Impacts of LEDs in the Red Spectrum on the Germination, Early Seedling Growth and Antioxidant Metabolism of Pea (Pisum sativum L.) and Melon (Cucumis melo L.)
Abstract
1. Introduction
2. Materials and Methods
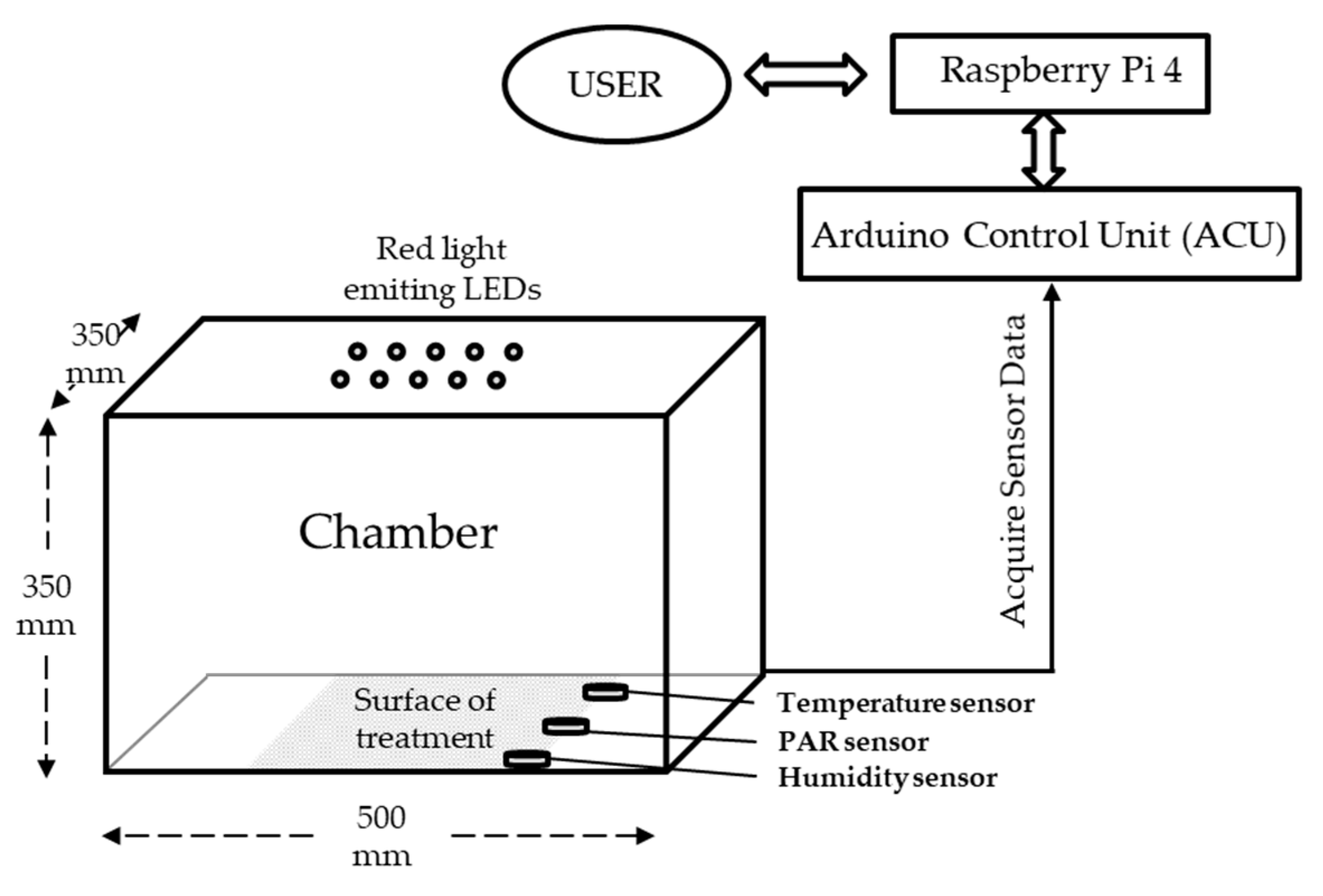
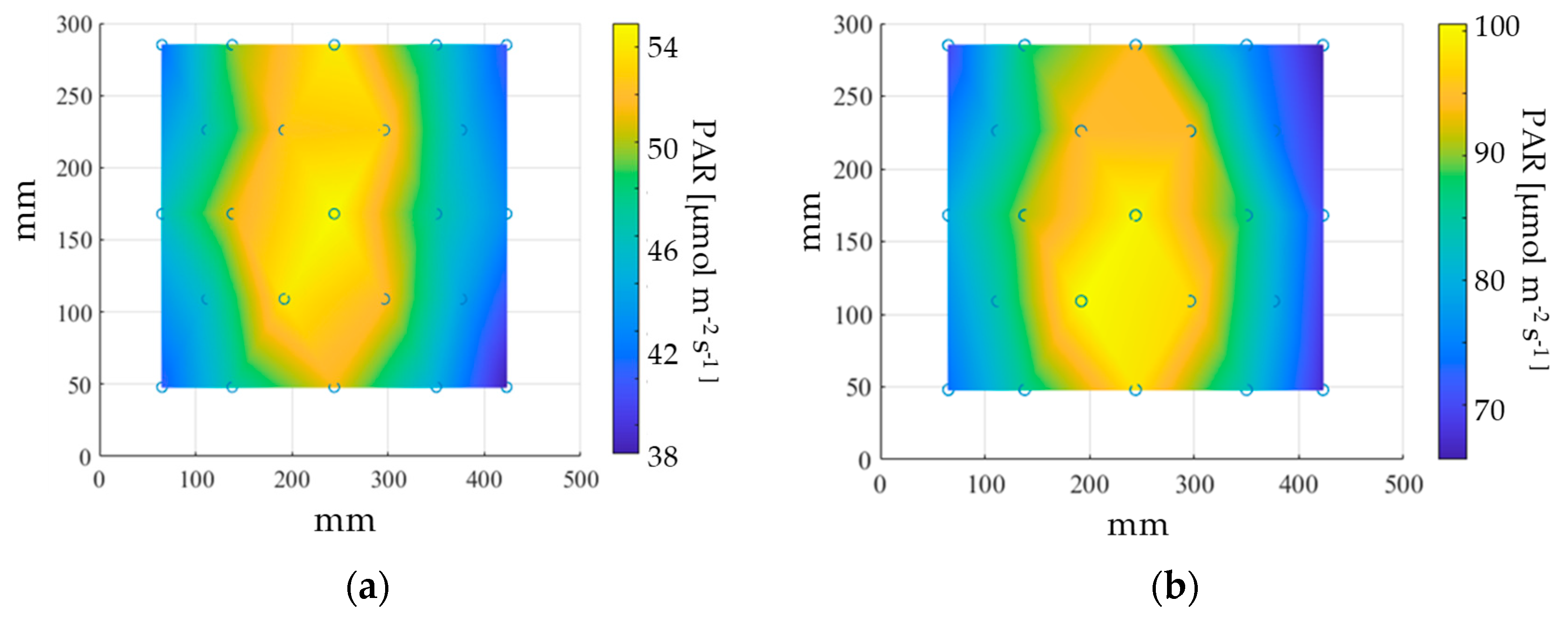
2.1. Experimental Chamber and Lighting Technology
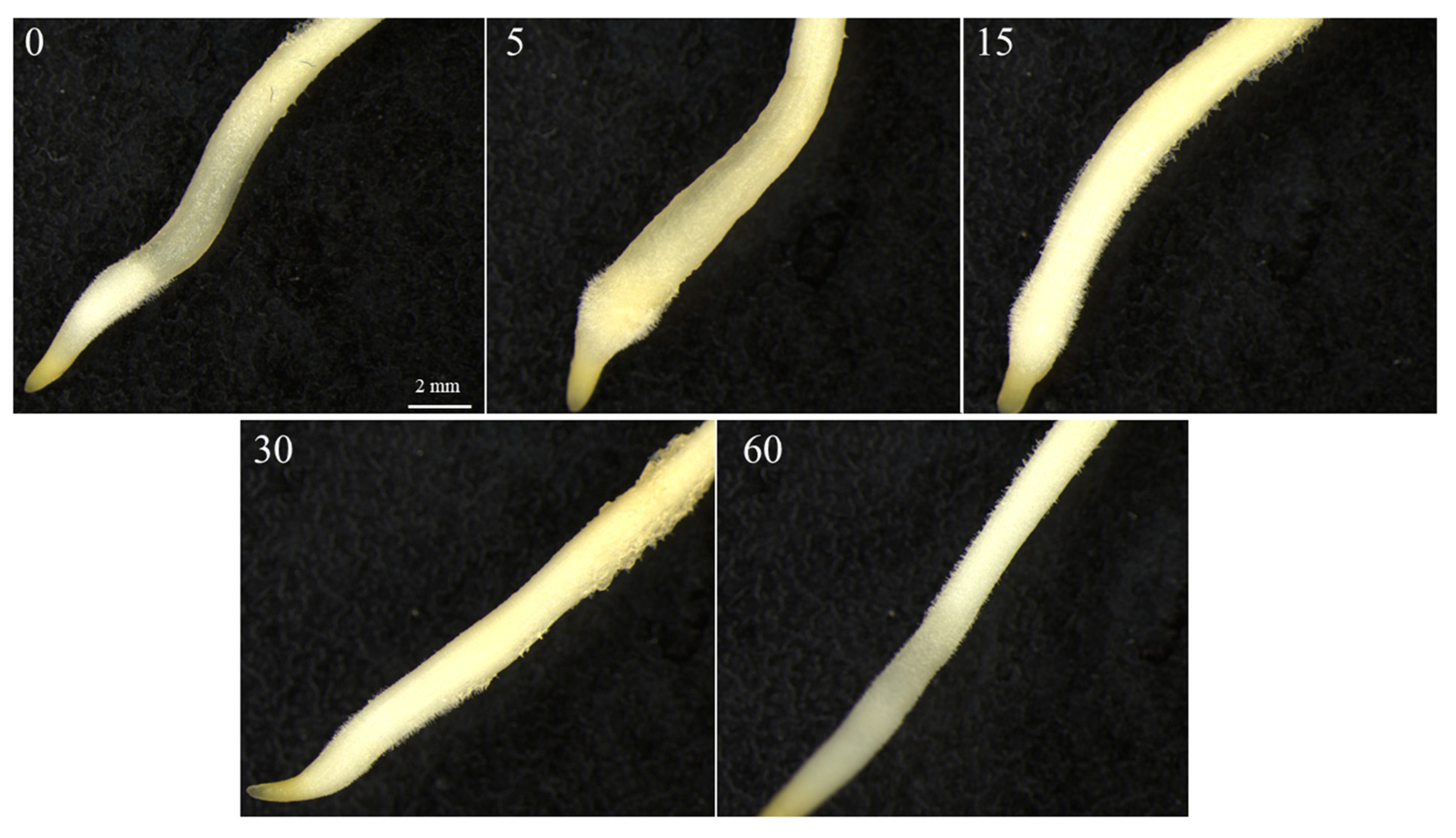
2.2. Red Light Treatment of Seeds in the MSPEC
2.3. Sample Preparation and Enzyme Activities Determination
2.4. Statitical Analyses
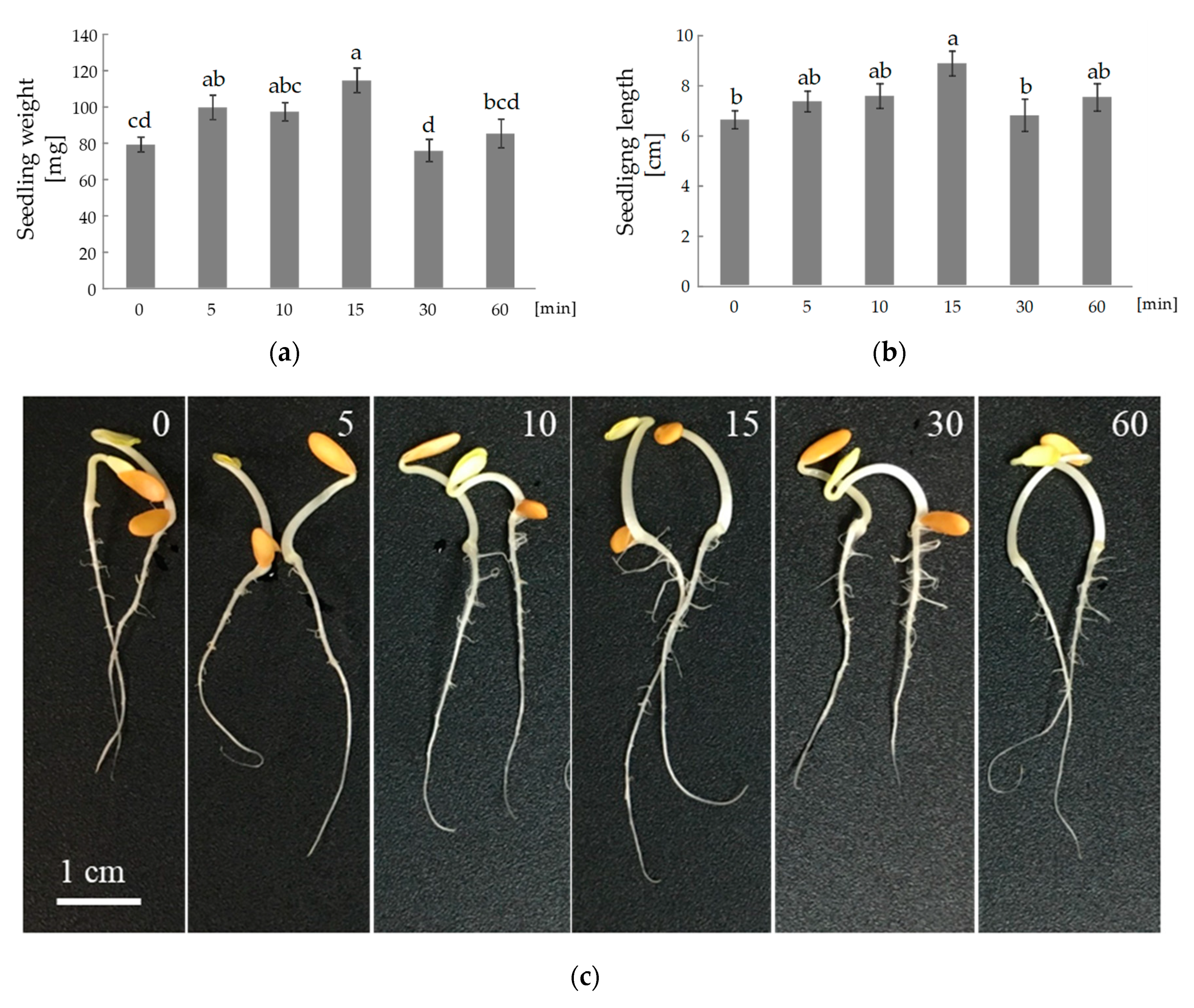
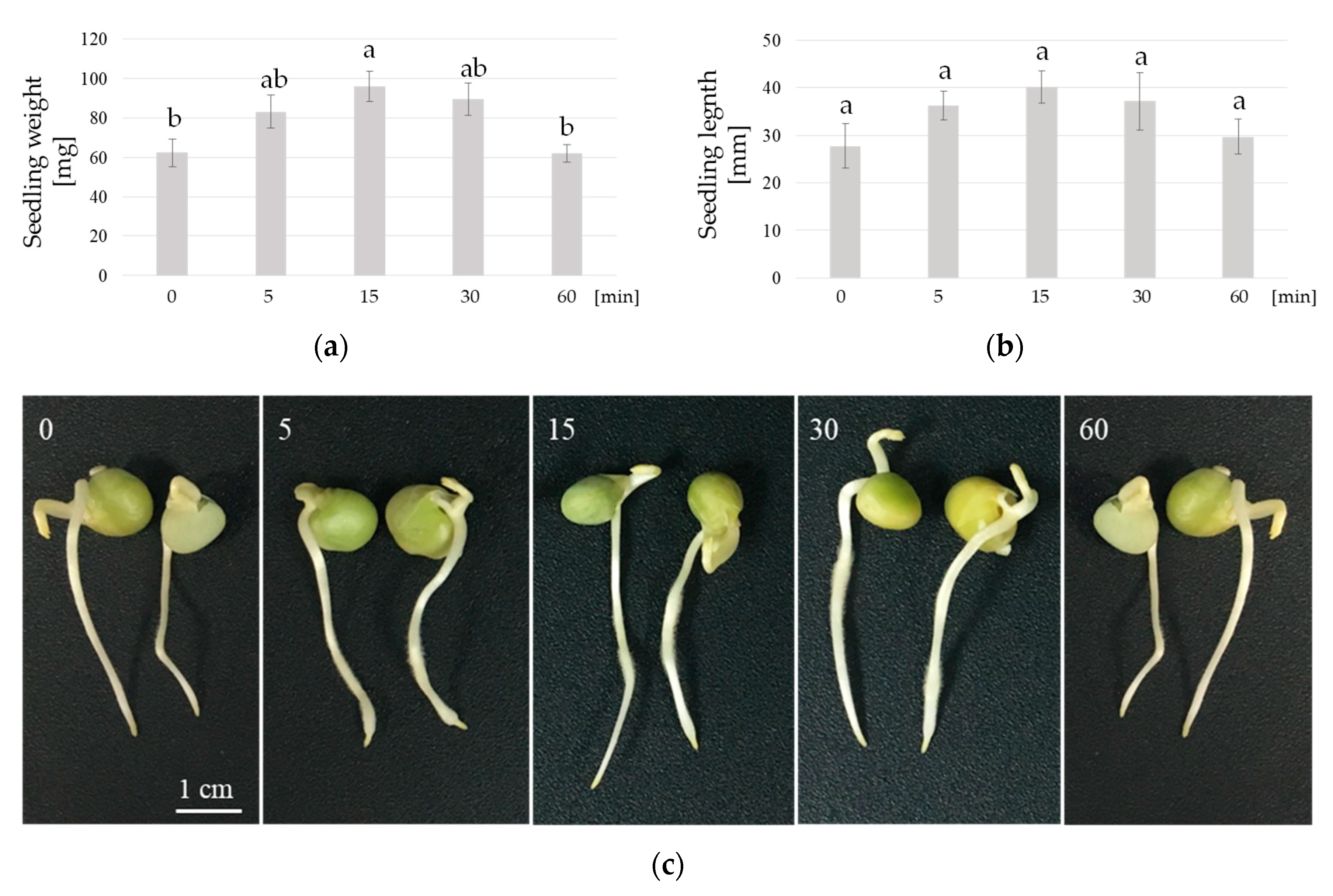
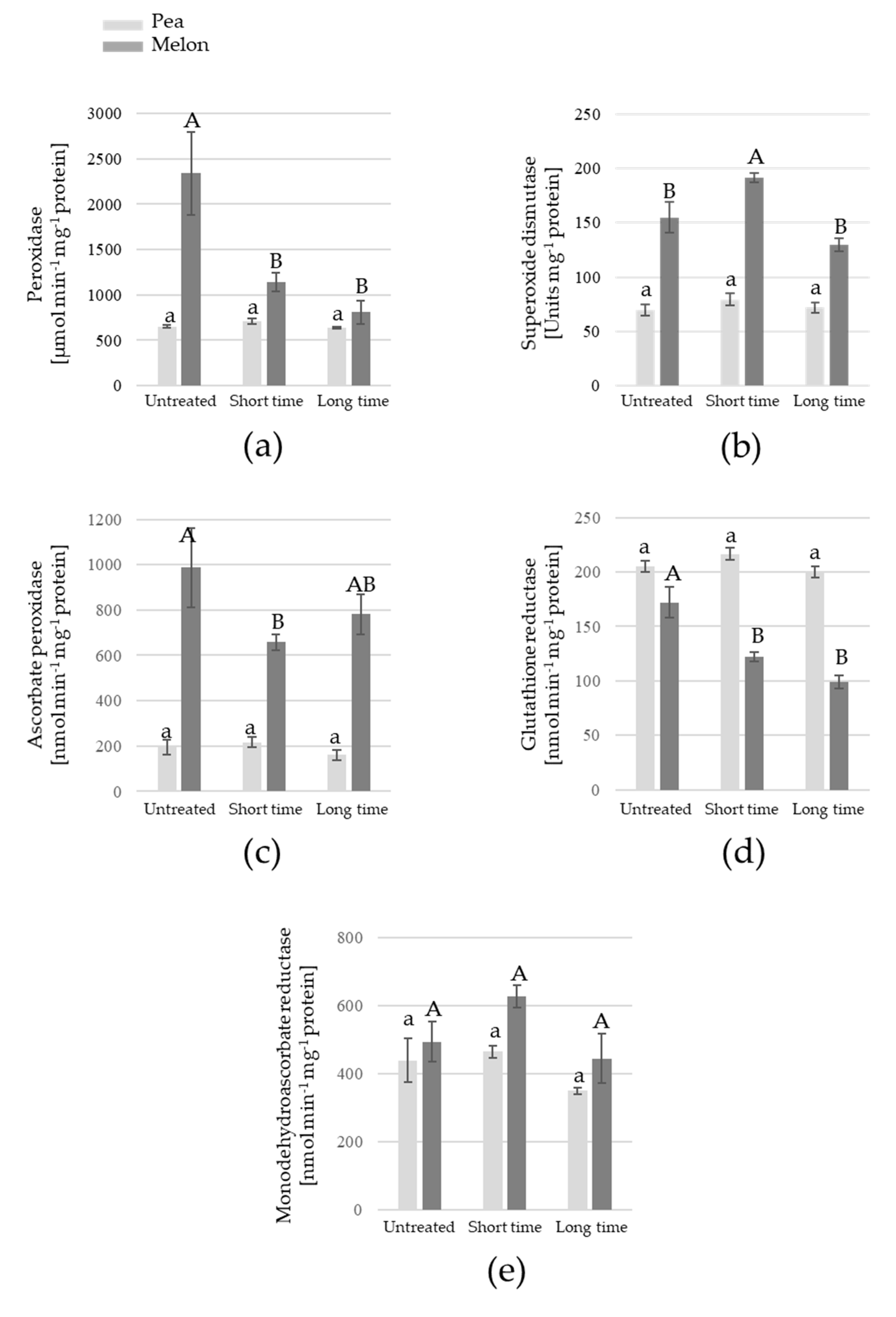
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Joosen, R.V.L.; Arends, D.; Li, Y.; Willems, L.A.J.; Keurentjes, J.J.B.; Ligterink, W.; Jansen, R.C.; Hilhorst, H.W.M. Identifying Genotype-by-Environment Interactions in the Metabolism of Germinating Arabidopsis Seeds Using Generalized Genetical Genomics. Plant Phys. 2013, 162, 553–566. [Google Scholar] [CrossRef]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Donohue, K. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology 2002, 83, 1006–1016. [Google Scholar] [CrossRef]
- Stutte, G.W. Light-emitting Diodes for Manipulating the Phytochrome Apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Bae, G.; Choi, G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, D.; Konjević, R. Light and nitrate interaction in phytochrome-controlled germination of Paulownia tomentosa seeds. Planta 1990, 181, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and Phytohormones: Working in Tandem for Plant Growth and Development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.L.; Borthwick, H.A. Photocontrol of germination and phytochrome reaction in dark-germinating seeds of Lactuca sativa L. Ann. Bot. 1964, 28, 9–24. [Google Scholar]
- Devlin, P. Plants wait for the lights to change to red. PNAS 2016, 113, 7301–7303. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Díaz-Vivancos, P.; Clemente-Moreno, M.J.; Albacete, A.; Faize, L.; Faize, M.; Pérez-Alfocea, F.; Hernández, J.A. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ. 2010, 33, 981–994. [Google Scholar] [CrossRef]
- Ibarra, S.E.; Auge, G.; Sánchez, R.A.; Botto, J.F. Transcriptional Programs Related to Phytochrome A Function in Arabidopsis Seed Germination. Mol. Plant. 2013, 6, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Neff, M.M.; Sanderson, L.; Tedor, D. Light-Mediated Germination in Lettuce Seeds: Resurrection of a Classic Plant Physiology Lab Exercise. Am. Biol. Teach. 2009, 71, 367–370. [Google Scholar] [CrossRef]
- Cresswell, E.G.; Grime, J.P. Induction of a light during seed development and its ecological consequences. Nature 1981, 291, 583–585. [Google Scholar] [CrossRef]
- Salisbury, F.B.; Ross, C.W. Plant Physiology, 4th ed.; Wadsworth Publishing: Belmont, CA, USA, 1992. [Google Scholar]
- Bradford, K.J.; May, D.M.; Hoyle, B.J.; Skibinski, Z.S.; Scott, S.J.; Tyler, K.B. Seed and Soil Treatments to Improve Emergence of Muskmelon from Cold or Crusted Soils. Crop. Sci. 1988, 28, 1001–1005. [Google Scholar] [CrossRef]
- Nerson, H.; Govers, A. Salt priming of muskmelon seeds for low-temperature germination. Sci. Hortic. 1986, 28, 85–91. [Google Scholar] [CrossRef]
- Sivritepe, H.Ö.; Sivritepe, N.; Eriş, A.; Turhan, E. The effects of NaCl pre-treatments on salt tolerance of melons grown under long-term salinity. Sci. Hortic. 2005, 106, 568–581. [Google Scholar] [CrossRef]
- Yeoung, Y.R.; Wilson, D.O.; Murray, G.A. Germination performance and loss of late-embryogenesis-abundant (LEA) proteins during muskmelon seed priming. Seed Sci. Technol. 1996, 24, 429–439. [Google Scholar]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Jones, M.A. Using light to improve commercial value. Hortic. Res. 2018, 5, 47. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthetic Characteristics of Rice Leaves Grown under Red Light with or without Supplemental Blue Light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef]
- Muleo, R.; Morini, S.; Casano, S. Photoregulation of growth and branching of plum shoots: Physiological action of two photosystems. In Vitro Cell. Dev. Biol.–Plant. 2001, 37, 609–617. [Google Scholar] [CrossRef]
- Jing, X.; Wang, H.; Gong, B.; Liu, S.; Wei, M.; Ai, X.; Li, Y.; Shi, Q. Secondary and sucrose metabolism regulated by different light quality combinations involved in melon tolerance to powdery mildew. Plant Physiol. Biochem. 2018, 124, 77–87. [Google Scholar] [CrossRef]
- Vidal, A.; Cantabella, D.; Bernal-Vicente, A.; Díaz-Vivancos, P.; Hernández, J.A. Nitrate- and nitric oxide-induced plant growth in pea seedlings is linked to antioxidative metabolism and the ABA/GA balance. J. Plant Physiol. 2018, 230, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.D. Impact of Light-Emitting Diodes (LEDs) and its Potential on Plant Growth and Development in Controlled-Environment Plant Production System. Curr. Biotechnol. 2016, 5, 28–43. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A. Influence of LED Lighting on In Vitro Plant Regeneration and Associated Cellular Redox Balance. In Light Emitting Diodes for Agriculture; Springer Nature: Singapore, 2017; pp. 273–303. [Google Scholar]
- Benson, E.E. Do free radicals have a role in plant tissue culture recalcitrance? In Vitro Cell. Dev. Biol.—Plant 2002, 36, 163–170. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell. Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Hernández, J.A.; Álvarez, S.; Barba-Espín, G.; Sánchez-Blanco, M.J. The long-term resistance mechanisms, critical irrigation threshold and relief capacity shown by Eugenia myrtifolia plants in response to saline reclaimed water. Plant Physiol. Biochem. 2017, 111, 244–256. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Nicolás, E.; Almansa, M.S.; Cantero-Navarro, E.; Albacete, A.; Hernández, J.A.; Díaz-Vivancos, P. Role of thioproline on seed germination: Interaction ROS-ABA and effects on antioxidative metabolism. Plant Physiol. Biochem. 2012, 59, 30–36. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Díaz-Vivancos, P.; Job, D.; Belghazi, M.; Job, C.; Hernández, J.A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011, 34, 1907–1919. [Google Scholar] [CrossRef]
- Hernández, J.A.; Jiménez, A.; Mullineaux, P.; Sevilla, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 862. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Kurniawan, B. Spectral quality affects morphogenesis on Anthurium plantlet during in vitro culture. Agrivita 2010, 32, 234–240. [Google Scholar]
- Choi, H.; Cho, H. Root hairs enhance Arabidopsis seedling survival upon soil disruption. Sci. Rep. 2019, 9, 11181. [Google Scholar] [CrossRef] [PubMed]
- Correll, M.J.; Kiss, J.Z. The roles of phytochromes in elongation and gravitropism of roots. Plant Cell Physiol. 2005, 46, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007, 50, 429–438. [Google Scholar] [CrossRef]
- Baťková, P.; Pospíšilová, J.; Synková, H. Production of reactive oxygen species and development of antioxidative systems during in vitro growth and ex vitro transfer. Biol. Plant. 2008, 52, 413–422. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Noguera-Vera, L.; Barba-Espín, G.; Piqueras, A.; Hernández, J.A. Antioxidant metabolism and chlorophyll fluorescence during the acclimatisation to ex vitro conditions of micropropagated Stevia rebaudiana Bertoni plants. Antioxidants 2019, 8, 615. [Google Scholar] [CrossRef]

| n° LED | PAR (µmol m−2 s−1) | Luminance (Lux) | Electrical Power (W) |
|---|---|---|---|
| 1 | 10.1 | 118 | 0.71 |
| 2 | 22.4 | 256 | 1.42 |
| 5 | 55 | 624 | 3.58 |
| 10 | 110 | 1283 | 7.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solano, C.J.; Hernández, J.A.; Suardíaz, J.; Barba-Espín, G. Impacts of LEDs in the Red Spectrum on the Germination, Early Seedling Growth and Antioxidant Metabolism of Pea (Pisum sativum L.) and Melon (Cucumis melo L.). Agriculture 2020, 10, 204. https://doi.org/10.3390/agriculture10060204
Solano CJ, Hernández JA, Suardíaz J, Barba-Espín G. Impacts of LEDs in the Red Spectrum on the Germination, Early Seedling Growth and Antioxidant Metabolism of Pea (Pisum sativum L.) and Melon (Cucumis melo L.). Agriculture. 2020; 10(6):204. https://doi.org/10.3390/agriculture10060204
Chicago/Turabian StyleSolano, Cristobal Javier, José A. Hernández, Juan Suardíaz, and Gregorio Barba-Espín. 2020. "Impacts of LEDs in the Red Spectrum on the Germination, Early Seedling Growth and Antioxidant Metabolism of Pea (Pisum sativum L.) and Melon (Cucumis melo L.)" Agriculture 10, no. 6: 204. https://doi.org/10.3390/agriculture10060204
APA StyleSolano, C. J., Hernández, J. A., Suardíaz, J., & Barba-Espín, G. (2020). Impacts of LEDs in the Red Spectrum on the Germination, Early Seedling Growth and Antioxidant Metabolism of Pea (Pisum sativum L.) and Melon (Cucumis melo L.). Agriculture, 10(6), 204. https://doi.org/10.3390/agriculture10060204








