Abstract
The effect of housing system on ovulation rate, leptin concentration, body weight, condition score and litter size of Żelażnieńska ewes was investigated. The observations were carried out during three successive years on 36 ewes between 2 and 4 years of age. The animals were divided into groups: the experimental group kept in a cold environment under an overhead shelter, and the control group kept in a warm barn. In both groups were ewes with similar age and reproductive performance nearing that of the flock. The average litter size was 1.53 and 1.59, respectively. This difference was not significant. The observation was carried out on the same ewes each year; thus, ewe age varied from 2 to 6 years. The ovulation rate was tasted by laparoscopy (L) on 16 September and 5 October. Blood was taken from each ewe after feeding one day before laparoscopy. The lower number of corpus lutea (p ≤ 0.01) and leptin concentration (p ≤ 0.01) at first L compared with second L was confirmed. The highest litter size (1.8) was shown by ewes at the age of 4 years (p ≤ 0.01). The Pearson correlation of ewe age and plasma leptin concentration was noted (p ≤ 0.05). The Spearman correlation of the condition score with ewe body weight (p ≤ 0.01) and with ewe age (p ≤ 0.05) was confirmed. Żelaźnieńska sheep may be housed in semiopen sheds with no negative impact on their reproduction. This may encourage breeders to develop this branch of livestock production avoiding the high expenses of construction of massive, warm barns.
1. Introduction
Local sheep breeds are capable of sustaining extensive farming conditions [1,2]. In Poland, one of these breeds is the Żelaźnieńska sheep, a cross of local Łowicz ewes with Leicester and Polish Merino rams. Since 1955, it has been selected for reproductive performance. The body weights of ewes and rams are 65 and 95 kg, respectively. Female and males reach sexual maturity at the age of 7 months. The average age at first parturition is 24 months. The average lifetime fecundity expressed as the number of lambs weaned per ewe per year reaches 1.5 [3,4].
Leptin, mainly produced in adipose tissue, is a protein involved in the central and/or peripheral regulation of body homeostasis, energy intake, storage and expenditure, fertility and immune functions [5]. Plasma leptin is released mainly from the adipose tissue and correlates with body fat [6,7,8]. As the adiposity count and mass increase, the peripheral concentration of leptin increases as well [9,10]. Authors [11] also stated that adiposity size may influence leptin synthesis and secretion due to larger cells containing more leptin mRNA. Once the animal reaches its mature size, most subsequent growth occurs in the form of adiposities; thus, it might be linked to the elevated concentration of plasma leptin [12,13].
Leptin has been considered as the key link between nutrition and reproduction, like the appropriate signal to inform the reproductive system about the metabolic status [14,15]. The stimulation by leptin of the hypothalamic–gonadotropic axis in ruminant species is observed predominantly in animals pre-exposed to a negative energy balance [16]. The ruminants (cattle, sheep and goats) in comparison to monogastric species are much less sensitive to nutritional deprivation due to the fact that they derive metabolizable energy primarily from volatile fatty acid production in the rumen [16]. The environmental conditions are important factors influencing reproductive performance [16,17]. Sheep kept in warm barn pens spend less energy on body warming, and thus store it up as a fat tissue [12,18,19]. Conversely, cold environments increase the maintenance requirements [20] and may reduce fat deposition. Cold environmental stress is known to cause foetal death in certain species [21,22,23]. However, an increase or decrease in the umbilical and/or uterine blood flow would respectively increase or decrease the rate at which the foetus loses heat via the placental rout [24]. The new trend in livestock production in Poland and in other European Union countries is to move from intensive to extensive management systems attributed with the maintenance of animals in semiopen sheds [1,2,25].
In this context, the objective of the presented study was to investigate whether the housing of ewes affects their reproductive performance and to improve the understanding of leptin′s effects, and also to see how husbandry strategies may integrate the adaptive capacities of sheep to their environment.
2. Materials and Methods
2.1. Climatic Conditions
The investigation was carried out in three successive years at the Sheep Experimental Station located in central Poland, with the mean annual temperature of 7.5 °C and the mean annual precipitation of 528 mm. The mean maximum temperature over 10 years in spring, summer, autumn and winter reached 23, 33, 23 and 11 °C, respectively, and the mean minimum temperature during these periods of the year dropped to −3, 6, −3 and −10 °C, respectively [26].
2.2. Animals
This study was approved by the third Local Animal Ethics Committee in Warsaw (25/2016).
The experiment was conducted on 36 ewes of Żelaźnieńska bred during 3 successive years.
In the first year of the experiment, in January, 36 ewes between 2 and 4 years of age were chosen from the flock. The animals were divided into two groups: experimental and control. In each group, there were 6 sheep of either 2, 3 or 4 years of age with reproductive performances compatible with the flock average. Body weight and condition score were evaluated on the day before the first laparoscopy as well as after weaning. The experimental group was kept under an overhead shelter (18 ewes), and the control one in a barn (18 ewes). The observation was carried out on the same ewes each year. Thus, during the three years of observation, ewe age varied from 2 to 6 years. In both groups, there were ewes with similar age and reproductive performance nearing that of the flock. The average litter size of the chosen ewes, expressed as a number of born lambs per number of lambing events, was 1.53 in the experimental group and 1.59 in the control one. This difference was not statistically significant. The rams came from ewes of the Żelaźnieńska breed, the reproductive performance of which was higher than the herd′s average.
2.3. Housing and Diet
During the experiment, the treated group was kept under an overhead shelter and the control one was kept in a barn. Both groups were kept on deep litter. The surface per ewe in both the overhead shelter and in the barn was 2 m2.
The overhead shelter was constructed of three wooden walls, with a wire netting and open front on the southern side, and an uninsulated tin roof. The control group was kept in the barn made of bricks with a tin ridge roof, equipped with a usable loft and gravity ventilation. The temperature never dropped below 12 °C at 75% relative humidity, while the winter air temperature under the overhead shelter dropped down to −10 °C.
All ewes were clipped to a coat length of about 5 mm one month before the lambing season.
The ewes were fed with farm-produced fodder (grass hay, corn grain, ground rapeseed, wheat bran, red carrot), and in the summer feeding period, both groups grazed 8 h per day in separate paddocks on the same pasture. During flushing and mating, both groups were fed with a fodder where one dose contained 220 g of protein and 7.6 MJ; during the fifth month of pregnancy, one dose contained 278 g of protein and 10.5 MJ; and during nursing, one dose contained 358 g of protein and 11.9 MJ.
Body condition score (BCS-5) of ewes on a five-point scale were assessed during the first laparoscopy and after weaning in each year.
2.4. Laparoscopy Procedures
The mating season was held at the typical term for that breed (September/October), implying parturition at the turn of February and March. The aim of the laparoscopy was to evaluate the ovarian activities during the reproductive season within three successive years. The first laparoscopy took place at the beginning of the reproduction season on 16 September 2017–2019, and the following one after 20 days. Each year, observation was carried out on the same ewes. The sheep were fasted for a period of 24 h before being placed on a table, and were given both anaesthetic (0.1 mL/kg of body weight) and atropine (0.3 mL/head) to help to prevent aspiration of saliva. Introduction of a laparoscope into a body cavity plus a small amount of air and then the manipulator enabled the retrieval of the ovaries. After the observation, the wound created by the introduction of the endoscope was closed with Michel′s buckle, and liquid penicillin was administered protectively (2 mL/head).
After the second laparoscopy, held on 5 October, the vaginal sponges were implemented (40 mg Cronolone) to synchronize the oestrus. The sponges were removed after 2 weeks, and then ewes were kept in the harems for one month (4 ewes per ram). The rams were at the age of 3–5 years. The younger ewes were in pens with older rams, whereas older ones were kept with younger rams. The pregnancy rates were 100%.
2.5. Plasma Leptin Immunoassays
Blood sampling and hormone assay blood samples were taken through the jugular from each ewe one day before laparoscopy at 10 am, and 2 h after feeding. Plasma was stored at the temperature of −20 °C. Plasma leptin concentrations were analysed with a specific enzyme immunoassay that has been validated for use in several ungulate species, including ovine samples [27]. The interassay coefficient of variation was 13.9%, and the limit of detection was 0.3 ng/mL.
2.6. Statistical Analysis
The distribution of corpora lutea and condition depending on the maintenance system and the observation time were tested using the nonparametric chi-square test. The data for plasma leptin concentration were analysed statistically by the analysis of variance and Tukey′s test of the SPSS 21.0 package software. The equation used was
where Yijkl is the dependent variable; μ the general mean; Ai the effect of the maintenance system (i = overhead shelter or barn); Bj the effect of period (j = 1st or 2nd laparoscopy); Ck the year of experiment; (Ai × Bj) the interaction between period and maintenance system; (Ai × Ck) the interaction between maintenance system and year of experiment; (Bj × Ck) the interaction period and year of experiment; and eijkl the random error.
Yijkl = μ + Ai + Bj + Ck + (Ai × Bj) + (Ai × Ck) + (Bj × Ck) + eijkl
The data for reproductive performance was analyzed using the following equation:
where Yijkl is the dependent variable, μ the general mean, Ai the effect of maintenance system (i = overhead shelter or barn), Bj the effect of ewe age (j = 2, 3, 4, 5 or 6 years), Ck the year of experiment, (Ai × Bj) the interaction between the maintenance system and ewe age, (Ai × Ck) the interaction between the maintenance system and year of experiment, and eijkl the random error.
Yijkl = μAi + Bj + Ck + (Ai × Bj) + (Ai + Ck) + eijkl
The relationships between the serum concentrations of leptin and ewe body weight and ewe age were quantified by the Pearson correlation coefficients. The relationships between serum concentrations of leptin and condition and litter size were evaluated by the Spearman correlation coefficients.
3. Results
In three years, during the second laparoscopy, the level of leptin increased on average by 1.48 ng/mL in comparison to the first one (p ≤ 0.01).
The distribution of corpora lutea in two successive cycles was significantly different (p ≤ 0.01). On 16 September, the number of corpora lutea was lower (Figure 1).
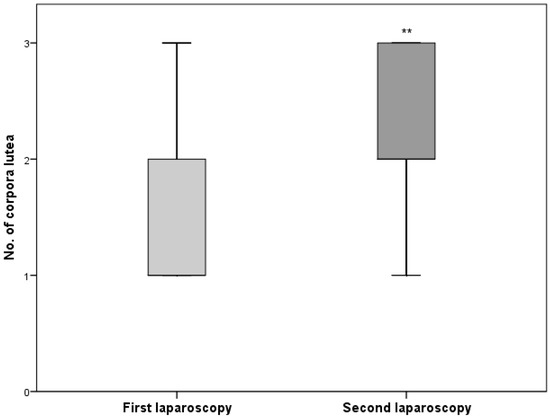
Figure 1.
The median distribution of corpora lutea in two successive laparoscopies. ** p ≤ 0.01.
In the current experiment, sheep kept in a cold environment were characterized by a lower number of corpora lutea in comparison to those staying in the warm barn (p ≤ 0.05). The distribution of corpora lutea in ewes kept under the overhead shelter and in the barn is demonstrated in Figure 2.
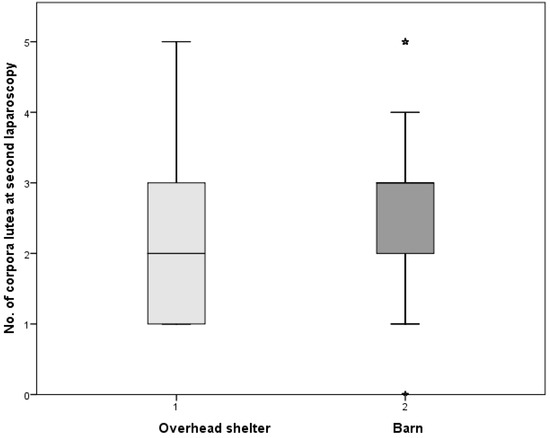
Figure 2.
The median distribution of corpora lutea in ewes kept under the overhead shelter and in the barn. * p ≤ 0.05.
There were no differences in the leptin concentration in the peripheral blood of ewes between the years of the experiment. During the second laparoscopy, the level of leptin increased in comparison to the first one (p ≤ 0.01). The ewes kept in the barn secreted more leptin compared to the experimental group, but the significance of the difference was not confirmed (Table 1). However, the interaction between the number of corpora lutea and leptin concentration in the year of observation and housing showed no differences.

Table 1.
The plasma leptin concentration, ewe body weight, body condition score and litter size of ewe kept under the overhead shelter and in the barn.
The analysis of litter size distribution indicated the best reproductive potential of ewes at the age of 4 years (Table 2). The litter size of 4-year-old ewes was 1.8 lambs and was higher in comparison to 2- and 3-year-old (p ≤ 0.01) and 5- and 6-year-old mothers (p ≤ 0.05). The youngest ewes secreted significantly less plasma leptin compared to other age groups (p ≤ 0.01). The difference between primiparous and 4-, 5- and 6-year-old ewes was significant (p ≤ 0.01).

Table 2.
The distribution of number of ewes in age groups, average plasma leptin concentration, litter size and live-born lambs of the ewes by age.
The correlation of leptin concentration with ewe age (p ≤ 0.05) as well as the litter size (p ≤ 0.01) was confirmed (Table 3).

Table 3.
Correlation of leptin concentration, litter size and body condition score with ewe age and ewe body weight.
The body weight and body condition scores were lower in the experimental group (p ≤ 0.05). The average body weight of ewes kept under the overhead shelter was 3.3 kg lower than those kept in the barn (Table 1). The decreasing body condition score of ewes, from 3.4 down to 2.7, kept under the overhead shelter in the second year of the experiment was noted. The difference was significant in the ewes of 2, 5 and 6 years of age (p ≤ 0.01).
The correlation of the body condition score with ewe body weight (p ≤ 0.01) and with ewe age (p ≤ 0.05) was confirmed (Table 3). However, in the following year, the interaction of the year, ewe age and maintenance system was not significant.
4. Discussion
Plasma melatonin concentration is a passive signal that provides information to the hypothalamus–pituitary–gonadal axis concerning the time of the year. Sheep are short-day breeders; their reproductive activity takes place when the length of daylight decreases [28]. The mating season for Żelaźnieńska sheep takes place in the autumn. The first laparoscopy was done at the beginning of the breeding season, which was characterized by smaller ovarian activity. The shortening day length caused an increase in the number of corpora lutea, which was observed during the second laparoscopy made 20 days later (Figure 1). Similar results were obtained by the authors in conducting an experiment on Polish lowland sheep [29]. In the current experiment, sheep kept in a cold environment were characterized by a lower number of corpora lutea in comparison to those staying in the warm barn (p ≤ 0.05). The distribution of corpora lutea in ewes kept under the overhead shelter and in the barn is shown in Figure 2. Leptin plays an important role in signalling nutritional status to the central reproductive axis of mammals and appears to be a permissive factor in the initiation of puberty at least [16,30]. The expression and secretion of leptin are correlated with body fat mass [6,7,8]. The body weight and body condition scores (Table 1) were lower for the ewes kept in the cold environment (p ≤ 0.05). Both groups received the same fodder (quantity and quality), but those from the semiopen shed needed more energy for body heating, which translated into a decrease in fat deposition. The increasing of leptin concentration during the second laparoscopy in comparison to the first one (p ≤ 0.01) could influence the larger number of corpora lutea observed in the second laparoscopy (p ≤ 0.01), as well as the greater ovarian activity in the group kept in the barn (Figure 2).
Leptin modulates a diverse range of biological functions, including energy homeostasis and reproduction [15,31,32]. The plasma leptin has a direct effect on the ovary, being a potent inhibitor of ovarian steroidogenesis [31,33]. It has also been shown to affect oocyte maturation [34], follicle rupture and corpus luteum formation [35]. Other authors, including Zhang et al. [36], have suggested that the role of leptin can be mediated by divergent modulation by gonadotropins.
The analysis of litter size distribution indicated the best reproductive potential of ewes at the age of 4 years (Table 2). These results are in accordance with findings of other authors [29,37]. In young females, the smaller litter size and notably lower number of corpora lutea were probably affected by lower levels of the hormone [37]. Pregnancy is an important factor that determines the amount of leptin in the bloodstream and depends on the number of foetuses, presenting higher values in the case of a multiple pregnancy [38]. It has also affected embryo implantation and pregnancy [16]. The lower concentration of leptin in young ewes (Table 2) could influence the fewer number of newborn lambs in 2- and 3-year-old ewes in comparison to older mothers. The difference between primiparous and 4-, 5- and 6-year-old ewes was significant (p ≤ 0.01). The leptin concentration may influence the number of embryo implantation events, resulting in the bigger litter size, which was confirmed by the correlation (Table 3) of leptin concentration and the litter size (p ≤ 0.01).
The body weight and body condition scores were lower in the ewes kept in the cold environment (p ≤ 0.05). Both groups received the same fodder (quantity and quality), but those from the semiopen shed needed more energy for body heating, which translated into a decrease of fat deposition [15]. Thermoregulatory strategies used by the pregnant ewe for thermoregulation during heat or cold exposure appear to protect the foetus from changes in its thermal environment [22]. The pregnant ewes may have access to other means of maintaining thermal homeostasis of their progeny during maternal cold exposure; for example, vasoconstriction of uterine and in-skin vessels [39]. Changes in the maternal metabolic environment may be important in enabling the newborn to effectively adapt to the extrauterine environment [23]. The observed lack of differences in reproductive performance between the ewes kept in the barn and under the overhead shelter as well as the survival rates of lambs reared in both maintenance systems confirms a good ability of Żelaźnieńska sheep to adapt to cold environments.
The correlation of the condition score with ewe body weight (p ≤ 0.01) and with ewe age (p ≤ 0.05) was confirmed (Table 3). This is in accordance with the findings of other authors [40,41]. Leptin is of prime importance in the regulation of metabolism and reduces body weight and increases energy expenditure [10]. According to some authors [3,13], leptin is a signal arising from adipose tissue which directly influences the central nervous system and peripheral organs, resulting in a better adaptation of body metabolism and physiological functions to the availability of metabolic energy. The interaction of the year, age of ewe and maintenance system was not confirmed, which may indicate good adaptation of the ewes to difficult environmental conditions.
5. Conclusions
The lack of difference between the plasma leptin and litter size of ewes kept under the overhead shelter and in the barn indicated a good adaptability of Żelaźnieńska sheep to harsh environmental conditions. The type of housing did not translate into a decrease of the ewes′ reproductive performance. The low temperature did not translate into a decrease of the ewes′ prolificacy. The local sheep breeds may be housed in semiopen sheds with no negative impact on the reproduction characteristics. This may encourage breeders to develop this branch of livestock production, avoiding the high expenses of construction and depreciation of massive, warm barns.
Author Contributions
Conceptualization, E.K., M.G. and M.K.-S.; methodology, E.K., M.G. and M.K.-S.; formal analysis, E.K. and M.K.-S.; investigation, E.K. and M.G.; resources, E.K.; data curation, E.K. and M.K.-S.; writing—original draft preparation, E.K.; writing—review and editing, E.K. and M.G.; visualization, M.K.-S.; supervision, E.K.; project administration, E.K. and M.G.; funding acquisition, E.K. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the State Committee for Scientific Research and cofinanced by the European Union from the European Regional Development Fund within the Innovative Economy Operational Programme.
Acknowledgments
We thank our project partners, the Sheep Experimental Station of Life Science University and the Institute of Genetics and Animal Breeding. The authors would like to thank Wladyslaw Janikowski for the English text correction.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dwyer, C.M.; Lawrence, A.B. A review of the behavioral and physiological adaptations of hill and lowland breeds of sheep that favor lamb survival. Appl. Anim. Behav. Sci. 2005, 92, 235–260. [Google Scholar] [CrossRef]
- González-García, E.; Gozzo de Figuereido, V.; Foulquie, D.; Jousserand, E.; Autran, P.; Camous, S.; Tesniere, A.; Bocquier, F.; Jouven, M. Circannual body reserve dynamics and metabolic profile changes in Romane ewes grazing on rangelands. Domest. Anim. Endocrin. 2014, 46, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kuźnicka, E. The effect of forced walking on slaughter value and meat quality of lambs: Linking up the meat chain: Ensuring quality and safety for the consumer. Anim. Sci. 2006, 1, 162–164. [Google Scholar]
- Kuźnicka, E.; Rant, W. The Ewe’s Reproductive Performance, Growth Rate and Carcass Quality of Lambs Kept ina Barn vs Those Kept under an Overhead Shelter. Asian Aust. J. Anim. Sci. 2013, 26, 211–217. [Google Scholar] [CrossRef]
- Chilliard, Y.; Delavaud, C.; Bonet, M. Leptin expression in ruminants: Nutritional and physiological regulations in relation with energy metabolism. Domest. Anim. Endocrin. 2005, 29, 3–22. [Google Scholar] [CrossRef]
- Altmann, M.; Sauerwein, H.; von Borell, E. Relationship between plasma leptin concentrations and car-cass composition in fattening mutton: A comparison withultrasound results. J. Anim. Physiol. Anim. Nutr. 2005, 89, 326–330. [Google Scholar] [CrossRef]
- Altmann, M.; Sauerwein, H.; von Borell, E. Plasmaleptin in growing lambs as a potential predictor for carcasscomposition and daily gain. Meat Sci. 2006, 74, 600–604. [Google Scholar] [CrossRef]
- Kuźnicka, E.; Gabryszuk, M.; Kunowska-Slósarz, M.; Gołębiewski, M.; Balcerak, M. Plasma leptin as a predictor for carcass compositionin growing lambs. Can. J. Anim. Sci. 2017, 97, 193–198. [Google Scholar]
- Considine, R.V. Weight regulation, leptin and growth hormone. Horm. Res. 1997, 48, 116–121. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Leptin. Ann. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef]
- Auwerx, J.; Staels, B. Leptin. Lancet 1998, 351, 737–742. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Ortigues-Marty, I.; Pethick, D.; Herpin, P.; Fernandez, X. Nutritional and hormonal regulation of energy metabolism in skeletal muscles of meat-producing animals. Livest. Prod. Sci. 1998, 56, 115–143. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Ortigues-Marty, I.; Vermorel, M. Manipulation of tissue energy metabolism in meatproducing ruminants—Review. Asian Austral. J. Anim. 2001, 14, 720–732. [Google Scholar] [CrossRef]
- Houseknecht, K.L.; Baile, C.A.; Matteri, R.L.; Spurlock, M.E. The biology of leptin: A review. J. Anim. Sci. 1998, 76, 1405–1420. [Google Scholar] [CrossRef]
- Clarke, L.J.; Henry, B.A. Leptin and reproduction. Rev. Reprod. 1999, 4, 48–55. [Google Scholar] [CrossRef]
- Zieba, D.A.; Amstalden, M.; Williams, G.L. Regulatory roles of leptin in reproduction and metabolism: A comparative review. Domest. Anim. Endocrin. 2005, 29, 166–185. [Google Scholar] [CrossRef]
- Lassoued, N.; Rekik, M.; Mahouachi, M.; Hamouda, M.B. The effect of nutrition prior to and during mating on ovulation rate, reproductive wastage and lambing rate in three sheep breeds. Small Ruminant Res. 2004, 52, 117–125. [Google Scholar] [CrossRef]
- Symonds, M.E.; Brayant, M.J.; Clarke, L.; Darby, C.J.; Lomax, M.A. Effect of maternal cold exposure on brown adipose tissue and thermogenesis in the neonatal lamb. J. Physiol. 1992, 455, 487–502. [Google Scholar] [CrossRef]
- Asakuma, S.; Morishita, H.; Sugino, T.; Kurose, Y.; Kobayashi, S.; Terashima, Y. Circulating leptin response to feeding and exogenous infusion of insulin in sheep exposed to thermoneutral and cold environments. Comp. Biochem. Physiol. Part A 2003, 134, 329–335. [Google Scholar] [CrossRef]
- Young, B.A. Ruminant cold stress: Effect on production. J. Anim. Sci. 1983, 57, 1601–1607. [Google Scholar] [CrossRef]
- Wentzel, D.K.; Viljoen, S.; Both, J.J. Physiological and endocrinological reactions to cold stress in the Angora goat. Agro Anim. 1997, 11, 19–22. [Google Scholar]
- Laburn, H.P.; Faurie, A.; Goelst, K.; Mitchell, D. Effects on fetal and maternal body temperatures of exposure of pregnant ewes to heat, cold, and exercise. J. Appl. Physiol. 2002, 92, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, D.P.; Mostyn, A.; Wilson, V.; Pearce, S.; Alves-Guerra, M.C.; Pecqueur, C.; Miroux, B.; Budge, H.; Stephenson, T.; Symonds, M.E. Different effects of maternal parity, cold exposure andnutrient restriction in late pregnancy on the abundance of mitochondrial proteins in kidney, liver and lung of postnatal sheep. Soc. Reprod. Fertil. 2007, 133, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.J.; Power, G.G. Engine and radiation: Fetal and placental interactions for heat dissipation. Exp. Physiol. 1997, 82, 403–414. [Google Scholar] [CrossRef]
- Kuźnicka, E.; Rant, W. Comparison of milk production and lamb growth in Żelaźnieńska sheep kept in a barn or under overhead shelter. Ann. Anim. Sci. 2008, 8, 175–183. [Google Scholar]
- IMGW. Institute of Meteorology and Water Management, Warsaw, Poland. Available online: www.imgw.pl (accessed on 10 April 2018).
- Sauerwein, H.; Heintges, U.; Hennies, M.; Selhorst, T.; Daxenberger, A. Growth hormone induced alterations of leptin serum concentrations in dairy cows as measured by a novel enzyme immunoassay. Livest. Prod. Sci. 2004, 87, 189–195. [Google Scholar] [CrossRef]
- Abecia, J.A.; Forcada, F.; Gonzales-Bulnez, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef]
- Kuźnicka, E.; Rant, W.; Radzik-Rant, A.; Kunowska-Slósarz, M.; Balcerak, M. The ovulation rate, plasma progesterone and estradiol concentration, and litter size of a local ewe breed kept in a barn vs. those kept under an overhead shelter. Archi. Anim. Breed. 2016, 59, 145–150. [Google Scholar] [CrossRef]
- Zieba, D.A.; Amstalden, M.; Maciel, M.N.; Keisler, D.H.; Raver, N.; Gertler, A.; Williams, G.L. Divergent effects of leptin on luteinizing hormone and insulin secretion are dose dependent. Exp. Biol. Med. 2003, 228, 325–330. [Google Scholar] [CrossRef]
- Spicer, L.J. Leptin: A possible metabolic signal affecting reproduction. Domest. Anim. Endocrin. 2001, 21, 251–270. [Google Scholar] [CrossRef]
- Spicer, L.J.; Francisko, C.C. The adipose obese gene product, leptin: Evidence of a direct inhibitory role in ovarian function. Endocrinology 1997, 138, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Kendall, N.R.; Gutierrez, C.G.; Scaramuzzi, R.J.; Baird, D.T.; Webb, R.; Campbell, B.K. Direct in vivo effects of leptin on ovarian steroidogenesis in sheep. Reproduction 2004, 128, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.; Zhu, H.; Dyce, P.W.; Petrik, J.; Li, J. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway. Endocrinology 2004, 145, 5355–5363. [Google Scholar] [CrossRef]
- Ruiz-Cortés, Z.T.; Martel-Kennes, Y.; G’evry, N.Y.; Downey, B.R.; Palin, M.F.; Murphy, B.D. Biphasic effects of leptin in porcine granulosa cells. Biol. Reprod. 2003, 68, 789–796. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Ma, H.; Qu, J.; Wang, Y.; Hou, L.; Liu, L.; Wu, X. The impairment of reproduction in db/db mice is not mediated by intraovarian efective leptin signaling. Fertil. Steril. 2012, 97, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Ishwar, A.K.; Mishra, S.N. Estimation of steroidal hormones in ovine during different stages of pregnancy. Vet. Pract. 2012, 13, 93–94. [Google Scholar]
- Kulcsar, M.; Danko, G.; Magdy, H.G.I.; Reiczigel, J.; Forgach, T.; Prohaczik, A.; Delavaud, C.; Magyar, K.; Chilliard, Y.; Solti, L.; et al. Pregnancy stage and number of fetuses may influence maternal plasma leptin in ewe. Acta. Vet. Hung. 2006, 54, 221–234. [Google Scholar] [CrossRef]
- Kawamura, T.; Gilbert, R.D.; Power, G.G. Effect of cooling and heating on the regional distribution of blood flow in fetal sheep. J. Dev. Physiol. 1986, 8, 11–21. [Google Scholar]
- Borg, R.C.; Notter, D.R.; Kott, R.W. Phenotypic and genetic associations between lamb growth traits and adult ewe body weights in western range sheep. J. Anim. Sci. 2009, 87, 3506–3514. [Google Scholar] [CrossRef]
- Sanson, D.W.; West, T.R.; Tatman, W.R.; Riley, M.L.; Judkins, M.B.; Moss, G.E. Relationship of body composition of mature ewes with condition score and body weight. J. Anim. Sci. 1993, 71, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).