The Effects of Root Temperature on Growth, Physiology, and Accumulation of Bioactive Compounds of Agastache rugosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Seedling Conditions
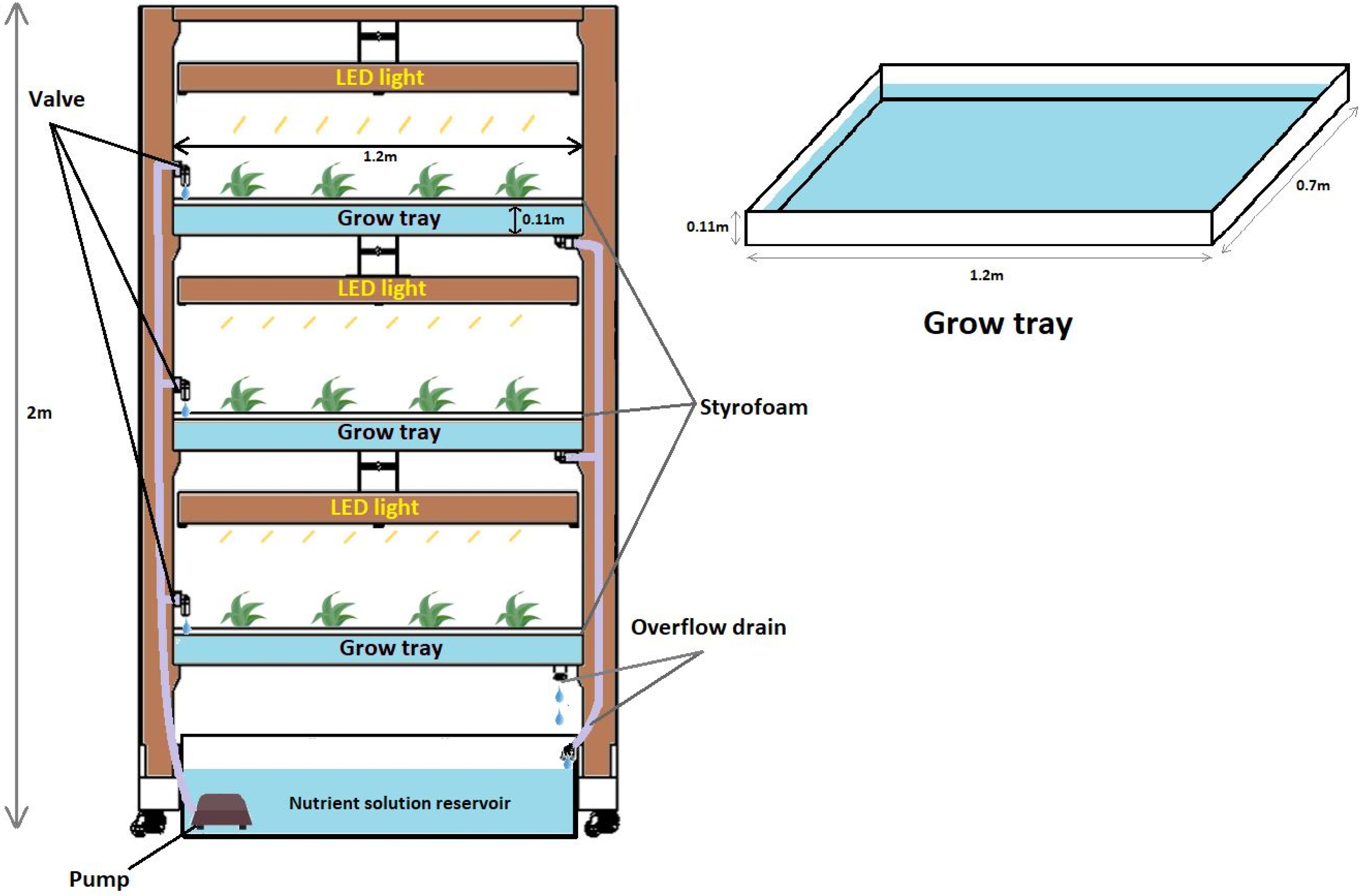
2.2. Root Zone Temperature Experiment and Growth Conditions
2.3. Measurements of Plant Growth Parameters
2.4. Measurements Chlorophyll fluorescence (Fv/Fm) and Relative Chlorophyll Values
2.5. Leaf Gas Exchange Measurement
2.6. Analysis of Rosmarinic Acid (RA), Tilianin, and Acacetin Concentrations and Contents
2.7. Statistical Analysis
3. Results and Discussion
3.1. Plant Growth Parameters
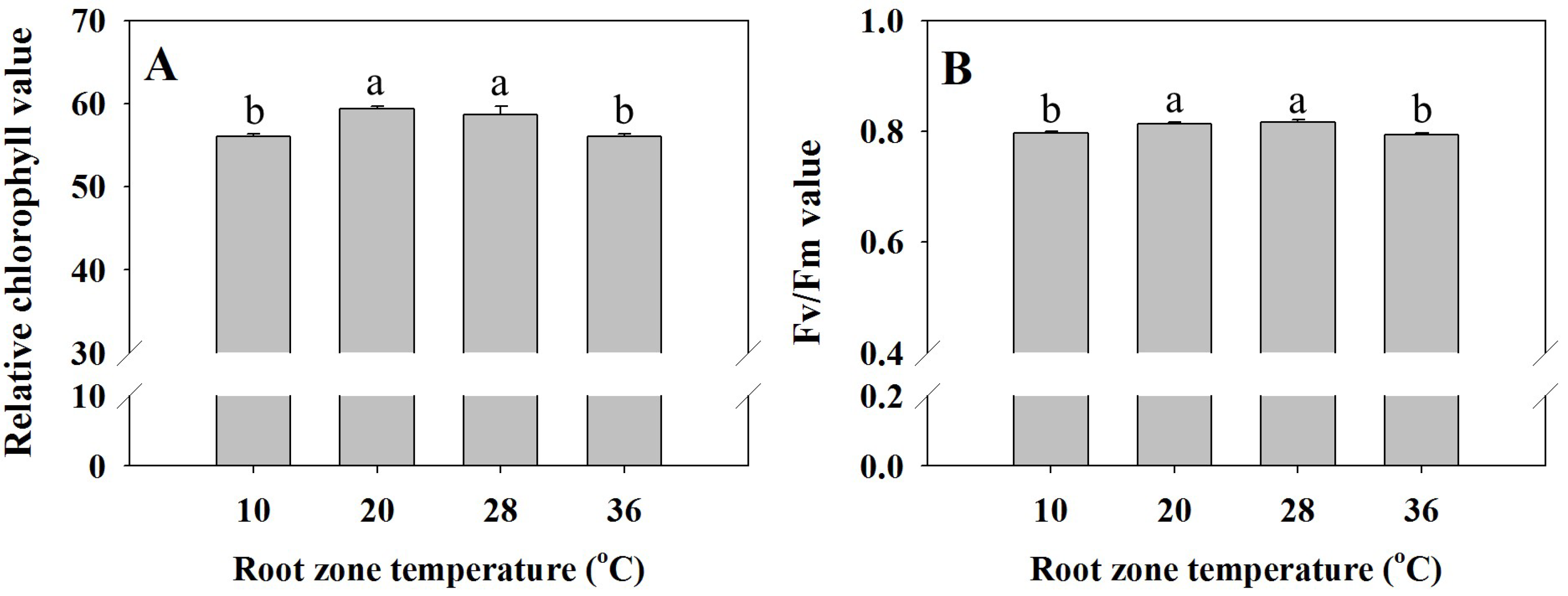
3.2. Chlorophyll Fluorescence (Fv /Fm) and Relative Chlorophyll Values
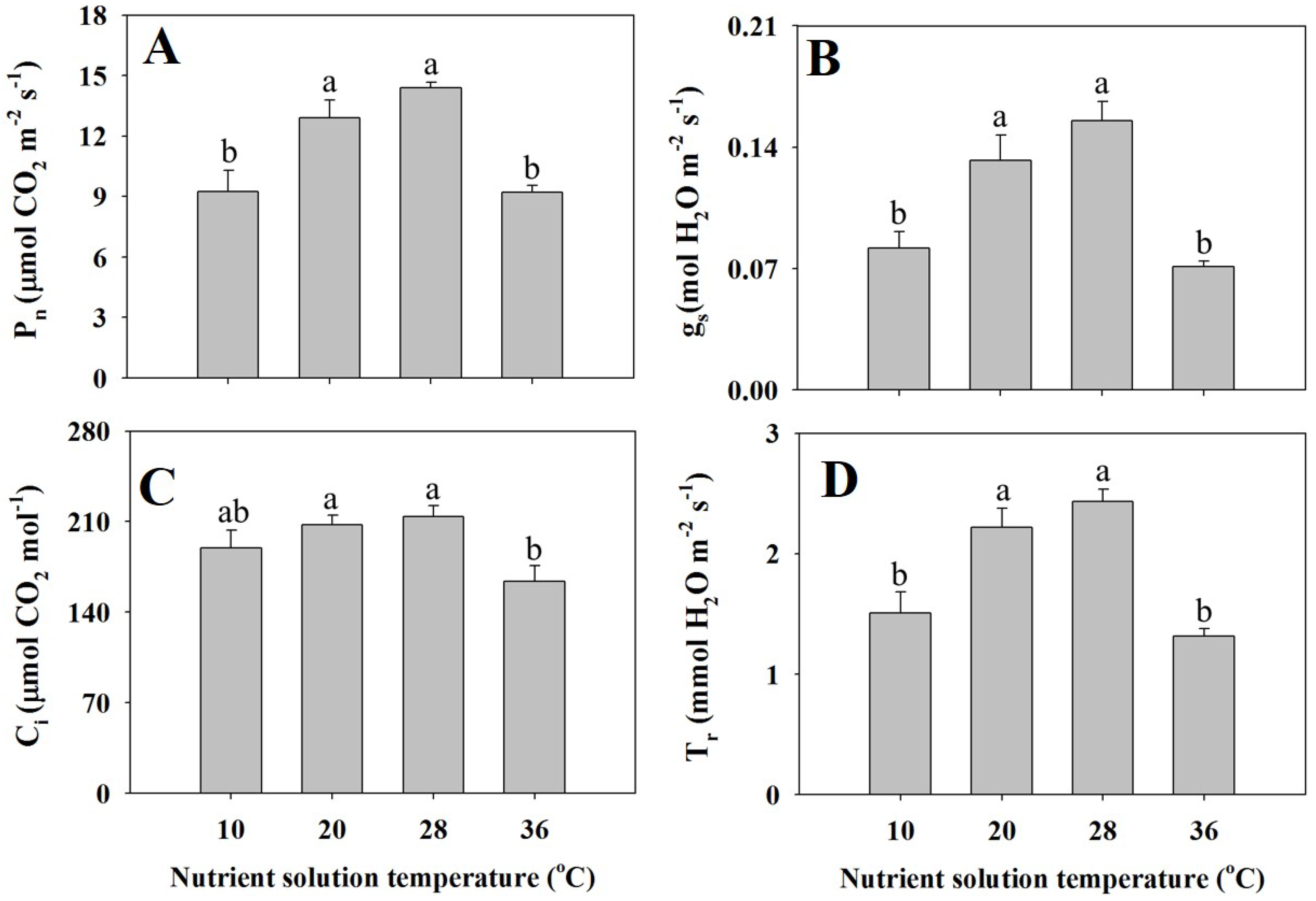
3.3. Leaf Gas Exchange Parameters
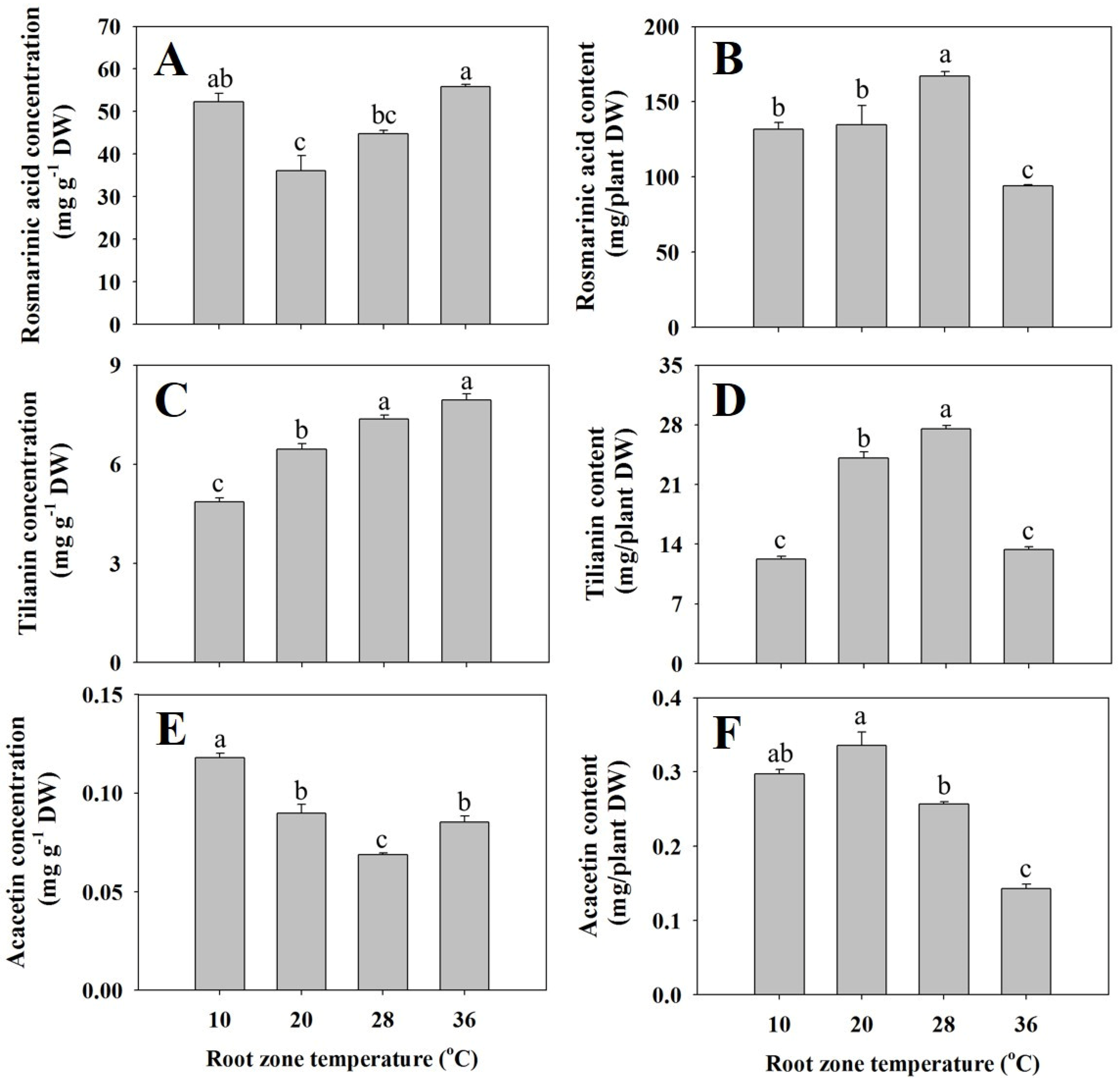
3.4. Rosmarinic Acid (RA), Tilianin, and Acacetin Concentrations and Contents
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zielinska, S.; Matkowski, A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem. Rev. 2014, 13, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.J.; Xu, S.F.; Yin, P.; Wang, W.; Song, X.Z.; Liu, F.H.; Xu, J.Q.; Zoccarato, I. Active components of common traditional Chinese medicine decoctions have antioxidant functions. J. Anim. Sci. 2011, 89, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Park, W.T.; Xu, H.; Park, N.I.; Park, S.U. Accumulation of tilianin and rosmarinic acid and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. J. Agric. Food Chem. 2012, 60, 5945–5951. [Google Scholar] [CrossRef] [PubMed]
- Yamani, H.; Mantri, N.; Morrison, P.D.; Pang, E. Analysis of the volatile organic compounds from leaves, flower spikes, and nectar of Australian grown Agastache rugosa. BMC Complement. Altern. Med. 2014, 14, 495. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: San Diego, CA, USA, 2015. [Google Scholar]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Takagaki, M. Optimization of photosynthetic photon flux density and root-zone temperature for enhancing secondary metabolite accumulation and production of coriander in plant factory. Agronomy 2019, 9, 224. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of root-zone temperature on growth and quality of hydroponically grown red leaf lettuce (Lactuca sativa L. cv. Red Wave). Am. J. Plant Sci. 2015, 6, 2350–2360. [Google Scholar] [CrossRef]
- Ogawa, E.; Hikosaka, S.; Goto, E. Effects of nutrient solution temperature on the concentration of major bioactive compounds in red perilla. J. Agric. Meteorol. 2018, 74, 71–78. [Google Scholar] [CrossRef]
- Hood, T.M.; Mills, H.A. Root-zone temperature affects nutrient uptake and growth of snapdragon. J. Plant Nutr. 1994, 17, 279–291. [Google Scholar] [CrossRef]
- Chung, S.J.; Chun, Y.T.; Kim, K.Y.; Kim, T.H. Root zone temperature effect in hydroponically grown cucumber plants: Growth and carbohydrate metabolism. Acta Hortic. 2002, 588, 53–57. [Google Scholar] [CrossRef]
- Qiuyan, Y.; Zengqiang, D.; Jingdong, M.; Xun, L.; Fei, D. Effects of root-zone temperature and N, P, and K supplies on nutrient uptake of cucumber (Cucumis sativus L.) seedlings in hydroponics. J. Soil Sci. Plant Nutr. 2012, 58, 707–717. [Google Scholar]
- Sakamoto, M.; Suzuki, T. Elevated root-zone temperature modulates growth and quality of hydroponically grown carrots. Agric. Sci. 2015, 6, 749–757. [Google Scholar] [CrossRef]
- Rhonda, M.B.S.; Henry, G.T.; Anthony, A. Effect of increasing root-zone temperature on growth and nutrient uptake by ‘gold star’ muskmelon plants. J. Plant Nutr. 1998, 21, 321–328. [Google Scholar]
- Bumgarner, N.R.; Scheerens, J.C.; Mullen, R.W.; Bennett, M.A.; Ling, P.P.; Kleinhenz, M.D. Root-zone temperature and nitrogen affect the yield and secondary metabolite concentration of fall- and spring-grown, high-density leaf lettuce. J. Sci. Food Agric. 2012, 92, 116–124. [Google Scholar] [CrossRef]
- Tan, L.P.; He, J.; Lee, S.K. Effects of root-zone temperature on the root development and nutrient uptake of Lactuca sativa L. “Panama” grown in an aeroponic system in the tropics. J. Plant Nutr. 2002, 25, 297–314. [Google Scholar] [CrossRef]
- Lam, V.P.; Kim, S.J.; Park, J.S. Optimizing the electrical conductivity of a nutrient solution for plant growth and bioactive compounds of Agastache rugosa in a plant factory. Agronomy 2020, 10, 76. [Google Scholar] [CrossRef]
- Wan, X.C.; Zwiazek, J.J.; Lieffers, V.J.; Landhausser, S.M. Hydraulic conductance in aspen (Populus tremuloides) seedlings exposed to low root temperatures. Tree Physiol. 2001, 21, 691–696. [Google Scholar] [CrossRef]
- Murai-Hatano, M.; Kuwagata, T.; Sakurai, J.; Nonami, H.; Ahamed, A.; Nagasuga, K.; Matsunami, T.; Fukushi, K.; Maeshima, M.; Okada, M. Effect of low root temperature on hydraulic conductivity of rice plants and the possible role of aquaporins. Plant Cell Physiol. 2008, 49, 1294–1305. [Google Scholar] [CrossRef]
- Yan, Q.Y.; Duan, Z.Q.; Mao, J.D.; Li, X.; Dong, F. Low root zone temperature limits nutrient effects on cucumber seedling growth and induces adversity physiological response. J. Integr. Agric. 2013, 12, 1450–1460. [Google Scholar] [CrossRef]
- Bugbee, B.; White, J. Tomato growth as affected by root-zone temperature and the addition of gibberellic acid and kinetin to nutrient solutions. J. Am. Soc. Hortic. Sci. 1984, 109, 121–125. [Google Scholar] [PubMed]
- Zhu, J.; Zhang, K.X.; Wang, W.S.; Gong, W.; Liu, W.C.; Chen, H.G.; Xu, H.H.; Lu, Y.T. Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol. 2015, 56, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Heckathorn, S.A.; Giri, A.; Mishra, S.; Bista, D. Heat stress and roots. In Climate Change and Plant Abiotic Stress Tolerance; Chapter 5; Wiley-VCH Verlag Gmb H & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Diaz-Perez, J.C.; Gitaitis, R.; Mandal, B. Effects of plastic mulches on root zone temperature and on the manifestation of tomato spotted wilt symptoms and yield of tomato. Sci. Hortic. 2007, 114, 90–95. [Google Scholar] [CrossRef]
- Malcolm, P.J.; Holford, P.; Barchia, I.; McGlasson, W.M. High and low root zone temperatures at bud-break reduce growth and influence dry matter partitioning in peach rootstocks. Sci. Hortic. 2014, 171, 83–90. [Google Scholar] [CrossRef]
- Ding, X.T.; Jiang, Y.P.; He, L.Z.; Zhou, Q.; Yu, J.Z.; Hui, D.F.; Huang, D.F. Exogenous glutathione improves high root-zone temperature tolerance by modulating photosynthesis, antioxidant and osmolytes systems in cucumber seedlings. Sci. Rep. 2016, 6, 35424. [Google Scholar] [CrossRef]
- Zarebanadkouki, M.; Trtik, P.; Hayat, F.; Carminati, A.; Kaestner, A. Root water uptake and its pathways across the root: Quantification at the cellular scale. Sci Rep. 2019, 9, 12979. [Google Scholar] [CrossRef]
- Odhiamboa, M.O.; Wang, X.C.; Antonioa, P.I.J.D.; Shia, Y.Y.; Zhaoa, B. Effects of root-zone temperature on growth, chlorophyll fluorescence characteristics and chlorophyll content of greenhouse pepper plants grown under cold stress in southern China. Russ. Agric. Sci. 2018, 44, 426–433. [Google Scholar] [CrossRef]
- Islam, M.Z.; Lee, Y.T.; Mele, M.A.; Choi, I.L.; Kang, H.M. The effect of phosphorus and rot zone temperature on anthocyanin of red romaine lettuce. Agronomy 2019, 9, 47. [Google Scholar] [CrossRef]
- Wan, X.C.; Landhausser, S.M.; Zwiazek, J.J.; Lieffers, V.J. Root water flow and growth of aspen (Populus tremuloides) at low root temperatures. Tree Physiol. 1999, 19, 879–884. [Google Scholar] [CrossRef]
- Hajihashemil, S.; Noedoostl, F.; Geuns, J.M.C.; Djalovic, L.; Siddique, K.H.M. Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef]
- Fujimura, S.; Suzuki, K.; Nagao, M.; Okada, M. Acclimation to root chilling increases sugar concentrations in tomato (Solanum lycopersicum L.) fruits. Sci. Hortic. 2012, 147, 34–41. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Guo, H.J.; Yuan, L.; Wei, J.N.; Zhang, W.H.; Ge, F. Plant stomatal closure improves aphid feeding under elevated CO2. Glob. Chang. Biol. 2015, 21, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Martin, R.; Gross, P.; Bogeat-Triboulot, M.B. Temperature effects on hydraulic conductance and water relations of Quercus robur L. J. Exp. Bot. 2000, 51, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Medori, M.; Michelini, L.; Nogues, I.; Loreto, F.; Calfapietra, C. The impact of root temperature on photosynthesis and isoprene emission in three different plant species. Sci. World J. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Lee, S.Y.; Xu, H.; Kim, Y.K.; Park, S.U. Rosmarinic acid production in hairy root cultures of Agastache rugosa Kuntze. World J. Microbiol. Biotechnol. 2008, 24, 969–972. [Google Scholar] [CrossRef]
- He, F.; Thiele, B.; Watt, M.; Kraska, T.; Ulbrich, A.; Kuhn, A.J. Effects of root cooling on plant growth and fruit quality of cocktail tomato during two consecutive seasons. J. Food Qual. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Singh, L.P.; Issenmann, B.; Caupin, F. Pressure dependence of viscosity in supercooled water and a unified approach for thermodynamic and dynamic anomalies of water. Proc. Natl. Acad. Sci. USA 2017, 114, 4312–4317. [Google Scholar] [CrossRef]

| RZT z (°C) | Leaf Length (cm) | Leaf Width (cm) | Number of Leaves (leaves) | Leaf Area (cm2) | Stem Length (cm) | SFW (g) | RFW (g) | Root Length (cm) | SDW (g) | RDW (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 7.92 b y | 7.29 b | 51.12 b | 563.71 b | 34.12 b | 16.56 b | 9.43 b | 29.56 b | 1.91 b | 0.54 b |
| 20 | 8.55 a | 8.39 a | 75.50 a | 819.20 a | 40.23 a | 24.49 a | 14.31 a | 49.99 a | 2.98 a | 0.77 a |
| 28 | 8.38 a | 8.17 a | 77.00 a | 810.25 a | 40.61 a | 25.43 a | 13.98 a | 51.15 a | 2.93 a | 0.79 a |
| 36 | 6.57 c | 6.02 c | 43.87 b | 402.77 c | 23.29 c | 11.93 c | 1.49 c | 3.80 c | 1.60 c | 0.17 c |
| Significance x | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| RZT Z (°C) | RA Concentration in Plant Organs (mg·g−1 DW) | Tilianin Concentration in Plant Organs (mg·g−1 DW) | Acacetin Concentration in Plant Organs (mg·g−1 DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Flowers | Stems | Roots | Leaves | Flowers | Stems | Roots | Leaves | Flowers | Stems | Roots | ||
| 10 | 5.122 cy | 5.948 a | 3.357 b | 37.915 a | 1.078 c | 2.606 c | 1.149 b | 0.033 b | ND | 0.086 a | 0.032 a | ND | |
| 20 | 5.496 bc | 5.890 a | 3.585 b | 21.083 b | 1.387 b | 3.485 b | 1.549 a | 0.027 b | ND | 0.057 b | 0.032 a | ND | |
| 28 | 5.781 b | 5.065 b | 3.316 b | 30.587 a | 1.858 a | 4.302 a | 1.183 b | 0.028 b | ND | 0.036 c | 0.032 a | ND | |
| 36 | 14.380 a | 6.485 a | 4.195 a | 30.819 a | 1.672 a | 4.309 a | 1.799 a | 0.643 a | ND | 0.057 b | 0.028 b | ND | |
| Significance x | *** | *** | *** | ** | *** | *** | *** | *** | ND | *** | ** | ND | |
| RZT z (°C) | RA Content in Plant Organs (mg/Plant Organs DW) | Tilianin Content in Plant Organs (mg/Plant Organs DW) | Acacetin Content in Plant Organs (mg/Plant Organs DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Flowers | Stems | Roots | Leaves | Flowers | Stems | Roots | Leaves | Flowers | Stems | Roots | ||
| 10 | 5.719 cy | 2.259 a | 1.544 c | 21.240 ab | 1.203 c | 0.990 b | 0.528 c | 0.018 b | ND | 0.032 a | 0.015 c | ND | |
| 20 | 10.497 b | 1.649 c | 2.761 a | 16.498 b | 2.649 b | 0.976 b | 1.193 a | 0.021 ab | ND | 0.016 b | 0.025 a | ND | |
| 28 | 10.773 b | 1.874 b | 2.409 b | 23.658 a | 3.463 a | 1.593 a | 0.860 b | 0.022 ab | ND | 0.013 bc | 0.023 b | ND | |
| 36 | 13.373 a | 1.360 d | 1.594 c | 5.029 c | 1.555 c | 0.903 b | 0.683 bc | 0.026 a | ND | 0.012 c | 0.011 d | ND | |
| Significance x | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, V.P.; Kim, S.J.; Bok, G.J.; Lee, J.W.; Park, J.S. The Effects of Root Temperature on Growth, Physiology, and Accumulation of Bioactive Compounds of Agastache rugosa. Agriculture 2020, 10, 162. https://doi.org/10.3390/agriculture10050162
Lam VP, Kim SJ, Bok GJ, Lee JW, Park JS. The Effects of Root Temperature on Growth, Physiology, and Accumulation of Bioactive Compounds of Agastache rugosa. Agriculture. 2020; 10(5):162. https://doi.org/10.3390/agriculture10050162
Chicago/Turabian StyleLam, Vu Phong, Sung Jin Kim, Gwon Jeong Bok, Jong Won Lee, and Jong Seok Park. 2020. "The Effects of Root Temperature on Growth, Physiology, and Accumulation of Bioactive Compounds of Agastache rugosa" Agriculture 10, no. 5: 162. https://doi.org/10.3390/agriculture10050162
APA StyleLam, V. P., Kim, S. J., Bok, G. J., Lee, J. W., & Park, J. S. (2020). The Effects of Root Temperature on Growth, Physiology, and Accumulation of Bioactive Compounds of Agastache rugosa. Agriculture, 10(5), 162. https://doi.org/10.3390/agriculture10050162





