Combined Application of Rhizosphere Bacteria with Endophytic Bacteria Suppresses Root Diseases and Increases Productivity of Black Pepper (Piper nigrum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Biomass production in a bioreactor (liquid product): The growth optimal conditions included a TSB media for 12–24 h for microbial breeding cultivation, and 8 h fermentation at a temperature of 35 °C, 150 rpm/m.
- Producing bacteria powder products: Liquid biomass products were mixed with a carrier material, such as talc, lactose, or corn starch, at a particular ratio, and then were dried on a tray for 5 days. Consequently, the mixed powder product was crushed, homogenized and stored in a sterilized vacuum bag.
2.2. Experimental Methods
- Formula 1: 2.5 g B. velezensis KN12 + 2.5 g B. amyloliquefaciens DL1 + 3.75 g B. velezensis DS29 + 3.75 g B. subtilis BH15 + 6.25 g B. subtilis V1.21 + 6.25 g B. cereus CS30.
- Formula 2: 2.5 g B. velezensis KN12 + 2.5 g B. amyloliquefaciens DL1 + 7.5 g B. velezensis DS29 + 7.5 g B. subtilis BH15 + 10 g B. subtilis V1.21 + 10 g B. cereus CS30.
- Formula 3: 5 g B. velezensis KN12 + 5 g B. amyloliquefaciens DL1 + 3.75 g B. velezensis DS29 + 3.75 g B. subtilis BH15 + 8.75 g B. subtilis V1.21 + 8.75 g B. cereus CS30.
- Formula 4: 5 g B. velezensis KN12 + 5 g B. amyloliquefaciens DL1 + 7.5 g B. velezensis DS29 + 7.5 g B. subtilis BH15 + 12.5 g B. subtilis V1.21 + 12.5 g B. cereus CS30.
- Formula 5: 7.5 g B. velezensis KN12 + 7.5 g B. amyloliquefaciens DL1 + 3.75 g B. velezensis DS29 + 3.75 g B. subtilis BH15 + 11.25 g B. subtilis V1.21; +11.25 g B. cereus CS30.
- Formula 6: 7.5 g B. velezensis KN12 + 7.5 g B. amyloliquefaciens DL1 + 7.5 g B. velezensis DS29 + 7.5 g B. subtilis BH15 + 15 g B. subtilis V1.21 + 15 g B. cereus CS30.
2.3. Sampling Method
2.4. Analytical Methods
2.4.1. Determination of Microbiological Density
2.4.2. Determination of the Density of Fungal Pathogens in the Soil
2.4.3. Measurement of Antagonistic Effect
2.4.4. Measurements of Chlorophyll Content and Photosynthesis Activity
2.4.5. Measurement of Growth Criteria
2.5. Data Analysis
3. Results and Discussion
3.1. Effect of Bio-Products Containing Rhizosphere Bacteria and Endophytic Bacteria on Total Microorganism Density in the Soil
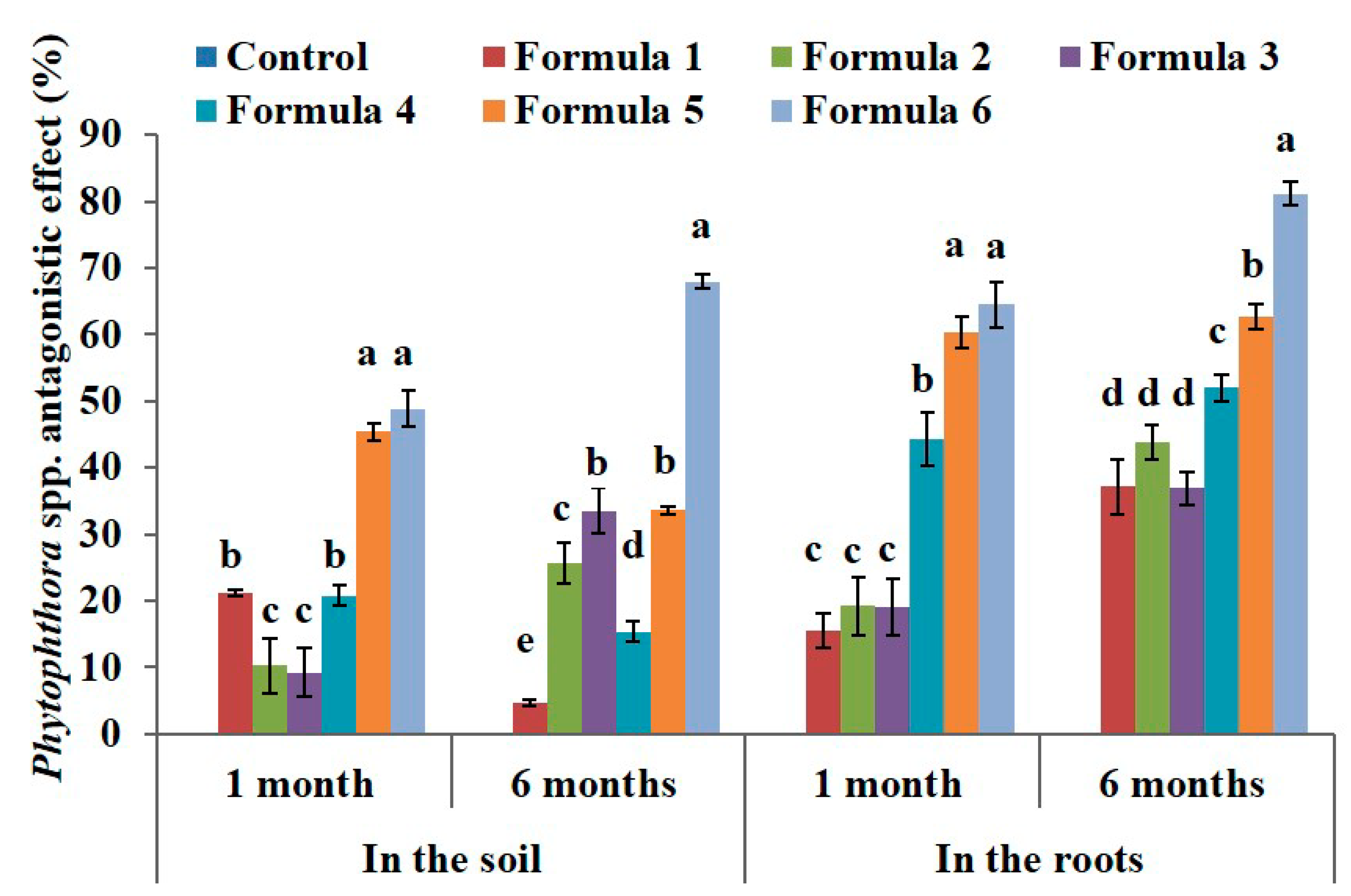
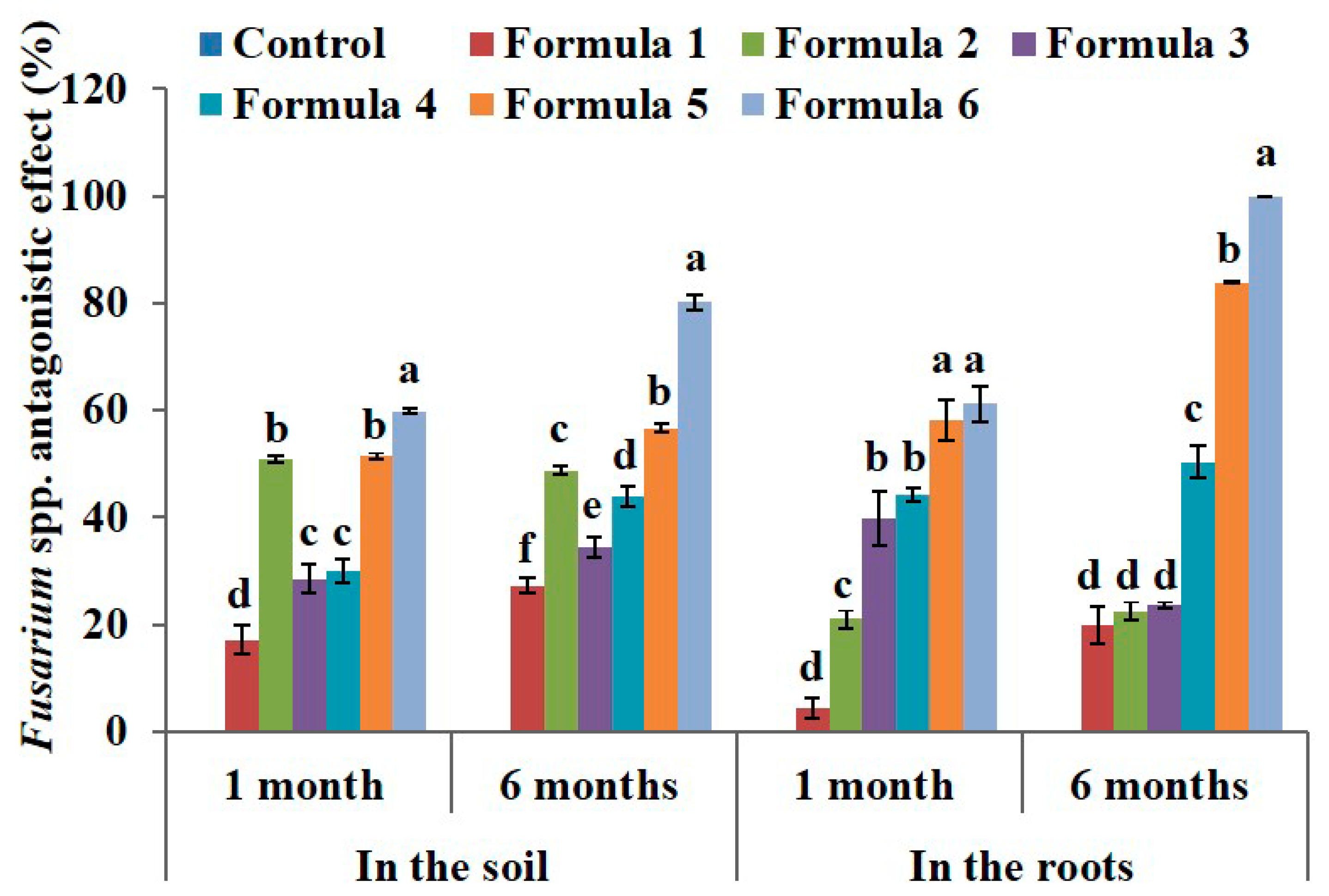
3.2. Effect of Bio-Products Containing Rhizosphere Bacteria and Endophytic Bacteria on Root Pathogens of Black Pepper
3.3. Effect of Bio-Products Containing Rhizosphere Bacteria and Endophytic Bacteria on the Growth of Black Pepper
3.4. Effect of Bio-Products Containing Rhizosphere Bacteria and Endophytic Bacteria on Crop Productivity and Quality of Black Pepper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnamoorthy, B.; Parthasarathy, V.A. Review: Improvement of black pepper. CAB Rev. 2010, 5, 1–12. [Google Scholar] [CrossRef]
- Acharya, S.G.; Momin, A.H.; Gajjar, A.V. Review of piperine as a bio-enhancer. Am. J. Pharm. Tech. Res. 2012, 2, 32–44. [Google Scholar]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Biological role of Piper nigrum L. (black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 3, 1945–1953. [Google Scholar] [CrossRef]
- Vietnam Pepper Association (VPA). Report; VPA: Ho Chi Minh, Vietnam, 2020. [Google Scholar]
- Kueh, T.K.; Sim, S.L. Slow decline of black pepper caused by root knot nematodes. In The International Workshop on Black Pepper Diseases; Wahid, P., Sitepu, D., Deciyanto, S., Superman, U., Eds.; Bander Lampung Indonesia Institute for Spices and Medicinal Crops: Bogor, Indonesia, 1992; pp. 198–207. [Google Scholar]
- Anandaraj, M.; Ramana, K.V.; Sarma, Y.R. Role of Phytophthora capsici in the slow decline disease of black pepper. J. Plantn. Crops 1996, 24, 166–170. [Google Scholar]
- Trudgill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef]
- Aravind, R.; Kumar, A.; Eapen, S.J.; Ramana, K.V. Endobacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: Isolation, identification and evaluation against Phytophthora capsici. Lett. Appl. Microbiol. 2009, 48, 58–64. [Google Scholar] [CrossRef]
- Trinh, T.H.T.; Wang, S.L.; Nguyen, V.B.; Tran, M.D.; Doan, C.T.; Vo, T.P.K.; Huynh, V.Q.; Nguyen, A.D. A potent antifungal rhizobacteria Bacillus velezensis RB.DS29 isolated from black pepper (Piper nigrum L.). Res. Chem. Intermed. 2019, 45, 5309–5323. [Google Scholar] [CrossRef]
- Anith, K.N.; Radhakrishnan, N.V.; Manomohandas, T.P. Screening of antagonistic bacteria for biological control of nursery wilt of black pepper (Piper nigrum). Microbiol. Res. 2003, 158, 91–97. [Google Scholar] [CrossRef]
- Trivedi, P.C. Bacteria in Agrobiology: Disease Management; Maheshwari, D.K., Ed.; Springer: Berlin, Germany, 2013; p. 349. [Google Scholar]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S.; Subedi, A. Sensitivity of Nematode Community Analysis to Agricultural Management Practices and Inoculation with Local Effective Microorganisms in the Southeastern United States. Soil Syst. 2019, 3, 41. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S. Impact of Inoculation with Local Effective Microorganisms on Soil Nitrogen Cycling and Legume Productivity Using Composted Broiler Litter. Appl. Soil Ecol. 2020, 154, 103567. [Google Scholar] [CrossRef]
- Ammar, M.; Hamad, M.; Hussien, S.; Mahmmoud, E.N. The Inhibitory Role of Effective Microorganisms (Em) on the Growth of Pathogenic Bacteria. Iraqi J. Vet. Sci 2020, 34, 153–158. [Google Scholar]
- Bahuguna, A.; Joe, A.-R.; Kumar, V.; Lee, J.S.; Kim, S.-Y.; Moon, J.-Y.; Cho, S.-K.; Cho, H.; Kim, M. Study on the Identification Methods for Effective Microorganisms in Commercially Available Organic Agriculture Materials. Microorganisms 2020, 8, 1568. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; CABI: Wallingford, UK, 2000; pp. 1–2. ISBN 9780851994215. [Google Scholar]
- Sikora, R.A.; Fernández, E. Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; Luc, M., Sikora, R.A., Bridge, J., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 319–376. [Google Scholar]
- Nair, K.P.P. The Agronomy and Economy of Turmeric and Ginger; Elsevier: Amsterdam, The Netherlands, 2013; pp. 139–157. [Google Scholar]
- Wiratno, M.S.; Ankardiansyah, P.P.; Ahmed, I.A.Y. Biological control of root-knot nematode (meloidogyne spp.) in pepper plants utilizing endophytic bacteria Pseudomona ssp. Micrococcussp. J. Pepper Ind. 2018, 9, 11–22. [Google Scholar]
- Nguyen, V.N.; Oh, I.J.; Kim, Y.J.; Kim, K.Y.; Kim, Y.C.; Park, R.D. Purification and characterization of chitinases from Paecilomyces variotii DG-3 parasitizing on Meloidogyne incognita eggs. J. Ind. Microbiol. Biotechnol. 2009, 36, 195–203. [Google Scholar] [CrossRef]
- Vicente, P.C.; Renata, S.C.D.P.; Eduardo, S.F. Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Ciênc. Agrotecnol. Lavras. 2010, 34, 525–535. [Google Scholar]
- Abdelnabby, H.M.; Mohamed, H.A.; AboAly, H.E. Nematode-antagonistic compounds from certain bacterial species. Egypt. J. Biol. Pest Control 2011, 21, 209–217. [Google Scholar]
- Gao, H.; Qi, G.; Yin, R.; Zhang, H.; Li, C.; Zhao, X. Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Sci. Rep. 2016, 24, 28756. [Google Scholar]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Elhady, A.; Giné, A.; Topalovic, O.; Jacquiod, S.; Sørensen, S.J.; Sorribas, F.J.; Heuer, H. Microbiomes associated with infective stages of root-knot and lesion nematodes in soil. PLoS ONE 2017, 12, e0177145. [Google Scholar] [CrossRef]
- Basyony, A.G.; Abo-Zaid, G.A. Biocontrol of the root-knot nematode, Meloidogyne incognita, using an eco-friendly formulation from Bacillus subtilis, lab. and greenhouse studies. Egypt. J. Biol. Pest Control 2018, 28, 87. [Google Scholar] [CrossRef]
- Tran, T.P.H.; Wang, S.-L.; Nguyen, V.B.; Tran, D.M.; Nguyen, D.S.; Nguyen, A.D. Study of Novel Endophytic Bacteria for Biocontrol of Black Pepper Root-knot Nematodes in the Central Highlands of Vietnam. Agronomy 2019, 9, 714. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Wang, S.L.; Trinh, T.H.T.; Tran, T.N.; Nguyen, V.B.; Doan, C.T.; Huynh, V.Q.; Vo, T.P.K. Plant growth promotion and fungal antagonism of endophytic bacteria for the sustainable production of black pepper (Piper nigrum L.). Res. Chem. Intermed. 2019, 45, 5325–5339. [Google Scholar] [CrossRef]
- Nguyen, V.L. Spread of Phytophthora capsici in Black Pepper (Piper nigrum) in Vietnam. Engineering 2015, 7, 506–513. [Google Scholar] [CrossRef]
- Toh, S.C.; Samuel, L.; Awang, A.S.A.H. Screening for antifungal- producing bacteria from Piper nigrum plant against Phytopthora capsici. Int. Food Res. J. 2016, 23, 2616–2622. [Google Scholar]
- Partelli, F.L. Nutrient of black pepper: A Brasilian experience. J. Spice Aromat. Crops 2009, 18, 73–83. [Google Scholar]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Parthasarathy, V.A.; Aipe, K.C. Physico-chemical, biochemical and microbial properties of the rhizospheric soils of tree species used as supports for black pepper cultivation in the humid tropics. Geoderma 2010, 158, 252–258. [Google Scholar] [CrossRef]
- Sheoran, N.; Nadakkakath, A.V.; Munjal, V.; Kundu, A.; Subaharan, K.; Venugopal, V.; Rajamma, S.; Eapen, S.J.; Kumar, A. Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiol. Res. 2015, 173, 66–78. [Google Scholar] [CrossRef]
- Jasim, B.; Jimtha, C.J.; Jyothis, M.; Radhakrishnan, E.K. Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul. 2013, 71, 1–11. [Google Scholar] [CrossRef]
- Munjal, V.; Nadakkakath, V.A.; Sheoran, N.; Kundu, A.; Venugopal, V.; Subaharan, K.; Rajamma, S.; Eapen, S.J.; Kumar, A. Genotyping and identification of broad spectrum antimicrobial volatiles in black pepper root endophytic biocontrol agent, Bacillus megaterium BP17. Biol. Control. 2016, 92, 66–76. [Google Scholar] [CrossRef]
- Ngo, V.A.; Wang, S.-L.; Nguyen, V.B.; Doan, C.T.; Tran, T.N.; Tran, D.M.; Nguyen, A.D. Phytophthora Antagonism of Endophytic Bacteria Isolated from Roots of Black Pepper (Piper nigrum L.). Agronomy 2020, 10, 286. [Google Scholar] [CrossRef]
- Ogunmwonyi, I.N.; Igbinosa, O.E.; Aiyegoro, O.A.; Odjadjare, E.E. Microbial analysis of different top soil samples of selected site in Obafemi Awolowo University, Nigeria. Sci. Res. Essay 2008, 3, 120–124. [Google Scholar]
- Maren, A.K. Identification of common Paenibacillus polymyxa and Bacillus Aspergillus species. In Pulished by the Pumilus Strains from Barley Rhizosphere; Central Bureau Voor Schimmelcutues, FEMS Microbiology Ecology: Utrecht, The Nethrlands, 2002; p. 107. [Google Scholar]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against the brow wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Rajalakshmi, K. Extraction and Estimation of Chlorophyll from Medicinal Plants. Int. J. Sci. Res. 2015, 4, 209–212. [Google Scholar]
- Getman-Pickering, Z.L.; Campbell, A.; Aflitto, N.; Grele, A.; Davis, J.K.; Ugine, T.A. LeafByte: A mobile application that measures leaf area and herbivory quickly and accurately. Methods Ecol. Evol. 2020, 11, 215–221. Available online: https://zoegp.science/leafbyte (accessed on 26 December 2020). [CrossRef]
- Olanrewaju, S.O.; Bernard, R.; Glick, B.R.; Babalola, O.O. Review: Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Lim, S.M.; Yoon, M.-Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Shin, T.S.; Park, H.W.; Yu, N.H.; Kim, Y.H.; Kim, J.-C. Diffusible and Volatile Antifungal Compounds Produced by an Antagonistic Bacillus Velezensis G341 against Various Phytopathogenic Fungi. Plant Pathol. J. 2017, 33, 488–498. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Christopher, A.D.; Michael, J.B.; Sofía, N.C. Bacillus Velezensis Rc 218 as a Biocontrol Agent to Reduce Fusarium Head Blight and Deoxynivalenol Accumulation: Genome Sequencing and Secondary Metabolite Cluster Profiles. Microbiol. Res. 2016, 192, 30–36. [Google Scholar] [CrossRef]
- Cai, X.-C.; Liu, C.-H.; Wang, B.-T.; Xue, Y.-R. Genomic and Metabolic Traits Endow Bacillus Velezensis Cc09 with a Potential Biocontrol Agent in Control of Wheat Powdery Mildew Disease. Microbiol. Res. 2016, 196, 89–94. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, A.D.; Nguyen, Q.V. Biodiversity of Soil Microorganisms and their Effects on Disease Management at Black Pepper Farms in Gia Lai Province. Asian J. Biol. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Mills, N. Biological Control—Ecology and Applications; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control. 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Nguyen, T.C.Q.; Tran, V.H.; Nguyen, T.H.; Le, V.T.; Vu, T.H.; Pham, T.M.T.; Phung, Q.T. SH-BV1—A Bio-product controlling nematodes and root fungal disease of black pepper and coffee in Dak Nong, Gia Lai. In Proceedings of the Second National Conference on Plant Science, Rome, Italy, 21–23 September 2020; pp. 960–965. [Google Scholar]
- Ann, Y.C. Rhizobacteria of Pepper (Piper Nigrum) and Their Antifungal Activities. Afr. J. Microbiol. Res. 2012, 19, 4185–4193. [Google Scholar]
- Jamal, Q.; Lee, Y.S.; Jeon, H.; Park, Y.S.; Kim, K.Y. Isolation and Biocontrol Potential of Bacillus Amyloliquefaciens Y1 against Fungal Plant Pathogens. KJSSF 2015, 48, 485–491. [Google Scholar] [CrossRef]
- Kröber, M.; Verwaaijen, B.; Wibberg, D.; Winkler, A.; Pühler, A.; Schlüter, A. Comparative Transcriptome Analysis of the Biocontrol Strain Bacillus Amyloliquefaciens Fzb42 as Response to Biofilm Formation Analyzed by Rna Sequencing. J. Biotechnol. 2016, 231, 212–223. [Google Scholar] [CrossRef]
- Dastager, S.G.; Deepa, C.K.; Pandey, A. Potential Plant Growth-Promoting Activity of Serratia Nematodiphila Nii-0928 on Black Pepper (Piper Nigrum L.). World J. Microbiol. Biotechnol. 2011, 27, 259–265. [Google Scholar] [CrossRef]
- Samaniego-Gámez, B.; Garruña, R.; Tun-Suárez, J.; Kantun-Can, J.; Reyes-Ramírez, A.; Cervantes-Díaz, L. Bacillus spp. Inoculation Improves Photosystem Ii Efficiency and Enhances Photosynthesis in Pepper Plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef]

| No. | Selected Bacterial Strains | Characteristics and Bioactivities of the Strains | References |
|---|---|---|---|
| 1 | Bacillus velezensis KN12 | Endophytic bacteria, Phytopthora antagonist | [36] |
| 2 | B. amyloliquefaciens DL1 | ||
| 3 | B. velezensis ĐS 29 | Rhizosphere bacteria, Phytopthora antagonist | [9] |
| 4 | B. subtilis BH 15 | ||
| 5 | B. subtilis V1.21 | Plant growth promoting activity | [28] |
| 6 | B. cereus CS30 |
| Treatment | Bio-Product-I (g/stump) | Bio-Product-II (g/stump) |
|---|---|---|
| Control | 0 | 0 |
| Formula 1 | 10 | 15 |
| Formula 2 | 10 | 30 |
| Formula 3 | 20 | 15 |
| Formula 4 | 20 | 30 |
| Formula 5 | 30 | 15 |
| Formula 6 | 30 | 30 |
| Treatment | Total Microorganisms (×107 CFU·g−1) | ||
|---|---|---|---|
| Before Treatment | 1-Month Treatment | 6-Month Treatment | |
| Control | 2.30 ± 0.08 | 3.90 ± 0.06 f | 1.91 ± 0.04 d |
| Formula 1 | 2.26 ± 0.09 | 4.13 ± 0.04 f | 2.57 ± 0.07 b |
| Formula 2 | 2.22 ± 0.16 | 7.75 ± 0.09 e | 2.36 ± 0.13 bc |
| Formula 3 | 1.84 ± 0.15 | 10.1 ± 0.14 d | 2.14 ± 0.04 cd |
| Formula 4 | 1.97 ± 0.04 | 11.5 ± 0.08 b | 2.5 ± 0.05 b |
| Formula 5 | 1.92 ± 0.10 | 11.0 ± 0.09 c | 2.11 ± 0.05 cd |
| Formula 6 | 2.15 ± 0.06 | 16.9 ± 0.17 a | 6.8 ± 0.20 a |
| ANOVA | NS | ** | ** |
| Treatment | Phytophthora Density in the Soil (×102 CFU·g−1) | Phytophthora Density in the Root (×101 CFU·g−1) | ||||
|---|---|---|---|---|---|---|
| Before Treatment | 1-Month Treatment | 6-Month Treatment | Before Treatment | 1-Month Treatment | 6-Month Treatment | |
| Control | 9.82 ± 0.06 b | 9.2 ± 0.12 c | 8.68 ± 0.1 f | 2.23 ± 0.06 | 2.8 ± 0.34 c | 1.94 ± 0.03 f |
| Formula 1 | 9.55 ± 0.07 ab | 7.05 ± 0.09 b | 8.05 ± 0.08 e | 2.25 ± 0.04 | 2.39 ± 0.11 bc | 1.23 ± 0.08 e |
| Formula 2 | 10.83 ± 0.41c | 9.08 ± 0.12 c | 7.11 ± 0.1 d | 2.16 ± 0.05 | 2.19 ± 0.1 b | 1.06 ± 0.07 cd |
| Formula 3 | 10.81 ± 0.45 c | 9.17 ± 0.04 c | 6.33 ± 0.09 c | 2.13 ± 0.06 | 2.17 ± 0.15 b | 1.17 ± 0.05 de |
| Formula 4 | 9.65 ± 0.11 ab | 7.17 ± 0.06 b | 7.22 ± 0.06 d | 2.2 ± 0.08 | 1.53 ± 0.06 a | 0.92 ± 0.04 c |
| Formula 5 | 8.98 ± 0.06 a | 4.59 ± 0.08 a | 5.27 ± 0.08 b | 2.32 ± 0.03 | 1.16 ± 0.07 a | 0.75 ± 0.03 b |
| Formula 6 | 9.5 ± 0.14 ab | 4.54 ± 0.18 a | 2.69 ± 0.08 a | 2.36 ± 0.14 | 1.04 ± 0.04 a | 0.39 ± 0.04 a |
| ANOVA | * | ** | ** | NS | ** | ** |
| Treatment | Fusarium Density in the Soil (×102 CFU·g−1) | Fusarium Density in the Roots (×101 CFU·g−1) | ||||
|---|---|---|---|---|---|---|
| Before Treatment | 1-Month Treatment | 6-Month Treatment | Before Treatment | 1-Month Treatment | 6-Month Treatment | |
| Control | 8.21 ± 0.05 a | 7.23 ± 0.05 g | 5.98 ± 0.09 g | 3.48 ± 0.07 | 3.44 ± 0.11 e | 3.98 ± 0.03 f |
| Formula 1 | 8.46 ± 0.2 a | 6.17 ± 0.09 f | 5.44 ± 0.05 f | 3.28 ± 0.09 | 3.1 ± 0.08 d | 3.01 ± 0.06 d |
| Formula 2 | 9.46 ± 0.12 b | 4.11 ± 0.08 c | 4.29 ± 0.1 c | 3.54 ± 0.06 | 2.77 ± 0.09 c | 3.15 ± 0.07 de |
| Formula 3 | 8.33 ± 0.22 a | 5.24 ± 0.07 d | 4.82 ± 0.05 e | 3.69 ± 0.11 | 2.19 ± 0.12 b | 3.22 ± 0.08 e |
| Formula 4 | 9.19 ± 0.18 b | 5.66 ± 0.08 e | 4.55 ± 0.08 d | 3.55 ± 0.14 | 1.96 ± 0.04 b | 2.01 ± 0.06 c |
| Formula 5 | 8.28 ± 0.1 a | 3.54 ± 0.06 b | 3.17 ± 0.06 b | 3.67 ± 0.09 | 1.53 ± 0.16 a | 0.68 ± 0.02 b |
| Formula 6 | 8.47 ± 0.19 a | 3.00 ± 0.07 a | 1.48 ± 0.08 a | 3.48 ± 0.11 | 1.33 ± 0.07 a | 0.0 ± 0.0 a |
| ANOVA | ** | ** | ** | NS | ** | ** |
| Treatment | Chl.a (mg·g−1 FW) | Chl.b (mg·g−1 FW) | Photosynthesis Intensity (μmol·m−2·s−1) |
|---|---|---|---|
| Control | 0.41 ± 0.001 g | 0.37 ± 0.001 e | 8.9 ± 0.91 c |
| Formula 1 | 0.52 ± 0.002 c | 0.33 ± 0.003 f | 10.4 ± 0.56 bc |
| Formula 2 | 0.49 ± 0.002 e | 0.38 ± 0.002 d | 10.87 ± 0.94 abc |
| Formula 3 | 0.45 ± 0.001 f | 0.42 ± 0.003 b | 10.43 ± 0.3 bc |
| Formula 4 | 0.50 ± 0.00 d | 0.40 ± 0.002 c | 10.4 ± 1.26 bc |
| Formula 5 | 0.56 ± 0.002 b | 0.41 ± 0.001 b | 11.9 ± 0.25 ab |
| Formula 6 | 0.57 ± 0.001 a | 0.43 ± 0.003 a | 13.03 ± 0.43 a |
| ANOVA | ** | ** | * |
| Treatment | Branch Length Increased (cm) | The Number of Increased Branches | Leaf Area (cm2) | The Percentage of Yellowing Symptom (%) |
|---|---|---|---|---|
| Control | 6.56 ± 0.29 | 2.44 ± 0.99 | 48.13 ± 0.89 b | 14.44 ± 1.11 d |
| Formula 1 | 10.19 ± 2.08 | 1.78 ± 0.44 | 52.56 ± 8.48 ab | 7.78 ± 1.11 bc |
| Formula 2 | 8.33 ± 1.61 | 3.67 ± 1.02 | 63.09 ± 3.78 ab | 11.11 ± 1.11 cd |
| Formula 3 | 10.25 ± 1.5 | 4.00 ± 0.38 | 52.83 ± 1.36 ab | 7.78 ± 1.11 bc |
| Formula 4 | 7.49 ± 1.09 | 1.33 ± 0.33 | 65.01 ± 7.70 a | 6.67 ± 1.92 ab |
| Formula 5 | 10.00 ± 0.19 | 3.56 ± 0.87 | 67.25 ± 2.57 a | 5.56 ± 1.11 ab |
| Formula 6 | 10.83 ± 0.25 | 4.56 ± 0.87 | 64.49 ± 1.33 a | 3.33 ± 0 a |
| ANOVA | NS | NS | * | ** |
| Treatment | Weight of 10 Spikes (g) | Number of Fruit/Spike (Fruit) | The Ratio of the Pepper Kernel | Dry Yield (Ton/ha) | (Weight g/L) |
|---|---|---|---|---|---|
| Control | 36.52 ± 1.85 d | 29.94 ± 2.42 | 0.434 ± 0.0043 | 3.30 ± 0.03 c | 573.33 ± 1.33 c |
| Formula 1 | 45.48 ± 0.42 bc | 39.42 ± 0.88 | 0.447 ± 0.0005 | 3.37 ± 0.02 b | 583.33 ± 2.85 abc |
| Formula 2 | 42.38 ± 0.72 c | 35.78 ± 2.45 | 0.435 ± 0.0115 | 3.43 ± 0.01 a | 586.00 ± 2.52 ab |
| Formula 3 | 47.94 ± 1.94 b | 37.75 ± 1.52 | 0.434 ± 0.0113 | 3.40 ± 0.01 ab | 582.33 ± 1.20 bc |
| Formula 4 | 46.18 ± 1.68 bc | 35.92 ± 4.32 | 0.454 ± 0.0105 | 3.42 ± 0.01 ab | 590.33 ± 6.39 ab |
| Formula 5 | 54.74 ± 1.69 a | 39.47 ± 1.08 | 0.453 ± 0.0086 | 3.40 ± 0.02 ab | 583.33 ± 2.91 abc |
| Formula 6 | 55.90 ± 0.73 a | 41.78 ± 1.93 | 0.489 ± 0.0095 | 3.45 ± 0.01a | 593.67 ± 1.20 a |
| ANOVA | ** | NS | NS | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, S.D.; Trinh, T.H.T.; Tran, T.D.; Nguyen, T.V.; Chuyen, H.V.; Ngo, V.A.; Nguyen, A.D. Combined Application of Rhizosphere Bacteria with Endophytic Bacteria Suppresses Root Diseases and Increases Productivity of Black Pepper (Piper nigrum L.). Agriculture 2021, 11, 15. https://doi.org/10.3390/agriculture11010015
Nguyen SD, Trinh THT, Tran TD, Nguyen TV, Chuyen HV, Ngo VA, Nguyen AD. Combined Application of Rhizosphere Bacteria with Endophytic Bacteria Suppresses Root Diseases and Increases Productivity of Black Pepper (Piper nigrum L.). Agriculture. 2021; 11(1):15. https://doi.org/10.3390/agriculture11010015
Chicago/Turabian StyleNguyen, Sy Dinh, Thi Huyen Trang Trinh, Trung Dzung Tran, Tinh Van Nguyen, Hoang Van Chuyen, Van Anh Ngo, and Anh Dzung Nguyen. 2021. "Combined Application of Rhizosphere Bacteria with Endophytic Bacteria Suppresses Root Diseases and Increases Productivity of Black Pepper (Piper nigrum L.)" Agriculture 11, no. 1: 15. https://doi.org/10.3390/agriculture11010015
APA StyleNguyen, S. D., Trinh, T. H. T., Tran, T. D., Nguyen, T. V., Chuyen, H. V., Ngo, V. A., & Nguyen, A. D. (2021). Combined Application of Rhizosphere Bacteria with Endophytic Bacteria Suppresses Root Diseases and Increases Productivity of Black Pepper (Piper nigrum L.). Agriculture, 11(1), 15. https://doi.org/10.3390/agriculture11010015








