Glycerol Monolaurate Enhances Reproductive Performance, Egg Quality and Albumen Amino Acids Composition in Aged Hens with Gut Microbiota Alternation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Reproductive Performance and Egg Quality
2.3. Sampling and Preparation
2.4. Serum Biochemical Indices and Sex Hormones
2.5. Yolk Fatty Acids Profile
2.6. Albumen Amino Acids Profile
2.7. 16S rRNA Sequencing and Analysis
2.8. Statistical Analysis
3. Results
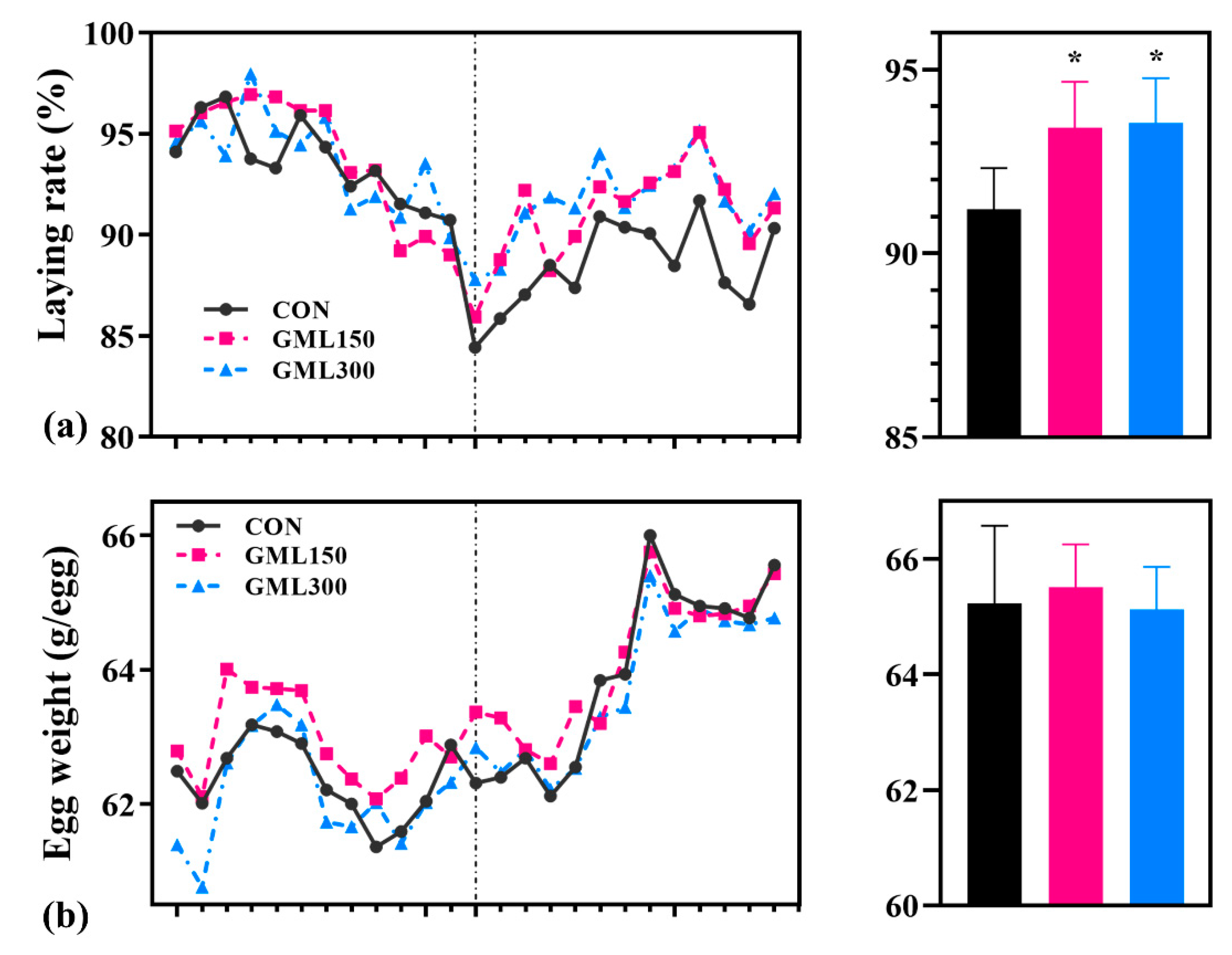
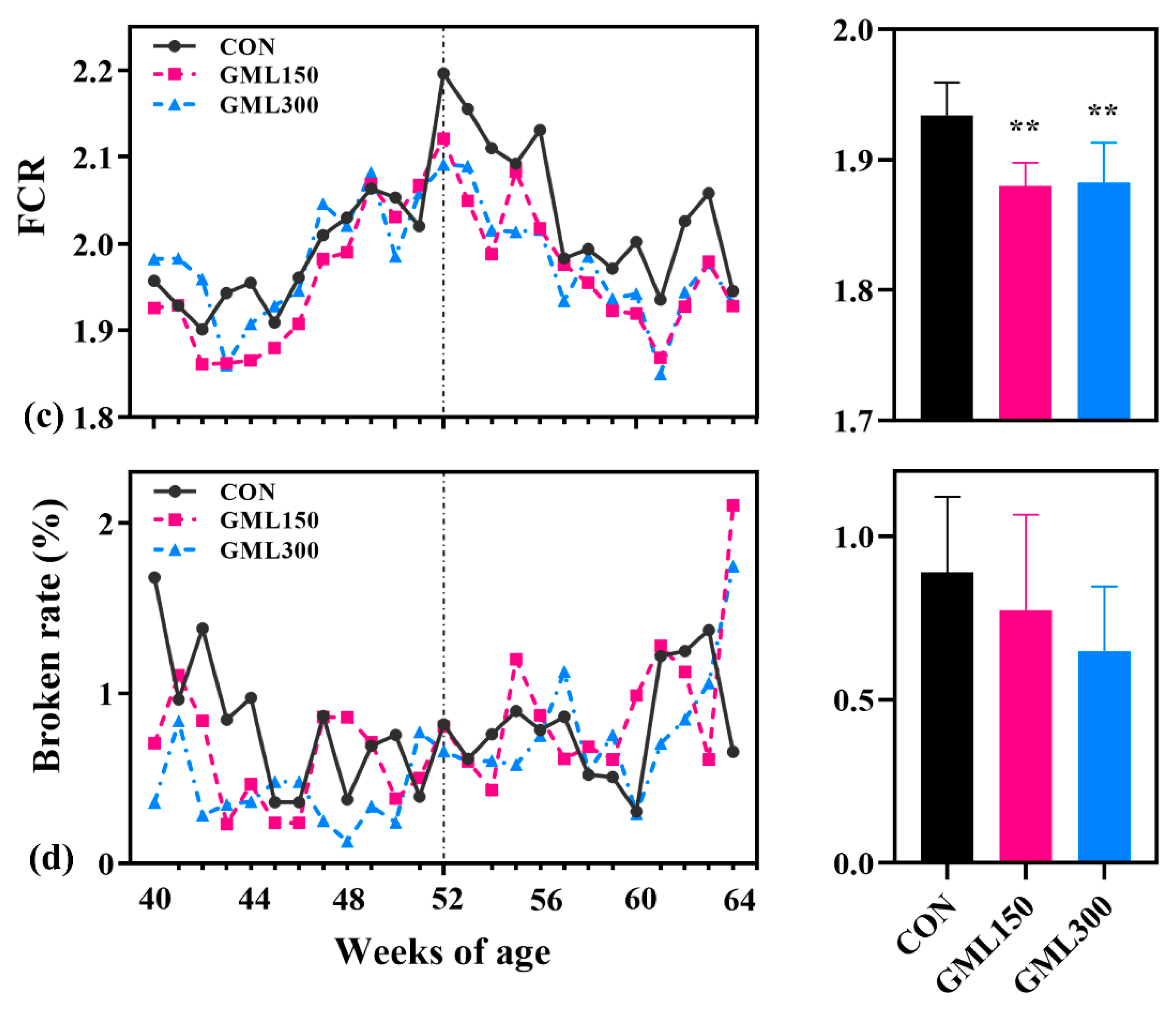
3.1. Dietary GML Improved the Reproductive Performance in Late Laying Period
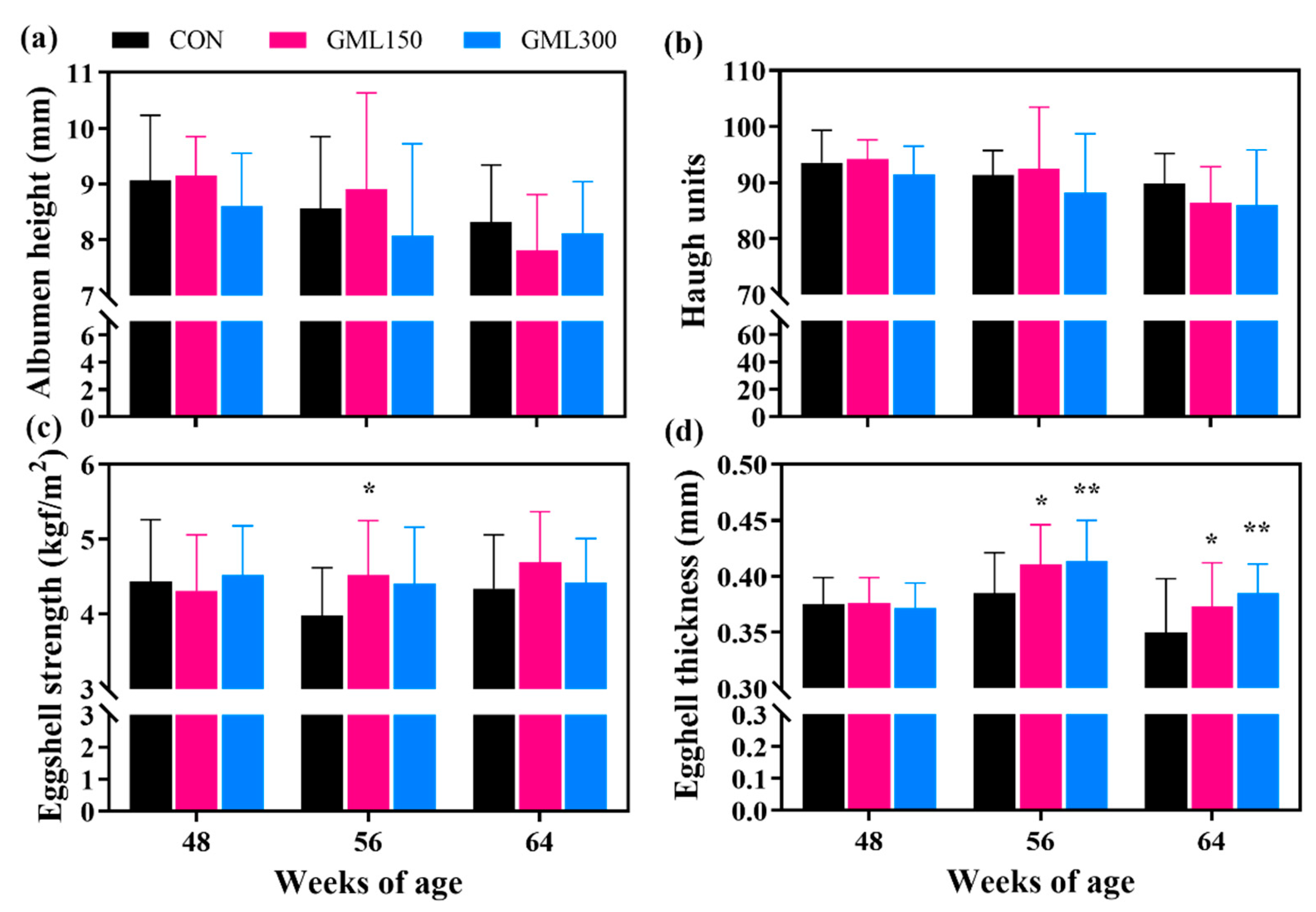
3.2. Dietary GML Improved Egg Quality in the Late Laying Period
3.3. Dietary GML Affected Serum Parameters
3.4. Effect of Dietary GML on the Yolk Fatty Acids Profile
3.5. Effect of Dietary GML on the Albumen Amino Acids Profile
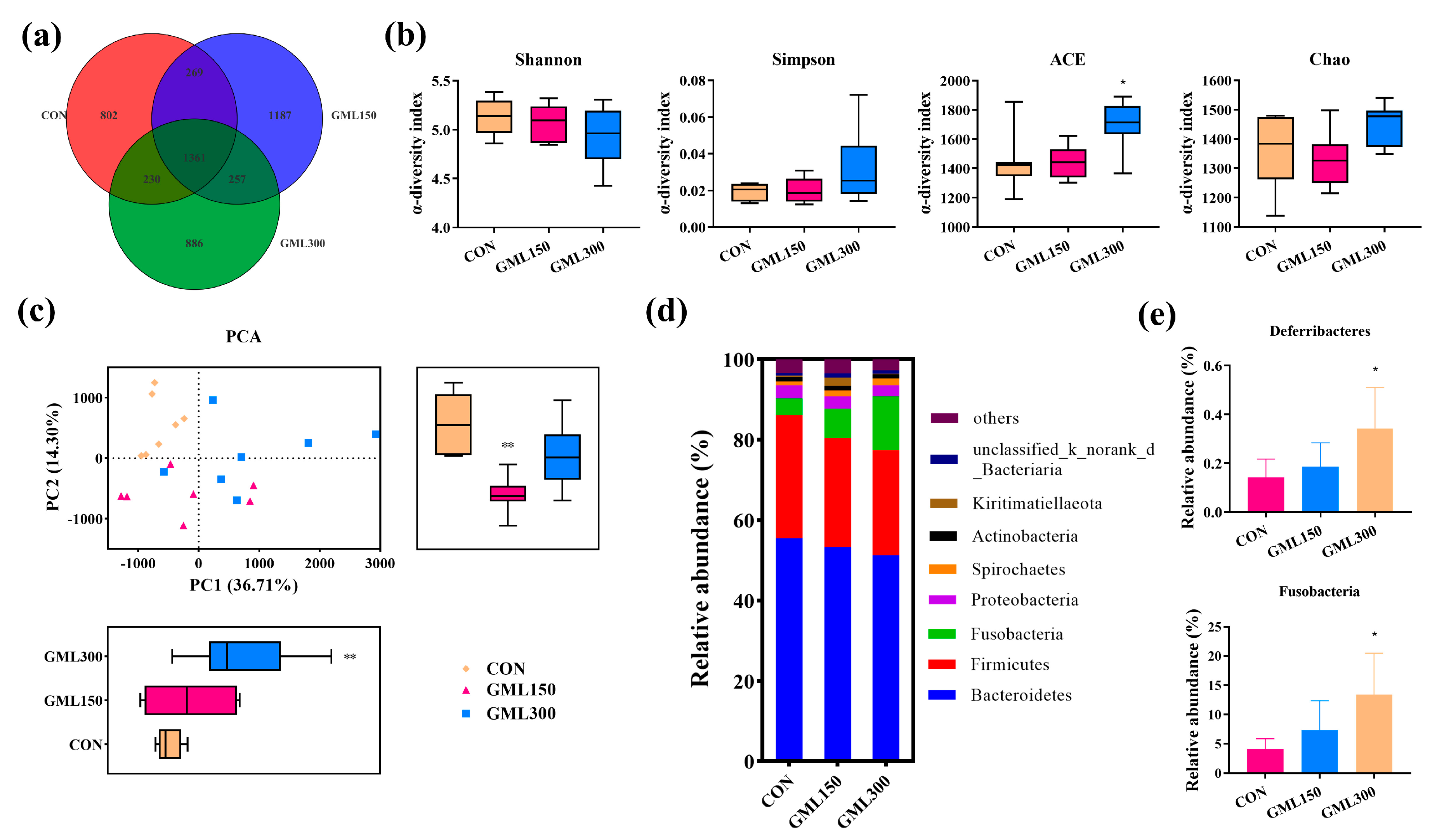
3.6. Dietary GML Altered the Gut Microbiota in Late Laying Period Hens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Li, Y.; Liu, H.N.; Suo, Y.L.; Hu, L.L.; Feng, X.A.; Zhang, L.; Jin, F. Effect of quercetin on performance and egg quality during the late laying period of hens. Br. Poult. Sci. 2013, 54, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Joyner, C.J.; Peddie, M.J.; Taylor, T.G. The effect of age on egg production in the domestic hen. Gen. Comp. Endocrinol. 1987, 65, 331–336. [Google Scholar] [CrossRef]
- Liu, H.K.; Long, D.W.; Bacon, W.L. Preovulatory luteinizing hormone surge interval in old and young laying turkey hens early in the egg production period. Poult. Sci. 2001, 80, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Bai, S.P.; Zhang, K.Y.; Ding, X.M.; Wang, J.P.; Zeng, Q.F.; Peng, H.W.; Lu, H.Y.; Bai, J.; Xuan, Y.; et al. Effects of Lonicera confusa and Astragali Radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult. Sci. 2019, 98, 4838–4847. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, C.J.; Yan, Y.Y.; Li, G.Q.; Shi, F.Y.; Wu, G.Q.; Liu, A.Q.; Yang, N. Genetic variations for egg quality of chickens at late laying period revealed by genome-wide association study. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef]
- Wen, Z.; Wu, Y.; Qi, Z.; Li, X.; Li, F.; Wu, X.; Yang, P. Rubber seed oil supplementation enriches n-3 polyunsaturated fatty acids and reduces cholesterol contents of egg yolks in laying hens. Food Chem. 2019, 301, 125198. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.A.; Lohakare, J.D. Effect of dietary supplementation of peppermint on performance, egg quality, and serum metabolic profile of Hy-Line Brown hens during the late laying period. Anim. Feed Sci. Technol. 2014, 197, 114–120. [Google Scholar] [CrossRef]
- Wang, X.C.; Wang, X.H.; Wang, J.; Wang, H.; Zhang, H.J.; Wu, S.G.; Qi, G.H. Dietary tea polyphenol supplementation improved egg production performance, albumen quality, and magnum morphology of Hy-Line Brown hens during the late laying period. J. Anim. Sci. 2018, 96, 225–235. [Google Scholar] [CrossRef]
- Baltic, B.; Starcevic, M.; Dordevic, J.; Mrdovic, B.; Markovic, R. Importance of medium chain fatty acids in animal nutrition. IOP Conf. Ser. Earth Environ. Sci. 2017, 85, 012048. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, M.; Zhang, H.; Li, Y.; Liu, M.; Feng, F. Antimicrobial emulsifier—Glycerol monolaurate induces metabolic syndrome, gut microbiota dysbiosis and systemic low-grade inflammation in low-fat diet fed mice. Mol. Nutr. Food Res. 2018, 63, 1700547. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, T.; Tsvetkova, V.; Najdenski, H.M. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: Individual effects and synergistic relationships. Pol. J. Microbiol. 2009, 58, 43–47. [Google Scholar] [PubMed]
- van der Aar, P.J.; Molist, F.; van der Klis, J.D. The central role of intestinal health on the effect of feed additives on feed intake in swine and poultry. Anim. Feed Sci. Technol. 2017, 233, 64–75. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; dos Reis, J.H.; Gebert, R.R.; Barreta, M.; Griss, L.G.; Casagrande, R.A.; de Cristo, T.G.; Santiani, F.; Campigotto, G.; Rampazzo, L.; et al. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: Impact on health, performance and meat quality. Microb. Pathog. 2019, 129, 161–167. [Google Scholar] [CrossRef]
- Mustafa, N.G. Biochemical trails associated with different doses of alpha-monolaurin in chicks. Adv. Anim. Vet. Sci. 2019, 7, 187–192. [Google Scholar] [CrossRef]
- Zhao, M.J.; Cai, H.Y.; Liu, M.Y.; Deng, L.L.; Li, Y.; Zhang, H.; Feng, F.Q. Dietary glycerol monolaurate supplementation for the modification of functional properties of egg white protein. J. Sci. Food Agric. 2019, 99, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, J.; Feng, F. Effects of glycerol monolaurate on production performance, egg quality, serum parameters, immune organ index and abdominal fat morphology of laying hens. China Poult. 2017, 39, 24–30. [Google Scholar]
- National Research Council, Nutrient Requirements of Poultry, 10th ed.; National Academies Press: Washington, DC, USA, 2017.
- Mrf, L.; Jks, T.; Kim, E.J.; Scollan, N.D. Beef, chicken and lamb fatty acid analysis—A simplified direct bimethylation procedure using freeze-dried material. Meat Sci. 2012, 92, 863–866. [Google Scholar]
- Gheshlaghi, R.; Scharer, J.M.; Moo-Young, M.; Douglas, P.L. Application of statistical design for the optimization of amino acid separation by reverse-phase HPLC. Anal Biochem. 2008, 383, 93–102. [Google Scholar] [CrossRef]
- Wang, J.P.; Kim, I.H. Effect of caprylic acid and Yucca schidigera extract on production performance, egg quality, blood characteristics, and excreta microflora in laying hens. Br. Poult. Sci. 2011, 52, 711–717. [Google Scholar] [CrossRef]
- Zeitz, J.O.; Fennhoff, J.; Kluge, H.; Stangl, G.I.; Eder, K. Effects of dietary fats rich in lauric and myristic acid on performance, intestinal morphology, gut microbes, and meat quality in broilers. Poult. Sci. 2015, 94, 2404–2413. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, X.T.; Hui, L.I.; Dong, X.Y.; Zhao, W. Effect of dietary γ-aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat-stressed Roman hens. Anim. Sci. J. 2012, 83, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Song, J.H.; Lee, J.C.; Lee, K.W. Age-related changes in egg quality of Hy-Line brown hens. Int. J. Poult. Sci. 2014, 13, 510–514. [Google Scholar] [CrossRef]
- Mertens, K.; Bamelis, F.; Kemps, B.; Kamers, B.; Verhoelst, E.; De Ketelaere, B.; Bain, M.; Decuypere, E.; De Baerdemaeker, J. Monitoring of eggshell breakage and eggshell strength in different production chains of consumption eggs. Poult. Sci. 2006, 85, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Leotoing, L.; Philippe, C.; Spilmont, M.; Coxam, V.; Wittrant, Y. Pros and cons of fatty acids in bone biology. Prog. Lipid Res. 2015, 58, 121–145. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Koreleski, J.; Arczewska, A. Laying performance and eggshell quality in laying hens fed diets supplemented with prebiotics and organic acids. Czech J. Anim. Sci. 2010, 55, 294–306. [Google Scholar] [CrossRef]
- Jiang, W.D.; Wu, P.; Tang, R.J.; Liu, Y.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res. Int. 2016, 89, 670–680. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Sentandreu, M.Á.; Lorenzo, J.M. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014, 56, 226–235. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Wang, Y.; Cao, J.X.; Chen, Y.J.; Liu, Y.; Sun, Y.Y.; Pan, D.D.; Ou, C.R. The effect of dry-cured salt contents on accumulation of non-volatile compounds during dry-cured goose processing. Poult. Sci. 2016, 95, 2160–2166. [Google Scholar] [CrossRef]
- Xiao, Z.; Ge, C.; Zhou, G.; Zhang, W.; Liao, G. 1H NMR-based metabolic characterization of Chinese Wuding chicken meat. Food Chem. 2019, 274, 574–582. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Liu, C.; Pan, D.; Ye, Y.; Cao, J. 1H NMR and multivariate data analysis of the relationship between the age and quality of duck meat. Food Chem. 2013, 141, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, B.; Yavari, Z.; Kordestani, A. Effects of dietary medium-chain fatty acids on performance, carcass characteristics, and some serum parameters of broiler chickens. Br. Poult. Sci. 2014, 55, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2013, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, Y.; Kitamura, N.; Kaneko, M.; Yoshioka, D.; Miki, T.; Nishlnarita, S.; Horie, T.; Hosokawa, N.; Iwasaki, Y.; Kumasaka, K. Multibacterial Sepsis in an Alcohol Abuser with Hepatic Cirrhosis. Intern. Med. 2003, 42, 208–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kollarcikova, M.; Kubasova, T.; Karasova, D.; Crhanova, M.; Cejkova, D.; Sisak, F.; Rychlik, I. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 2019, 98, 2347–2353. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, R.; Wu, N.; Zhu, G.; Yang, C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. microbiol. Biotechnol. 2019, 103, 461–472. [Google Scholar] [CrossRef]
- Xu, Q.; Yuan, X.; Gu, T.; Li, Y.; Dai, W.; Shen, X.; Song, Y.; Zhang, Y.; Zhao, W.; Chang, G. Comparative characterization of bacterial communities in geese fed all-grass or high-grain diets. PLoS ONE 2017, 12, e0185590. [Google Scholar] [CrossRef]
- Zhou, B.-H.; Jia, L.-S.; Wei, S.-S.; Ding, H.-Y.; Yang, J.-Y.; Wang, H.-W. Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken cecum. Poult. Sci. 2020, 99, 1297–1305. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Li, J.; Xing, T.; Jiang, Y.; Zhang, L.; Gao, F. Dietary resistant starch modifies the composition and function of caecal microbiota of broilers. J. Sci. Food Agric. 2020, 100, 1274–1284. [Google Scholar] [CrossRef]
- Mo, Q.; Fu, A.; Deng, L.; Zhao, M.; Li, Y.; Zhang, H.; Feng, F. High-dose glycerol monolaurate up-regulated beneficial indigenous microbiota without inducing metabolic dysfunction and systemic inflammation: New insights into its antimicrobial potential. Nutrients. 2019, 11, 1981. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, H.; Jiang, Z.; Li, Y.; Zhong, H.; Zhang, H.; Feng, F. Glycerol-monolaurate-mediated attenuation of metabolic syndrome is associated with the modulation of gut microbiota in high-fat-diet-fed mice. Mol. Nutr. Food Res. 2019, 63, 1801417. [Google Scholar] [CrossRef] [PubMed]
| Ingredients, % | 40–58 Week of Age | 58–64 Week of Age |
|---|---|---|
| Corn | 62.90 | 64.90 |
| Soybean meal | 23.55 | 21.48 |
| Limestone | 7.93 | 7.93 |
| Salt | 0.30 | 0.30 |
| Fish oil | 0.03 | 0.03 |
| Rapeseed oil | 0.59 | 0.66 |
| 1 Premix | 4.70 | 4.70 |
| Total | 100 | 100 |
| Calculated/analyzed values | ||
| 2 Metabolizable energy (MJ/kg) | 11.85 | 11.98 |
| 3 Crude protein, % | 16.10 | 15.80 |
| Crude fat, % | 2.86 | 2.91 |
| 3 Calcium, % | 3.73 | 3.78 |
| Total phosphorus, % | 0.60 | 0.57 |
| Lysine, % | 0.81 | 0.75 |
| Methionine + Cysteine, % | 0.65 | 0.60 |
| Methionine, % | 0.35 | 0.32 |
| Parameters | CON | GML150 | GML300 | SEM | p Value |
|---|---|---|---|---|---|
| TG (mM) | 10.57 ± 2.17 | 13.88 ± 2.88 | 17.86 ± 3.16 ** | 0.77 | 0.001 |
| TC (mM) | 4.96 ± 0.75 | 5.194 ± 0.89 | 6.78 ± 0.37 ** | 0.22 | 0.001 |
| HDL-C (mM) | 2.69 ± 0.68 | 2.74 ± 0.83 | 3.23 ± 0.83 | 0.12 | 0.122 |
| LDL-C (mM) | 0.74 ± 0.08 | 0.88 ± 0.24 | 0.89 ± 0.08 * | 0.08 | 0.038 |
| Ca (mM) | 1.32 ± 0.03 | 1.35 ± 0.08 | 1.41 ± 0.04 ** | 0.01 | 0.001 |
| AKP (mM) | 26.20 ± 8.07 | 27.61 ± 5.64 | 26.58 ± 5.57 | 1.57 | 0.821 |
| Glucose (mM) | 16.55 ± 2.91 | 15.47 ± 3.87 | 18.43 ± 3.33 | 0.54 | 0.074 |
| Total protein (g protein/L) | 28.64 ± 1.86 | 29.86 ± 2.31 | 29.21 ± 4.43 | 0.46 | 0.562 |
| T-AOC (U/mL) | 364.83 ± 38.44 | 435.56 ± 78.60 | 457.92 ± 76.39 * | 19.01 | 0.008 |
| FSH (ng/mL) | 32.32 ± 10.78 | 40.65 ± 15.52 | 91.30 ± 49.05 *** | 12.34 | 0.001 |
| LH (pg/mL) | 487.95 ± 124.54 | 620.84 ± 163.80 | 1254.40 ± 475.56 *** | 8.34 | 0.001 |
| E2 (pg/mL) | 120.39 ± 19.71 | 147.58 ± 57.43 | 404.29 ± 288.13 ** | 40.68 | 0.001 |
| g/100 g | CON | GML150 | GML300 | SEM | p Value |
|---|---|---|---|---|---|
| Umami taste amino acids | |||||
| Aspartic acid | 5.32 ± 1.47 | 7.58 ± 0.58 * | 7.44 ± 0.87 * | 0.41 | 0.018 |
| Glutamic acid | 4.83 ± 1.33 | 5.86 ± 0.66 | 5.45 ± 0.85 | 0.27 | 0.286 |
| Sweet taste amino acids | |||||
| Serine | 3.50 ± 0.66 | 3.88 ± 0.40 | 3.29 ± 0.35 | 0.14 | 0.247 |
| Glycine | 2.80 ± 0.33 | 3.04 ± 0.21 | 2.87 ± 0.19 | 0.07 | 0.344 |
| Alanine | 5.23 ± 0.66 | 5.87 ± 0.55 | 5.69 ± 0.10 | 0.15 | 0.166 |
| Σ FAA | 20.80 ± 3.06 | 26.18 ± 2.00 * | 24.79 ± 2.95 * | 0.85 | 0.014 |
| EAA | |||||
| Lysine | 5.44 ± 1.21 | 6.08 ± 0.84 | 4.89 ± 0.29 | 0.25 | 0.198 |
| Methionine | 3.22 ± 0.43 | 3.39 ± 0.19 | 3.22 ± 0.20 | 0.08 | 0.615 |
| Threonine | 2.72 ± 0.43 | 2.80 ± 0.64 | 2.72 ± 0.56 | 0.13 | 0.955 |
| Leucine | 8.29 ± 0.80 | 8.83 ± 0.66 | 8.50 ± 0.44 | 0.17 | 0.441 |
| Isoleucine | 5.16 ± 0.57 | 5.53 ± 0.51 | 5.35 ± 0.40 | 0.13 | 0.514 |
| Arginine | 3.86 ± 0.97 | 4.58 ± 0.47 | 4.37 ± 0.48 | 0.19 | 0.270 |
| Valine | 5.86 ± 0.96 | 6.70 ± 0.86 | 6.70 ± 0.34 | 0.22 | 0.190 |
| Phenylalanine | 5.30 ± 0.54 | 5.58 ± 0.42 | 5.44 ± 0.43 | 0.12 | 0.646 |
| Histidine | 1.58 ± 0.23 | 1.67 ± 0.19 | 1.60 ± 0.23 | 0.05 | 0.784 |
| Σ EAA | 41.43 ± 5.72 | 43.41 ± 5.41 | 42.05 ± 3.82 | 1.62 | 0.794 |
| Proline | 5.33 ± 0.69 | 5.50 ± 0.52 | 5.02 ± 0.33 | 0.14 | 0.460 |
| Tyrosine | 2.90 ± 0.50 | 3.07 ± 0.07 | 2.79 ± 0.59 | 0.11 | 0.680 |
| Cysteine | 0.71 ± 0.87 | 1.99 ± 1.64 | 1.40 ± 0.94 | 0.39 | 0.476 |
| Σ AA | 72.42 ± 6.07 | 80.88 ± 5.44 * | 76.24 ± 4.32 | 2.18 | 0.074 |
| Genus | Relative Abundance (%) | ||||
|---|---|---|---|---|---|
| CON | GML150 | GML300 | SEM | p Value | |
| p_Bacteroidetes | |||||
| Rikenellaceae_RC9_gut_group | 8.035 ± 3.252 | 12.690 ± 4.134 * | 9.991 ± 3.346 | 0.859 | 0.078 |
| p_Fusobacteria | |||||
| Fusobacterium | 4.124 ± 1.759 | 7.332 ± 5.010 | 13.430 ± 7.030 * | 1.362 | 0.010 |
| p_Firmicutes | |||||
| Ruminiclostridium_9 | 1.314 ± 0.405 | 0.898 ± 0.219 * | 0.887 ± 0.174 * | 0.074 | 0.017 |
| Megasphaera | 0.347 ± 0.238 | 0.282 ± 0.554 | 0.075 ± 0.084 * | 0.077 | 0.048 |
| Negativibacillus | 0.103 ± 0.053 | 0.121 ± 0.051 | 0.049 ± 0.031 ** | 0.012 | 0.031 |
| norank_f_Erysipelotrichaceae | 0.082 ± 0.047 | 0.024 ± 0.023 * | 0.062 ± 0.047 | 0.010 | 0.031 |
| GCA-900066225 | 0.057 ± 0.033 | 0.025 ± 0.019 * | 0.058 ± 0.028 | 0.007 | 0.012 |
| Ruminococcaceae_UCG-009 | 0.057 ± 0.023 | 0.046 ± 0.0.11 | 0.032 ± 0.011 * | 0.004 | 0.012 |
| Family_XIII_AD3011_group | 0.056 ± 0.029 | 0.037 ± 0.016 | 0.027 ± 0.016 * | 0.005 | 0.061 |
| Angelakisella | 0.053 ± 0.019 | 0.033 ± 0.014 * | 0.030 ± 0.010 * | 0.004 | 0.046 |
| Eubacterium_nodatum_group | 0.043 ± 0.007 | 0.024 ± 0.016 * | 0.019 ± 0.010 *** | 0.003 | 0.006 |
| p_Deferribacteres | |||||
| Mucispirillum | 0.142 ± 0.075 | 0.185 ± 0.099 | 0.343 ± 0.167 * | 0.031 | 0.043 |
| p_Verrucomicrobia | |||||
| norank_f_Pedosphaeraceae | 0.043 ± 0.021 | 0.104 ± 0.059 * | 0.085 ± 0.038 * | 0.010 | 0.036 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, C.; Li, Y.; Feng, F. Glycerol Monolaurate Enhances Reproductive Performance, Egg Quality and Albumen Amino Acids Composition in Aged Hens with Gut Microbiota Alternation. Agriculture 2020, 10, 250. https://doi.org/10.3390/agriculture10070250
Liu T, Li C, Li Y, Feng F. Glycerol Monolaurate Enhances Reproductive Performance, Egg Quality and Albumen Amino Acids Composition in Aged Hens with Gut Microbiota Alternation. Agriculture. 2020; 10(7):250. https://doi.org/10.3390/agriculture10070250
Chicago/Turabian StyleLiu, Tao, Chuang Li, Yang Li, and Fengqin Feng. 2020. "Glycerol Monolaurate Enhances Reproductive Performance, Egg Quality and Albumen Amino Acids Composition in Aged Hens with Gut Microbiota Alternation" Agriculture 10, no. 7: 250. https://doi.org/10.3390/agriculture10070250
APA StyleLiu, T., Li, C., Li, Y., & Feng, F. (2020). Glycerol Monolaurate Enhances Reproductive Performance, Egg Quality and Albumen Amino Acids Composition in Aged Hens with Gut Microbiota Alternation. Agriculture, 10(7), 250. https://doi.org/10.3390/agriculture10070250






