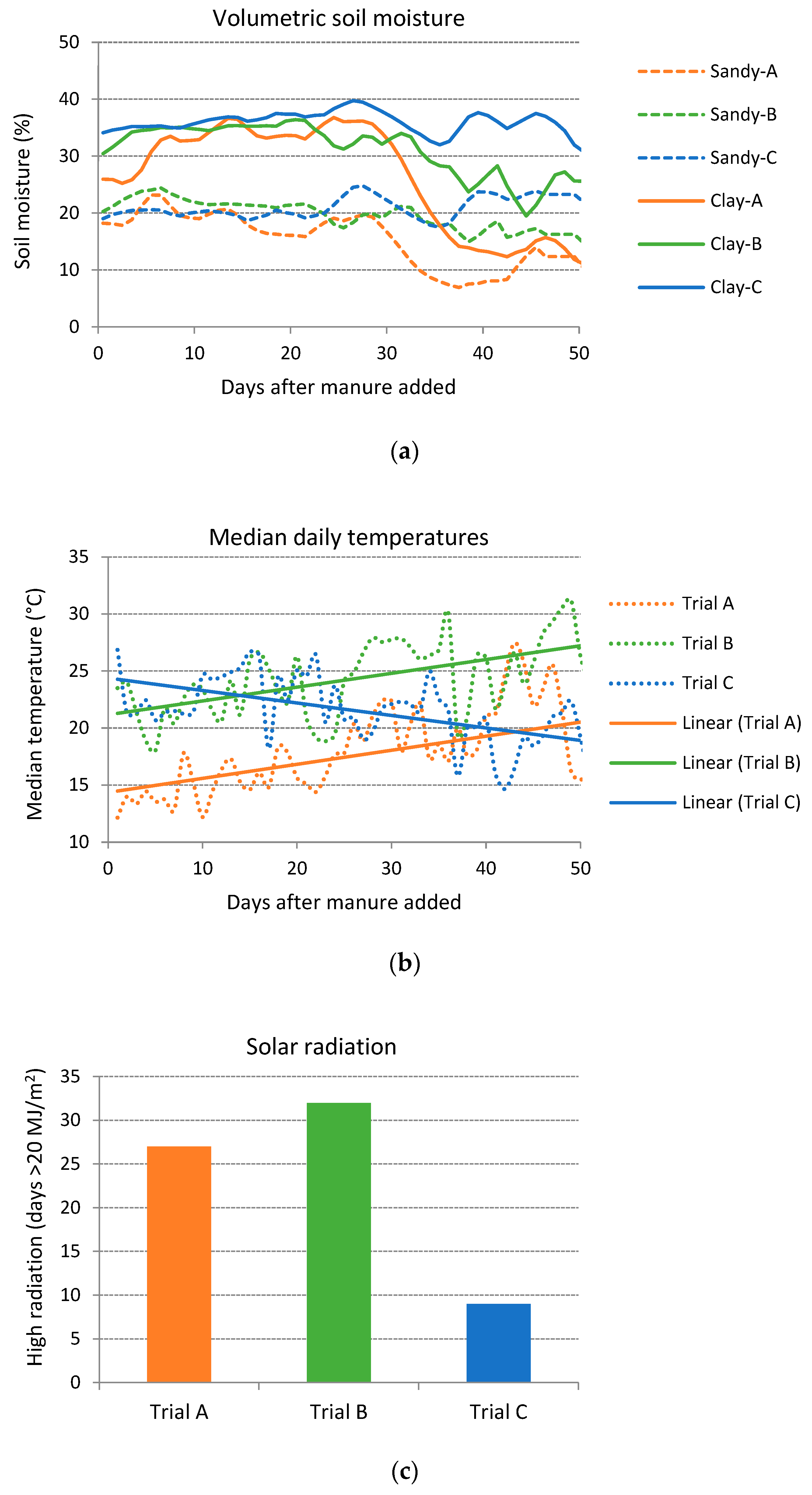
3.2. Climate and Soil Moisture
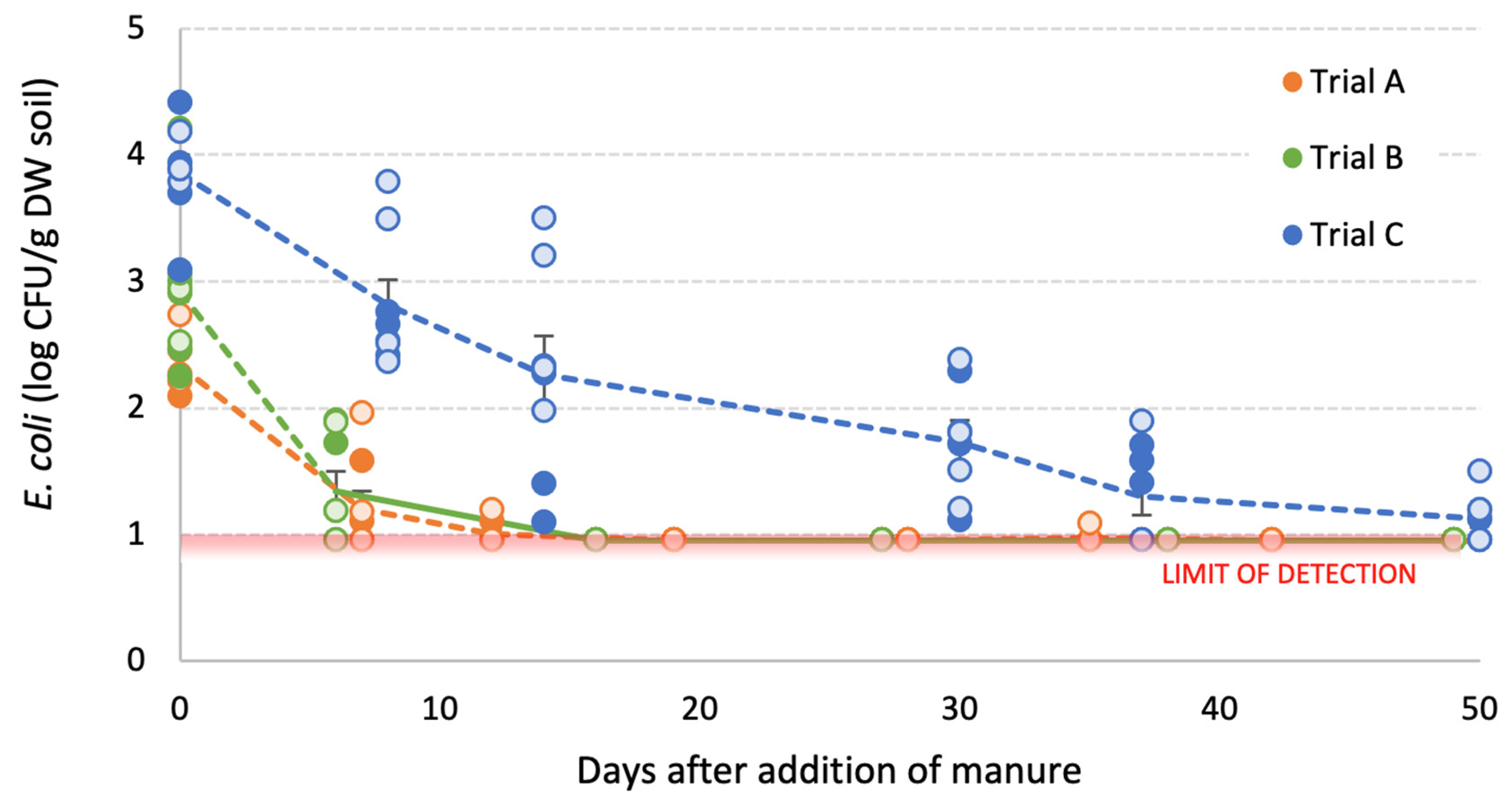
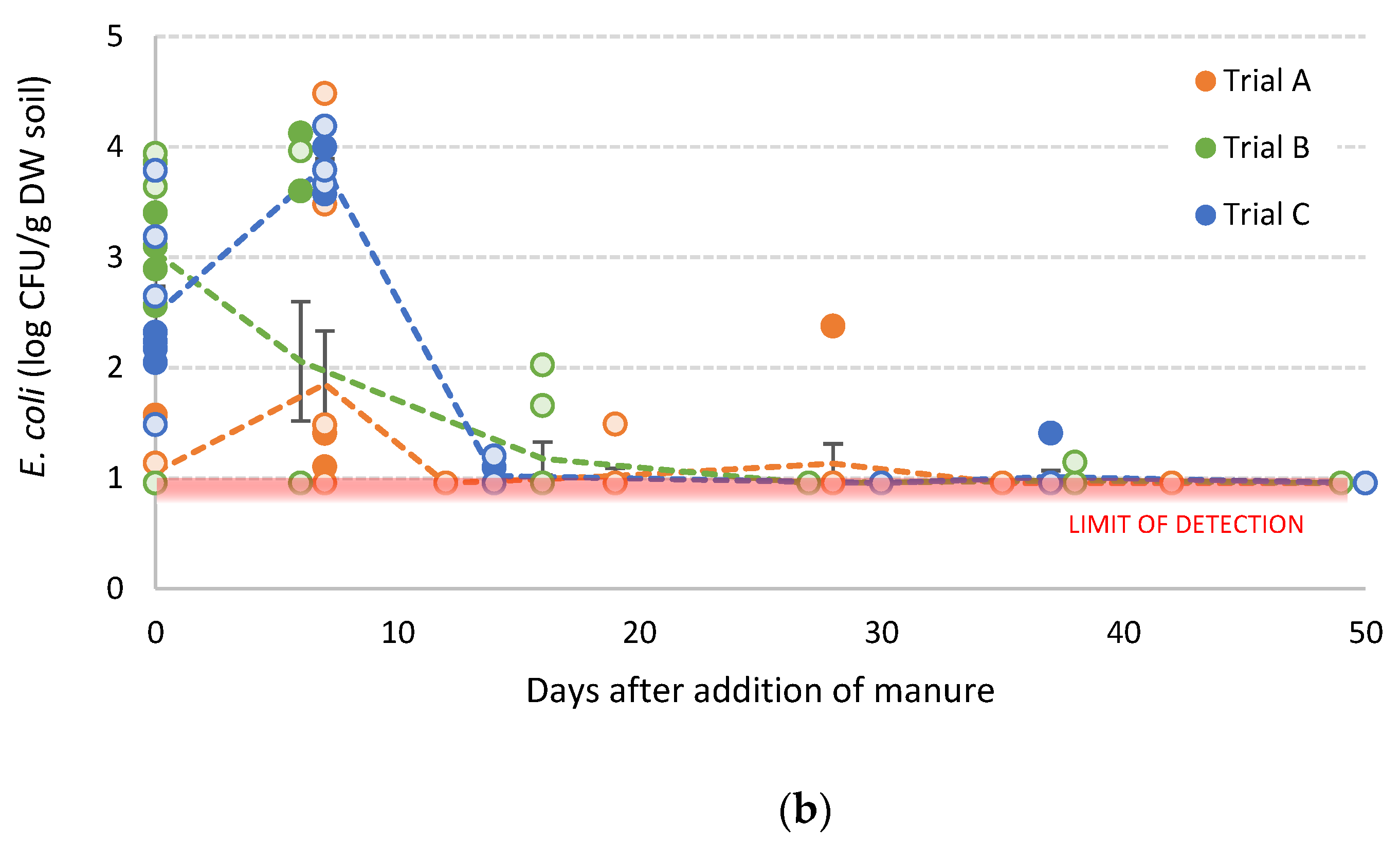
3.3. Survival of E. coli in Manure-Amended Soils
3.3.1. Inoculated Dairy Cow Manure
3.3.2. Poultry Litter
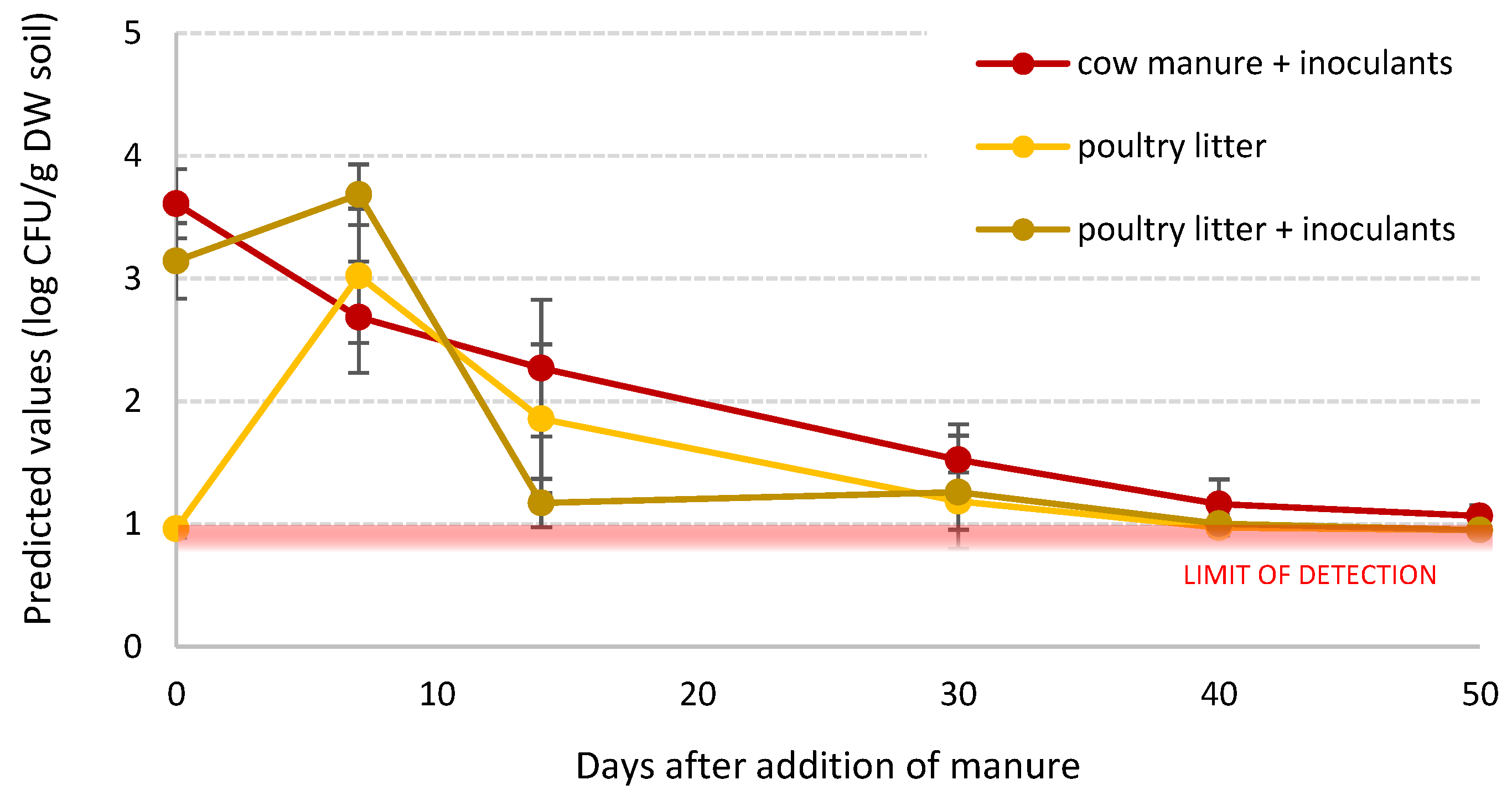
3.3.3. Generalised E. coli Population Model
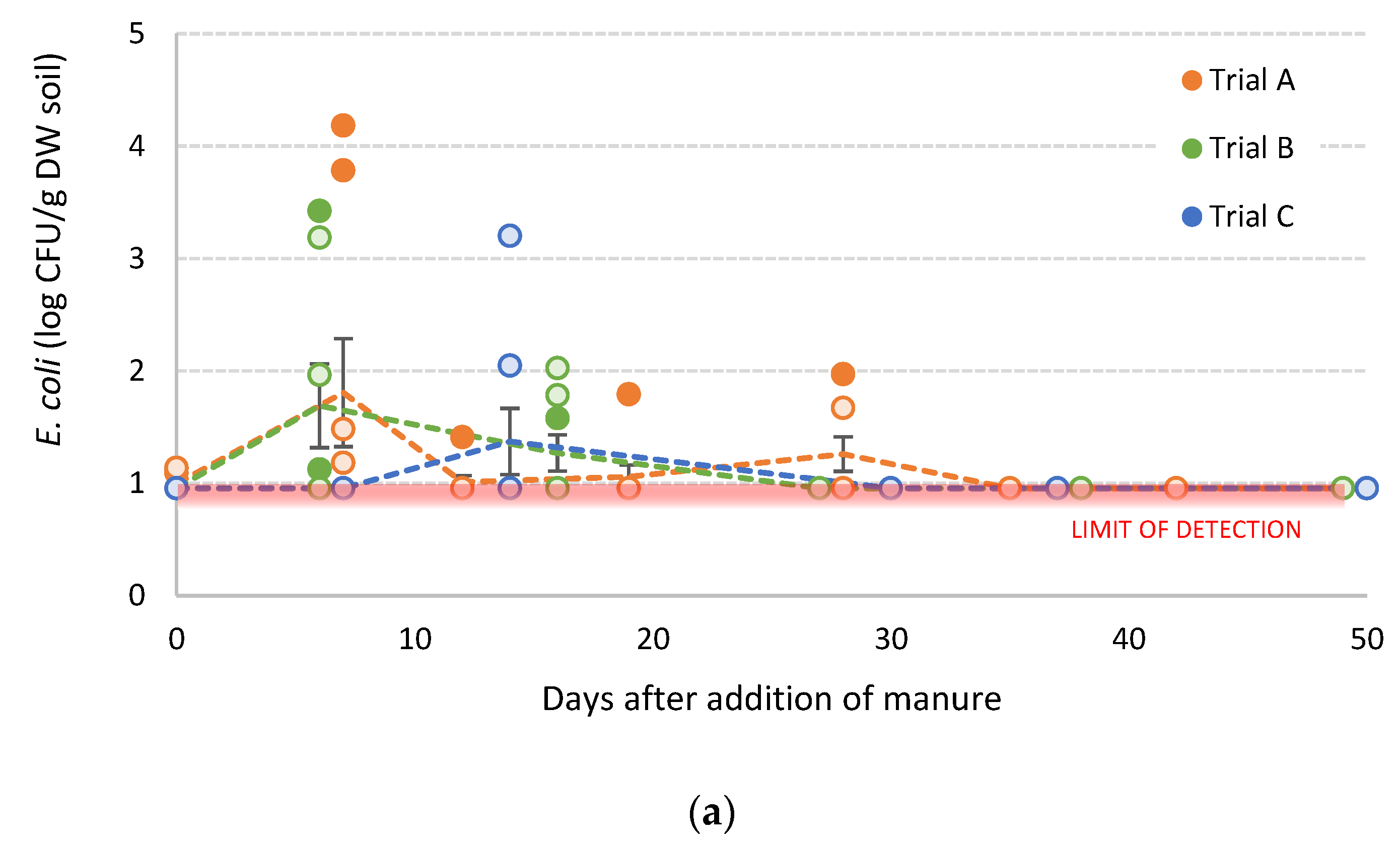
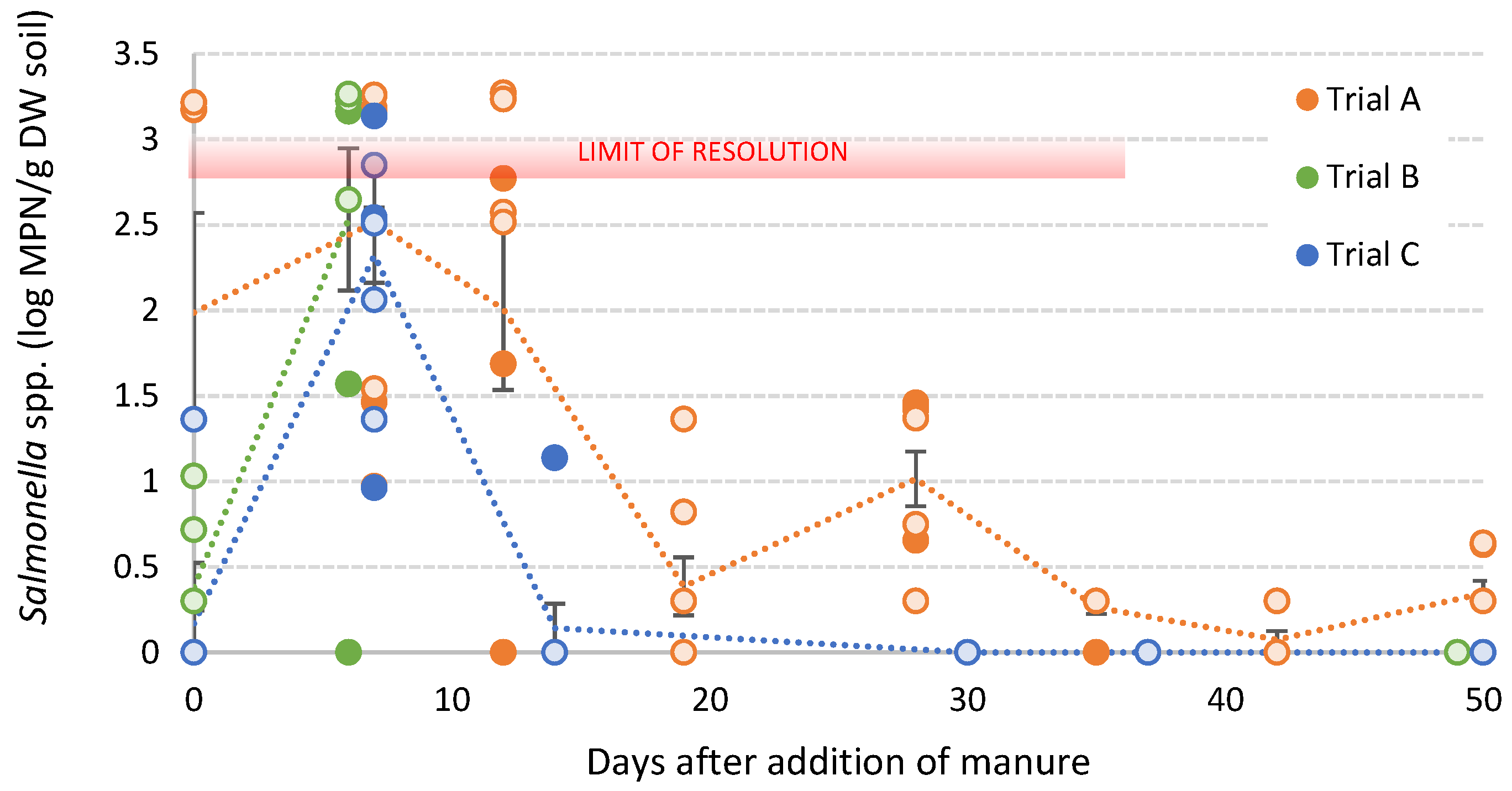
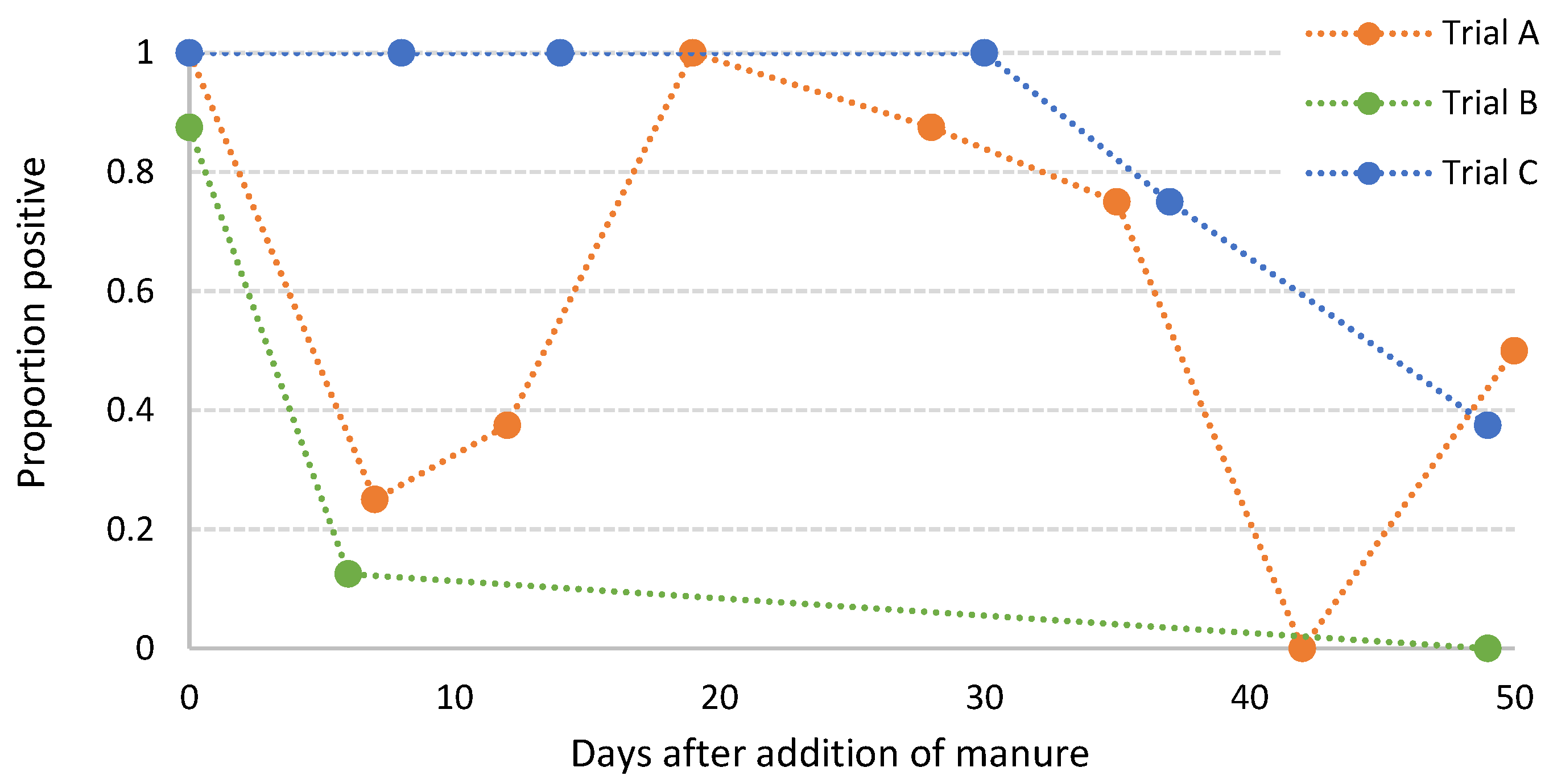
3.4. Survival of S. sofia in Poultry Litter Amended Soils
3.5. Survival of L. innocua in Dairy Cow Manure-Amended Soils
3.6. Pathogen Detection on Lettuce
3.7. Lettuce Yield and Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bogaard, A.; Fraser, R.; Heaton, T.H.E.; Wallace, M.; Vaiglova, P.; Charles, M.; Jones, G.; Evershed, R.P.; Styring, A.K.; Andersen, N.H.; et al. Crop manuring and intensive land management by Europe’s first farmers. Proc. Natl. Acad. Sci. USA 2013, 110, 12589–12594. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Millner, P.D.; Hashem, F.; Vinyard, B.T.; East, C.; Handy, E.T.; White, K.; Stonebraker, R.; Cotton, C.P. Survival of Escherichia coli in Manure-Amended soils is Affected by Spatiotemporal, Agricultural, and Weather Factors. Appl. Environ. Microbiol. 2019, 85, e02392-18. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Alaboudi, A.R.; Al-Quran, H.N.; Al-Nabulsi, A.A. Decontamination and survival of Enterobacteriaceae on shredded iceberg lettuce during storage. Food Microbiol. 2018, 73, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Shirron, N.; Kisluk, G.; Zelikovich, Y.; Eivin, I.; Shimoni, E.; Yaron, S. A comparative study assaying commonly used sanitizers for antimicrobial activity against indicator bacteria and a salmonella typhimurium strain on fresh produce. J. Food Prot. 2009, 72, 2413–2417. [Google Scholar] [CrossRef]
- Prado-Silva, L.; Cadavez, V.; Gonzales-Barron, U.; Rezende, A.C.B.; Sant’Ana, A.S. Meta-analysis of the effects of sanitizing treatments on Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes inactivation in fresh produce. Appl. Environ. Microbiol. 2015, 81, 8008–8021. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Sharma, M.; Kniel, K.E. Human enteric pathogen internalization by root uptake into food crops. Foodborne Pathog. Dis. 2012, 9, 396–405. [Google Scholar] [CrossRef]
- Islam, M.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Persistence of Enterohemorrhagic Escherichia coli O157:H7 in Soil and on Leaf Lettuce and Parsley Grown in Fields Treated with Contaminated Manure Composts or Irrigation Water. J. Food Prot. 2004, 67, 1365–1370. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Walters, L.D.; Moore, A.; Crookes, K.M.; Avery, S.M. Effect of length of time before incorporation on survival of pathogenic bacteria present in livestock wastes applied to agricultural soil. Appl. Environ. Microbiol. 2004, 70, 5111–5118. [Google Scholar] [CrossRef]
- Ongeng, D.; Geeraerd, A.H.; Springael, D.; Ryckeboer, J.; Muyanja, C.; Mauriello, G. Fate of Escherichia coli O157:H7 and Salmonella enterica in the manure-amended soil-plant ecosystem of fresh vegetable crops: A review. Crit. Rev. Microbiol. 2015, 41, 273–294. [Google Scholar] [CrossRef]
- Tran, D.T.Q.; Bradbury, M.I.; van Ogtrop, F.F.; Bozkurt, H.; Jones, B.J.; McConchie, R. Environmental drivers for persistence of Escherichia coli and salmonella in manure-amended soils: A meta-analysis. J. Food Prot. 2020, 83, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Warriner, K.; Huber, A. Die Off Rates of Human Pathogens in Manure Amended Soils under Natural Climatic Conditions Using a Novel Sentinel Chamber System; Technical Report; The Center for Produce Safety: Woodland, CA, USA, 2014. [Google Scholar]
- Shah, M.K.; Bradshaw, R.; Nyarko, E.; Handy, E.T.; East, C.; Millner, P.D.; Bergholz, T.M.; Sharma, M. Salmonella enterica in soils amended with heat-treated poultry pellets survived longer than bacteria in unamended soils and more readily transferred to and persisted on spinach. Appl. Environ. Microbiol. 2019, 85, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ongeng, D.; Muyanja, C.; Ryckeboer, J.; Geeraerd, A.H.; Springael, D. Rhizosphere effect on survival of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in manure-amended soil during cabbage (Brassica oleracea) cultivation under tropical field conditions in Sub-Saharan Africa. Int. J. Food Microbiol. 2011, 149, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Reynnells, R. Importance of Soil Amendments: Survival of Bacterial Pathogens in Manure and Compost Used as Organic Fertilizers. Microbiol. Spectr. 2016, 4, 1–13. [Google Scholar] [CrossRef]
- Pang, H.; Mokhtari, A.; Chen, Y.; Oryang, D.; Ingram, D.T.; Sharma, M.; Millner, P.D.; Van Doren, J.M. A Predictive Model for Survival of Escherichia coli O157:H7 and Generic E. coli in Soil Amended with Untreated Animal Manure. Risk Anal. 2020, 40, 1367–1382. [Google Scholar] [CrossRef]
- Nyberg, K.A.; Vinnerås, B.; Ottoson, J.R.; Aronsson, P.; Albihn, A. Inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium in manure-amended soils studied in outdoor lysimeters. Appl. Soil Ecol. 2010, 46, 398–404. [Google Scholar] [CrossRef]
- Jensen, A.N.; Storm, C.; Forslund, A.; L Baggesen, D.; Dalsgaard, A. Escherichia coli contamination of lettuce grown in soils amended with animal slurry. J. Food Prot. 2013, 76, 1137–1144. [Google Scholar] [CrossRef]
- Ma, J.; Mark Ibekwe, A.; Crowley, D.E.; Yang, C.H. Persistence of Escherichia coli O157 and non-O157 strains in agricultural soils. Sci. Total Environ. 2014, 490, 822–829. [Google Scholar] [CrossRef]
- Franz, E.; Semenov, A.V.; Termorshuizen, A.J.; De Vos, O.J.; Bokhorst, J.G.; Van Bruggen, A.H.C. Manure-amended soil characteristics affecting the survival of E. coli O157:H7 in 36 Dutch soils. Environ. Microbiol. 2008, 10, 313–327. [Google Scholar] [CrossRef]
- Kothary, M.H.; Babu, U.S. Infective dose of foodborne pathogens in volunteers: A review. J. Food Saf. 2001, 21, 49–68. [Google Scholar] [CrossRef]
- Teunis, P.; Takumi, K.; Shinagawa, K. Dose Response for Infection by Escherichia coli O157:H7 from Outbreak Data. Risk Anal. 2004, 24, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P.; Frank, S.A. Pathogenesis, virulence, and infective dose. PLoS Pathog. 2007, 3, 1372–1373. [Google Scholar] [CrossRef] [PubMed]
- Ingham, S.C.; Fanslau, M.A.; Engel, R.A.; Breuer, J.R.J.E.; Breuer, J.R.J.E.; Wright, T.H.; Reith-Rozelle, J.K.; Zhu, J. Evaluation of fertilization-to-planting and fertilization-to-harvest intervals for safe use of noncomposted bovine manure in wisconsin vegetable production. J. Food Prot. 2005, 68, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.S.; Bech, T.B. Soil survival of Salmonella and transfer to freshwater and fresh produce. Food Res. Int. 2012, 45, 557–566. [Google Scholar] [CrossRef]
- Solomon, E.B.; Yaron, S.; Matthews, K.R. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 2002, 68, 397–400. [Google Scholar] [CrossRef]
- Islam, M.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Survival of Escherichia coli O157:H7 in soil and on carrots and onions grown in fields treated with contaminated manure composts or irrigation water. Food Microbiol. 2005, 22, 63–70. [Google Scholar] [CrossRef]
- Franz, E. Ecology and Risk Assessment of E. coli O157:H7 and Salmonella Typhimurium in the Primary Production Chain of Lettuce; Wageningen University: Wageningen, The Netherlands, 2007. [Google Scholar]
- Natvig, E.E.; Ingham, S.C.; Ingham, B.H.; Cooperband, L.R.; Roper, T.R. Salmonella enterica serovar typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Appl. Environ. Microbiol. 2002, 68, 2737–2744. [Google Scholar] [CrossRef]
- Franz, E.; Van Diepeningen, A.D.; De Vos, O.J.; Van Bruggen, A.H.C. Effects of cattle feeding regimen and soil management type on the fate of Escherichia coli O157:H7 and Salmonella enterica serovar typhimurium in manure, manure-amended soil, and lettuce. Appl. Environ. Microbiol. 2005, 71, 6165–6174. [Google Scholar] [CrossRef]
- Fresh Produce Safety Centre Australia and New Zealand. Guidelines for Fresh Produce Food Safety; FPSC A-NZ: Camperdown, Australia, 2019. [Google Scholar]
- Ramos, T.M.; Jay-Russell, M.T.; Millner, P.D.; Shade, J.; Misiewicz, T.; Sorge, U.S.; Hutchinson, M.; Lilley, J.; Pires, A.F.A. Assessment of Biological Soil Amendments of Animal Origin Use, Research Needs, and Extension Opportunities in Organic Production. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Integrated Farm Assurance: All Farm Base-Crops Base-Fruit and Vegetables-Control Points and Compliance Criteria-Version 5.1; GlobalG.A.P.: Cologne, Germany, 2017.
- Bennett, R. PMA News, 2018; 1.
- Fresh Produce Standards, Red Tractor Assurance; London, UK, 2017.
- Commodity Specific Food Safety Guidelines for the Production and Harvest of Lettuce and Leafy Greens; California Leafy Greens Marketing Association: Sacramento, CA, USA, 2019; p. 86.
- Chinivasagam, H.N.; Redding, M.; Runge, G.; Blackall, P.J. Presence and incidence of food-borne pathogens in australian chicken litter. Br. Poult. Sci. 2010, 51, 311–318. [Google Scholar] [CrossRef]
- Barlow, R.S.; McMillan, K.E.; Duffy, L.L.; Fegan, N.; Jordan, D.; Mellor, G.E. Prevalence and antimicrobial resistance of salmonella and escherichia coli from Australian cattle populations at slaughter. J. Food Prot. 2015, 78, 912–920. [Google Scholar] [CrossRef]
- Klein, M.; Brown, L.; Tucker, R.W.; Ashbolt, N.J.; Stuetz, R.M.; Roser, D.J. Diversity and abundance of zoonotic pathogens and indicators in manures of feedlot cattle in Australia. Appl. Environ. Microbiol. 2010, 76, 6947–6950. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huber, A.; Dunfield, K.; Murray, K.; Wu, F.; Warriner, K. Comparative persistence of Salmonella and Escherichia coli O157:H7 in loam or sandy loam soil amended with bovine or swine manure. Can. J. Microbiol. 2018, 64, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Mcconchie, R. Remediation of Soil Contaminated by Salmonella Enterica to Expedite Plant of Replant of Vegetables; University of Sydney Faculty of Science Life and Environmental Sciences: Sydney, Australia, 2018. [Google Scholar]
- Brennan, F.P.; Moynihan, E.; Griffiths, B.S.; Hillier, S.; Owen, J.; Pendlowski, H.; Avery, L.M. Clay mineral type effect on bacterial enteropathogen survival in soil. Sci. Total Environ. 2014, 468–469, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Phan-Thien, K.; Metaferia, M.H.; Bell, T.L.; Bradbury, M.I.; Sassi, H.P.; van Ogtrop, F.F.; Suslow, T.V.; McConchie, R. Effect of soil type and temperature on survival of Salmonella enterica in poultry manure-amended soils. Lett. Appl. Microbiol. 2020, 71, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Cools, D.; Merckx, R.; Vlassak, K.; Verhaegen, J. Survival of E. coli and Enterococcus spp. derived from pig slurry in soils of different texture. Appl. Soil Ecol. 2001, 17, 53–62. [Google Scholar] [CrossRef]
- Neher, D.A.; Cutler, A.J.; Weicht, T.R.; Sharma, M.; Millner, P.D. Composts of poultry litter or dairy manure differentially affect survival of enteric bacteria in fields with spinach. J. Appl. Microbiol. 2019, 126, 1910–1922. [Google Scholar] [CrossRef]
- Chinivasagam, H.N.; Tran, T.; Blackall, P.J. Impact of the Australian litter re-use practice on Salmonella in the broiler farming environment. Food Res. Int. 2012, 45, 891–896. [Google Scholar] [CrossRef]
- Fegan, N.; Higgs, G.; Duffy, L.L.; Barlow, R.S. The Effects of Transport and Lairage on Counts of Escherichia Coli O157 in the Feces and on the Hides of Individual Cattle. Foodborne Pathog. Dis. 2009, 6, 1113–1120. [Google Scholar] [CrossRef]
- Çekiç, S.K.; De, J.; Jubair, M.; Schneider, K.R. Persistence of indigenous Escherichia coli in raw bovine manure-amended soil. J. Food Prot. 2017, 80, 1562–1573. [Google Scholar] [CrossRef]
- Buchrieser, C.; Rusniok, C.; Kunst, F.; Cossart, P.; Glaser, P.; Frangeul, L.; Amend, A.; Baquero, F.; Berche, P.; Bloecker, H.; et al. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: Clues for evolution and pathogenicity. FEMS Immunol. Med. Microbiol. 2003, 35, 207–213. [Google Scholar] [CrossRef]
- Reed-Jones, N.L.; Marine, S.C.; Everts, K.L.; Micallef, S.A. Effects of cover crop species and season on population dynamics of Escherichia coli and Listeria innocua in soil. Appl. Environ. Microbiol. 2016, 82, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, A.; Spor, A.; Jolivet, C.; Piveteau, P.; Hartmann, A. Biotic and Abiotic Soil Properties Influence Survival of Listeria monocytogenes in Soil. PLoS ONE 2013, 8, e75969. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, H.P.; Casey, P.G.; Cotter, J.; Gahan, C.G.M.; Hill, C. Factors affecting survival of Listeria monocytogenes and Listeria innocua in soil samples. Arch. Microbiol. 2011, 193, 775–785. [Google Scholar] [CrossRef]
- Dowe, M.J.; Jackson, E.D.; Mori, J.G.; Bell, C.R. Listeria monocytogenes survival in soil and incidence in agricultural soils. J. Food Prot. 1997, 60, 1201–1207. [Google Scholar] [CrossRef]
| Trial | Manure Added | Lettuce Planted | Testing (Days from Start) | Notes | |
|---|---|---|---|---|---|
| Soil | Lettuce | ||||
| A | 21/9/18 | 28/9/18 | 0, 7, 12, 19, 28, 35, 42, 50 | 42 * | |
| B | 11/12/18 | 17/12/18 | 0, 6, 16, 27, 38, 49 | 27; 38; 49 * | |
| C | 19/2/19 | 26/2/19 | 0, 7, 14, 30, 37, 50 | 37; 50 * | Boxes with poultry litter re-inoculated at 6 days |
| Treatment | Trial | Moisture Content (%) | E. coli (Log CFU/g) | Salmonella spp. (Log MPN/g) | Listeria spp. (D/ND) |
|---|---|---|---|---|---|
| Poultry litter | A | 43 | <1 | ND | ND |
| B | 30 | <1 to 3.3 | ND | ND | |
| C | 28 | <1 | ND | ND | |
| Poultry litter + E. coli + S. sofia | A | 43 | <1 | >3.0 | ND |
| B | 30 | 4.0 to 4.5 | 1.5 to 3.0 | ND | |
| C | 28 | 4.2 to 4.4 | 1.4 to 3.0 | ND | |
| Cow manure | A | 28 | <1 | ND | ND |
| B | 40 | <1 | ND | ND | |
| C | 36 | <1 | ND | ND | |
| Cow manure + E. coli + L. innocua | A | 28 | 2.8 to 3.0 | ND | Detected |
| B | 40 | 4.7 to 5.2 | ND | Detected | |
| C | 36 | 5.0 to 5.1 | ND | Detected |
| Source | F * | df1 ** | df2 ** | Significance |
|---|---|---|---|---|
| Corrected model | 41.13 | 32 | 29 | 0.000 |
| Trial (season) | 5.685 | 2 | 6 | 0.045 |
| Soil type | 2.513 | 1 | 5 | 0.176 |
| Manure type | 157.973 | 4 | 104 | 0.000 |
| Time | 154.491 | 5 | 19 | 0.000 |
| Manure × time | 60.606 | 20 | 40 | 0.000 |
| Trial | Days after Addition of Manure | |||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 30 | 40 | 50 | |
| A | 0.11 | 0.90 | 0.06 | 0.13 | 0.03 | 0.02 |
| B | 0.84 | 1.00 | 0.72 | 0.87 | 0.55 | 0.44 |
| C | 0.42 | 0.98 | – | – | – | 0.10 |
| Trial | Days from Start | Detections | Treatment |
|---|---|---|---|
| A | 42 | None | – |
| B | 27 | 1 × E. coli log 2.4 CFU/g−1 | Poultry litter |
| 38 | 1 × E. coli log 2.2 CFU/g−1 | Inoculated cow manure | |
| 49 | 1 × E. coli log 4.6 CFU/g−1 | Unamended control | |
| C | 37 | 2 × Listeria spp. detected | Inoculated cow manure |
| 50 | None | – |
| Trial | Clay Loam | Sand | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poultry | Cow | Unamended | Poultry | Cow | Unamended | |||||||
| A | 464.4 | b | 479.0 | b | 534.5 | a | 510.8 | a | 438.3 | b | 388.8 | c |
| B | 629.4 | a | 613.8 | a | 620.8 | a | 664.6 | a | 594.8 | b | 566.7 | b |
| C | 532.0 | ns | 564.8 | ns | 529.2 | ns | 567.6 | a | 298.4 | b | 354.0 | b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekman, J.; Goldwater, A.; Bradbury, M.; Matthews, J.; Rogers, G. Persistence of Human Pathogens in Manure-Amended Australian Soils Used for Production of Leafy Vegetables. Agriculture 2021, 11, 14. https://doi.org/10.3390/agriculture11010014
Ekman J, Goldwater A, Bradbury M, Matthews J, Rogers G. Persistence of Human Pathogens in Manure-Amended Australian Soils Used for Production of Leafy Vegetables. Agriculture. 2021; 11(1):14. https://doi.org/10.3390/agriculture11010014
Chicago/Turabian StyleEkman, Jennifer, Adam Goldwater, Mark Bradbury, Jim Matthews, and Gordon Rogers. 2021. "Persistence of Human Pathogens in Manure-Amended Australian Soils Used for Production of Leafy Vegetables" Agriculture 11, no. 1: 14. https://doi.org/10.3390/agriculture11010014
APA StyleEkman, J., Goldwater, A., Bradbury, M., Matthews, J., & Rogers, G. (2021). Persistence of Human Pathogens in Manure-Amended Australian Soils Used for Production of Leafy Vegetables. Agriculture, 11(1), 14. https://doi.org/10.3390/agriculture11010014