1. Introduction
Leonardite is a product of atmospheric oxidation (part of the weathering process) of lignite (brown coal). This conversion occurs on a large scale, significantly impacting lignite properties in a negative manner, i.e., leading to structural weakness, excessive fragility, and loss of other inherent qualities of parental coal. As described in many literature works, leonardite represents sediments enriched in humic acids, which occur at shallow depths [
1,
2]. The oxygen exposure of lignite leads to various heterogeneous oxidation reactions, mainly by impacting the aliphatic moieties, rather than the aromatic ones [
3]. Although the details of the oxidation mechanisms of lignite are unclear, it is possible to propose that the introduction of additional carboxyl, hydroxyl, amino, and nitro groups plays a crucial role [
4].
As a result of weathering, the valuable properties of the parental coal as a fuel source deteriorate. In many cases, it cannot be used for energy production due to the low calorific value and extreme fragmentation. For this reason, leonardite is not taken into account when calculating coal reserves and is commonly marked as off-balance or run-of-mine coal [
5].
Compared with high-rank coals, such as bituminous coal and anthracite, the low-rank coals (lignite, leonardite, or some others) typically have a low energy content (10 to 20 MJ/kg), low carbon content (60%–70%), and retain great fractions of moisture (up to 70%) [
6].
Such low-rank coals from outcrops or abandoned surface mines create the need for finding alternative solutions to their disposal. They are usually dispersed over large areas, which complicates their utilization. The reserves of such mineral sediments are very large and may reach 500 billion tons worldwide [
7].
Low-rank coals, however, are the anticipated source of humic substances that can be used for rehabilitation and reclamation of degraded lands. The possibility of using leonardite for soil amendments and conditioners, including the production of humic products, has been documented in previous studies [
8,
9]. Oxidized and metamorphosed coals contain substantial amounts of humic acids, having properties and a composition close to those of the humic acids found in usual soils and sediments [
10,
11,
12]. Leonardite contains 25%–85% humic acids, while soils on average contain only 1%–5% humic acids [
13,
14,
15]. This circumstance offers a novel and robust way to study the possibility of producing favorable humic products from coal discards.
Low-rank coals, being one of the reserves of nutrients in the soil, are known to contain the elements necessary for the growth and development of plants [
5]. Published studies show that oxidized coals improve the physical properties of soil by increasing its sorption ability due to organic humified substances, subsequently improving the mineral nutrition of plants and their provision with microelements [
4,
12,
16].
Distribution and stability of different forms of nitrogen in the soil are determined mainly by the microbiological activity of soil. Several recent studies indicate that low-rank coals and their products increase the crop yields by improving the microbe-mediated biochemical properties of soil [
17,
18,
19]. The input of coal-based substances has a great effect on the enzymatic activity and dynamics of the mineral nitrogen forms in soil.
With respect to plants, the stimulating and protecting effects of coal-derived humic substances have been illustrated in many comprehensive studies, showing their positive effect on crop yields and soil fertility [
20,
21]. Yet extensive experience needs to be gained in the practical application of various types of humic-rich coal residues in a wide variety of soil and climatic conditions. For example, one potential application of oxidized coal is its use in its raw/crude form as humic-based soil amendments for different crops and soil management [
1]. There are also many contradictory opinions regarding the influence of carbon-based substances on soil health and fertility, as well as their effects on plant growth and productivity. Coal-based organic amendments promote the binding characteristics of heavy metals, which can be both positive and negative, depending on the level of trace elements in the soil and their physiological role [
22,
23].
The reported effects of humic acid dosage on potato plants’ growth and yield are not always consistent. Several studies [
20,
24,
25] have shown that the supplementation of humic substances in appropriate concentrations can stimulate potato growth and enhance tuber formation. The beneficial effects of humic acids include, firstly, better nitrogen compound uptake by potato, thus promoting soil nutrient utilization and secondly, an increase in the availability and uptake of potassium, calcium, magnesium, and phosphorus, as well as trace minerals [
26,
27].
Applications of leonardite directly and leonardite-derived humic substances as soil amendments/conditioners and plant stimulants are expected to improve the physicochemical and biological aspects of soil and promote plant growth. However, researches are still limited in terms of how leonardite-based amendments affect the soil microbial community structure and potato plant growth. Therefore, the objectives of this research were (a) to characterize leonardite and humic substances extracted from it and (b) to investigate their impact on the soil microbiome, as well as (c) to examine the effects on potato growth and tuber yield. The experimental results presented here should provide further insights into the rational utilization of low-rank coal for sustainable production of crops.
2. Materials and Methods
2.1. Leonardite and Humic Acid Extraction
2.1.1. Leonardite Sample Collection and Charachterization
Leonardite collected from the Oi-Karagay coal basin in Almaty region, Kazakhstan (43°11′35.5″ N 80°35′42.8″ E) was selected for this study. Coal sampling was carried out according to the technique described by Dai et al. [
28] and stored at 4 °C in sealed plastic bags. The ultimate (comprehensive quantitative analysis of various elements, including carbon, hydrogen, sulfur, oxygen, and nitrogen) and proximate (major physical properties, such as heating value, moisture, volatile compounds, ash content) analyses of the leonardite samples were performed in accordance with ASTM standards (ASTM D3176-15: Standard Practice for Ultimate Analysis of Coal and Coke [
29] and ASTM 5373-16: Standard Test Methods for Determination of Carbon, Hydrogen, and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke [
30]).
2.1.2. Extraction of Humic Acid
The pulverized and sieved, to a particle size of <0.2 mm, parental leonardite (PL) was treated with 0.25M NaOH by constant stirring at 20 °C for 12 h, after which it was centrifuged at 2500×
g for 10 min, where the soluble humic acid was separated from the insoluble humin sediments. In the following stage, humic acid was precipitated by adjusting the pH to 2.0 using 2M HCl. The solution was allowed to sediment for 24 h, followed by centrifugation at 2500×
g for 10 min, then washed 3 times with dH
2O, and dried at 60 °C in a drying cabinet [
31]. The resulting solid product was further referred to as LHA (leonardite-derived humic acid). The humic acid yield was calculated on an air-dried basis according to the formula [
32]:
where ε is the yield of humic acid, %; M
YM = the mass of leonardite, g; M
CY = the mass of the residual coal, g; and M
ad = the water content in raw coal, %.
2.2. Characterization of Parental Leonardite and LHA
The parental leonardite (PL) and LHA were characterized by Fourier-transform infrared spectroscopy (FTIR), Raman spectroscopy, and elemental analysis. Preparation and analysis of the samples were carried out in full accordance with the device manufacturer’s protocols.
FTIR spectroscopy was performed using a Nicolet 6700 FTIR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The IR spectra of the samples were recorded in the range between 400 and 4000 cm−1 with 32 scanning times at a 4 cm−1 resolution.
The Raman spectra of the samples were characterized by an automated AFM-Raman Solver Spectrum system (NT-MDT Spectrum Instruments, Moscow, Russian Federation) system using a diode laser with a wavelength 473 nm. The laser beam was focused on a 2-μm spot diameter with the Mitutoyo 100 × lens (NA = 0.7).
The elemental composition of the samples was determined using a Vario EL cube Elemental Analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany). The difference to 100% was assigned to the oxygen content.
2.3. Soil Collection, Characterization, and Treatment
Dark-chestnut soil was obtained from the Botanical Garden in Almaty city, Kazakhstan (43°13’07.9” N 76°54’49.6” E). Soil samples were randomly collected in the 0–20-cm depth at least 5 m from the nearest trees. The soils were then air-dried and sieved to 2 mm, pooled on-site, and stored at 4 °C for a maximum of 2 weeks before starting the experiments. The physicochemical properties of the soil were characterized according to Berndt-Michael Wilke [
33].
The pH values of each soil group were tested 3 months after amendment application and before plant cultivation. pH measurements were performed on suspensions of 5 g of air-dried soil samples in 25 mL of fresh dH2O using the 781 pH-meter (Metrohm AG, Herisau, Switzerland).
The soil was amended with LHA (such samples are further named SLHA, i.e., soil (S) with LHA) and with PL directly (further referred to as SPL, i.e., soil (S) with PL), thus representing two treatments, in addition to the control represented by untreated soil. In detail, LHA (dry weight basis) was applied to the soil at 1 g·kg−1 upon mixing. The PL dose was determined according to the soil characterization to supplement the nutrient content of the soil. Freshly mined leonardite, passed through a 2-mm mesh sieve, was mixed with soil (ratio 1.5 g to 1 kg, both dry weight).
2.4. Microbial Diversity Analysis
2.4.1. DNA Extraction and PCR Amplification
Microbial DNA was extracted from all soil sample groups (SLHA, SPL, and control) using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s protocols. The final DNA concentration and purity were determined by a NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and the DNA quality was checked by 1% agarose gel electrophoresis. The V3-V4 hypervariable regions of the bacteria 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by a thermocycler PCR system (GeneAmp 9700, ABI, Waltham, MA, USA). The PCR reactions were conducted using the following program: 3 min of denaturation at 95 °C, 27 cycles of 30 s at 95 °C, 30 s for annealing at 55 °C, and 45 s for elongation at 72 °C, and a final extension at 72 °C for 10 min. PCR reactions were performed in triplicate in a 20-μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu Polymerase, and 10 ng of template DNA. The resultant PCR products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using QuantiFluor™-ST (Promega, Waltham, MA, USA) according to the manufacturer’s protocol.
2.4.2. Illumina MiSeq Sequencing
Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) according to the standard protocols established by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
2.4.3. Processing of Sequencing Data
Raw fastq files were demultiplexed, quality filtered by Trimmomatic, and merged by FLASH using the following criteria: (I) The reads were truncated at any site receiving an average quality score <20 over a 50-bp sliding window. (II) Primers were exactly matched, allowing 2 nucleotide mismatching, and reads containing ambiguous bases were removed. (III) Sequences that overlapped longer than 10 bp were merged according to their overlapping sequence. Operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff using UPARSE (version 7.1
http://drive5.com/uparse/). Chimeric sequences were identified and removed using UCHIME algorithm (
https://drive5.com/uchime). The taxonomy of each 16S rRNA gene sequence was analyzed by the RDP Classifier algorithm (
http://rdp.cme.msu.edu/) using the Silva (SSU123) 16S rRNA database with a confidence threshold of 70%.
The soil microbiome diversity within samples (α-diversity) was assessed by the Shannon index, Simpson index, Chao1 richness index and ACE richness index, using QIIME (1.9.1 pro). All bacterial community analyses were performed using the free online platform Majorbio I-Sanger Cloud Platform (
www.i-sanger.com).
2.5. Greenhouse Experiments
The Agata table potato cultivar (
Solanum tuberosum L. cv.
Agata) was chosen for this experiment. In the first quarter of the year, single potato tubers of high sanitary quality were planted in 6-L plastic pots (
n = 15) filled with a sandy loam soil in the greenhouse. The soil was pasteurized with steam to ensure pathogen and weed seed destruction as described in [
34]. Then, 10 g of Bionic’s organic fertilizer (contains N (150 g/m
3), P
2O
5 (130 g/m
3), and K
2O (210 g/m
3)) were mixed with 1.5 kg of soil at potting.
The potato plants were harvested when growth stage 909 (the BBCH scale (German: Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie) was achieved [
35,
36]. The plants yielded enough tubers to be further planted in three different soil types. Undamaged healthy tubers of approximately the same size were selected in order to produce uniform plants. In the second quarter of the year, the tubers were randomly and blindly (to avoid unintentional manipulation) planted in 10-L plastic pots filled with (I) a control soil, (II) SLHA-soil (amended with 1 g/kg-1 LHA), and (III) SPL-soil (supplemented with 1.5 g/kg
−1 PL). Plants were cultivated with 15 replicates per soil type. One tuber was planted per pot and the first sprout that emerged on the soil surface was regarded as the main stem. All plants in separated pots were blindly and randomly placed in possibly equal illumination conditions. An automatic temperature control system in the greenhouse was set at 23 °C with a relative humidity of 50% during the day and at 21 °C with a 35% relative humidity during the night. All plants were equally well-watered in a blinded manner: 1 L of water was added to each pot every third day. Like in the previous stage, the plants were harvested when growth stage 909 of the BBCH scale was reached [
35].
2.6. Plant Measurements
Phenotypic growth and yield data were recorded at the harvest time for selected potato plants in each treatment. The observations on growth parameters, including the number of stems (009 of the BBCH scale) and plant height (805 of the BBCH scale), were recorded according to the study by Hack et al. [
35]. The number of tubers and their weight, as well as the yield, were measured at maturity. Harvested tubers were weighted and manually sorted into three categories (small: <80 g; medium: 81–150 g; and large: >151 g) for counting.
2.7. Statistical Analysis
Most measurement values represent mean values ± standard deviation (SD). The analysis of treatments on potato growth and tuber yield was conducted using the one-way analysis of variance (ANOVA) method (SPSS Statistics, version 26.0, Chicago, IL, USA). The significance of differences among means was evaluated by using Duncan’s multiple range test with the significance level of 0.05. Statistical differences between the microbial communities associated with each treatment (control, SPL, and SLHA) were determined by two-sided Fisher’s exact test (n = 13 per each group).
4. Discussion
Maintaining soil functional integrity and sustainability is a high priority in intensive agriculture development. Long-term application of non-renewable chemical fertilizers and pesticides has a negative impact on soil health and causes environmental problems. Therefore, current concern in agriculture is related to the gradual replacement of chemicals with organic amendments and improvement of their efficiency by adopting proper application techniques [
5,
49]. Leonardite, due to the presence of humic acids in it, can be suitable for soil amendment [
2,
50].
In our study, the technical characterization of raw leonardite samples confirmed that they correctly reckoned among low-rank coals with a low calorific value. However, the measured high humic acid content (71.1%) in the leonardite samples indicated its potential value for soil amendment. Elemental characterization confirmed that LHA had a higher nitrogen content, and therefore great potential to stimulate biological activity in soil [
51]. Besides, the O/C, and N/C ratios demonstrated that LHA is rich in oxygen- and nitrogen-containing groups. These data are comparable with other reported results for different coal-derived humic acids [
39,
41].
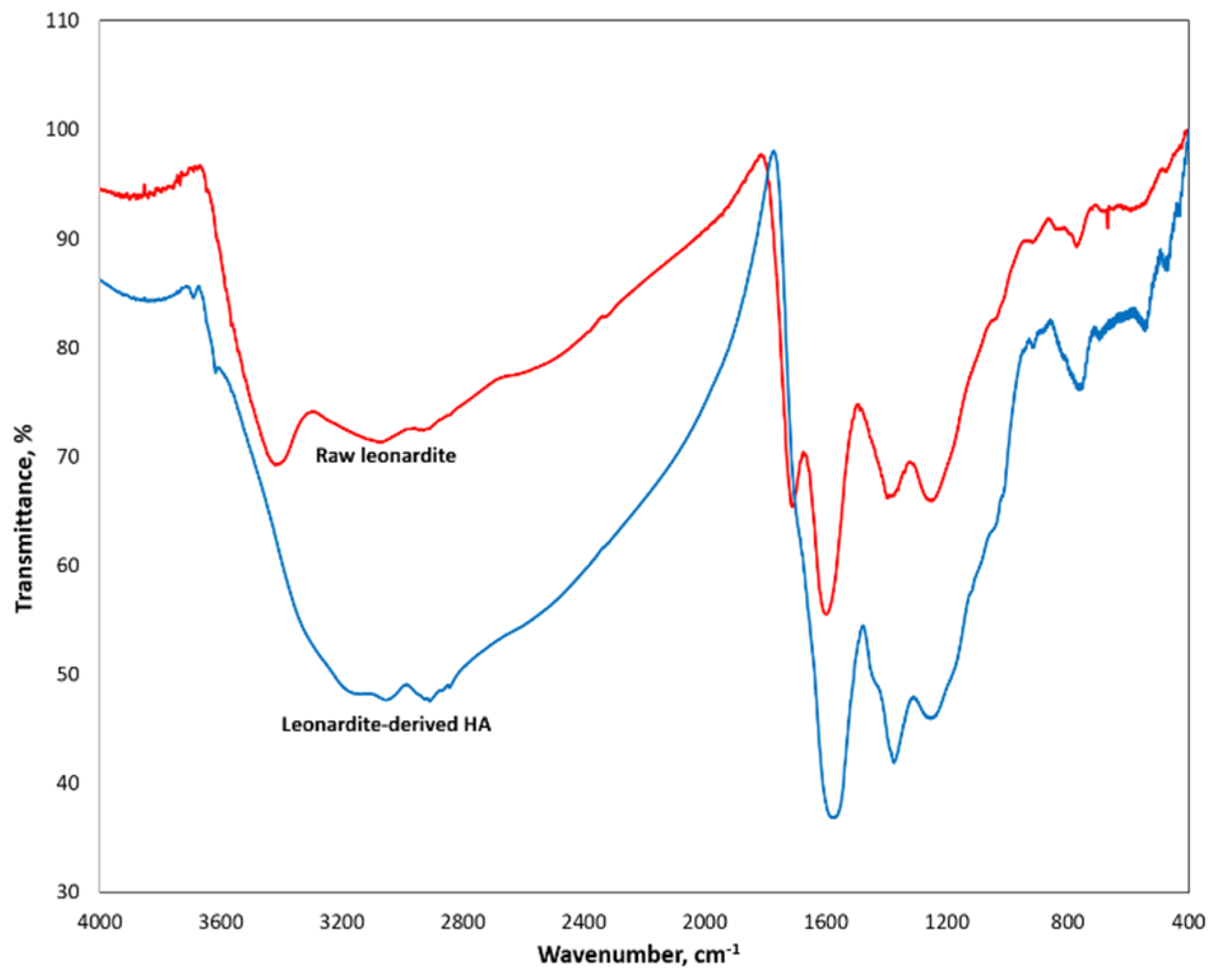
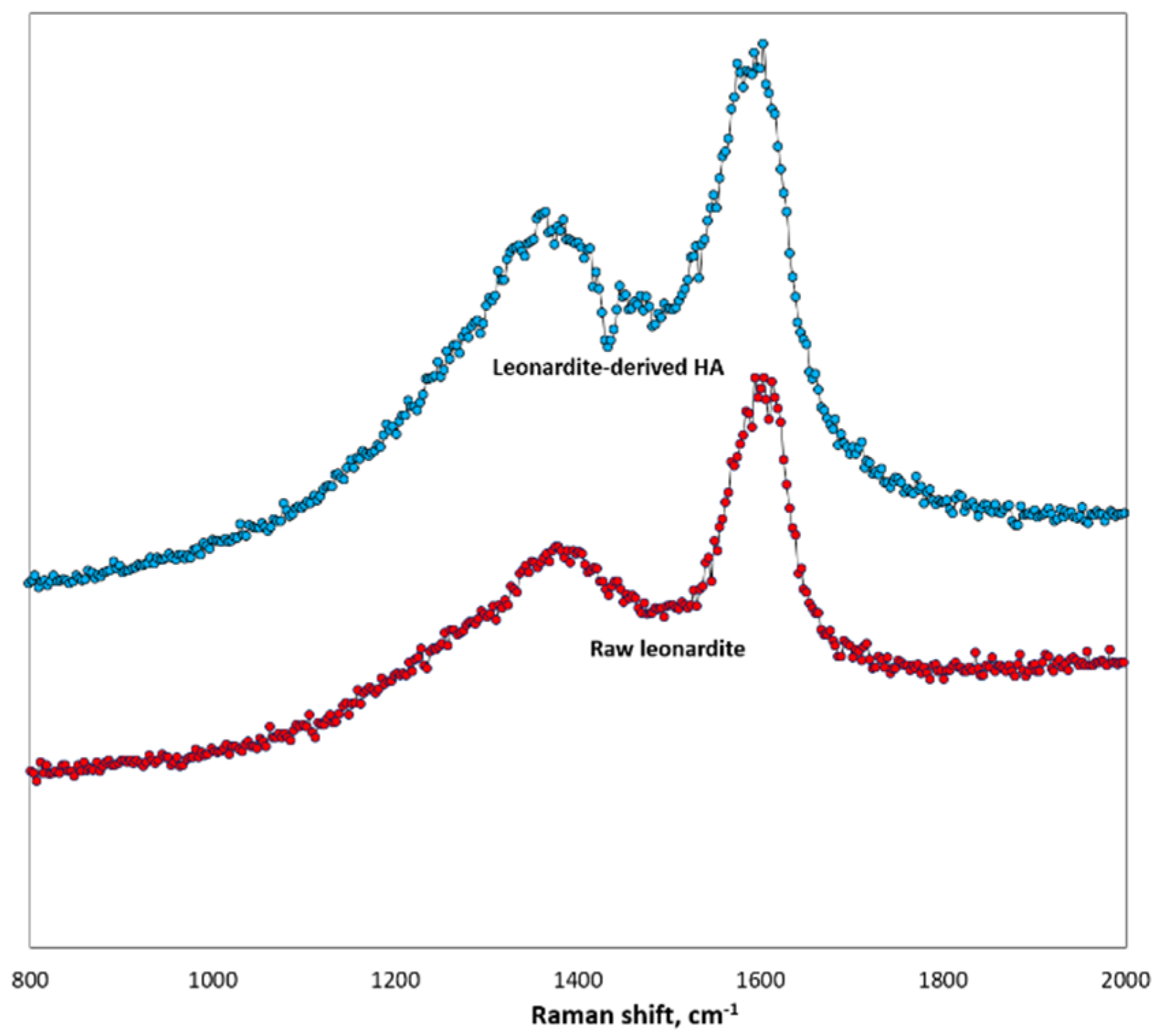
According to FTIR analysis, the LHA and PL samples had similar spectra. Their main absorption peaks were at 3050, 2900, 1260−1240, and 1070−1020 cm
−1 and attributed to aromatic C–H, aliphatic C–H, carboxylic C–O, and polysaccharide C–O–C functional groups [
42]. However, the intensity of absorption bands around 1600 and 3100 cm
−1 were greater for LHA, reflecting a larger amount of N-containing groups.
The reported concentrations of humic substances used for soil treatment vary significantly. Chen and Aviad [
52] estimated the average dosage for field applications as 75 kg humic substances per hectare (the values ranged between 20–225 kg·ha
−1) based on the midpoint average benefits of humus application. Thus, using leonardite with a 70% humic substances content, the amount required would be approximately 110 kg·ha
−1, laying in the range 30–350 kg·ha
−1. In contrast with commercially available humic substances, which have been extensively studied in greenhouse conditions, data for leonardite-derived humic substances are scarce. The applicable concentration of leonardite-based soil amendments may be very variable, thus complicating the determination of effective treatment rates. In addition, the structural/compositional characteristics of mineral-derived humic substances may differ from those of soil [
53,
54]. Other factors, such as the methods of extraction/purification, pretreatment, and application of humic acids, may also have considerable influence on the overall crop outcome [
20,
55]. We hope that our data reported here could contribute to the better clarity and uniformity of the values.
In our case, the 1 g·kg
−1 LHA dosage was chosen for further analysis steps for the following reasons: (1) The HA rates in the pot condition may be higher than in the field trials, (2) the tested soils had a relatively low organic matter content and were treated just with a single dose of HA throughout the experiment, and (3) the bioavailability of leonardite-derived humic acids may differ from those of soil and peat. Likewise, Asik et al. [
56] suggested treating saline soil with leonardite-derived humic acid at a dose of 1 g·kg
−1 for wheat growth and productivity. In our case, 1.5 g·kg
−1 PL was used due to the fact that it had a high yield potential of humic acid (71.1%).
The effect of raw leonardite as a soil amendment may significantly vary with the origin and dose of the leonardite applied, the environmental conditions, the species of plant, and the soil type to which it is applied. According to Akinremi et al., the agronomic productivity of canola plant increased when 3.3 g·kg
−1 of leonardite was applied to the soil [
1].
The impact of humic acids on the uptake of essential anionic macronutrients (such as nitrate, sulphate, and phosphate) has been discussed elsewhere [
57,
58], indicating the great role of pH. Our results indicated that the soil pH three months after the treatment remained above 7.0 for the SLHA and the control group, while the pH in the SPL group changed to lower values. It is well known that a shift in soil pH leads to alterations in the soil microbial communities [
13,
59]. The effects of organic matter on the soil ecosystem are primarily attributed to metabolism activation of soil microbial communities. Available organic matter in the soil ecosystem is decomposed by microorganisms retaining C and N in their biomass and releasing CO
2, CH
4, and NO
2 into the atmosphere [
45]. Many chemical transformations of humic-based soil amendments are mediated by heterotrophic microorganisms [
60,
61]. Studies show that the introduction of humic substances into the soil usually affects the community composition and numbers of soil bacteria and to a lesser extent soil fungi, actinomycetes, and microalgae [
13,
62]. To date, metagenomic approaches have become a valuable method of choice in establishing a microbial population structure and diversity. Studies on the effect of coal-based humic substances on the soil microbiome are scarce. However, published data obtained by using 16S rRNA gene-based phylogenetic microarrays revealed a great impact of commercial humic products on the resident bacterial community in various soil profiles [
17,
21,
63].
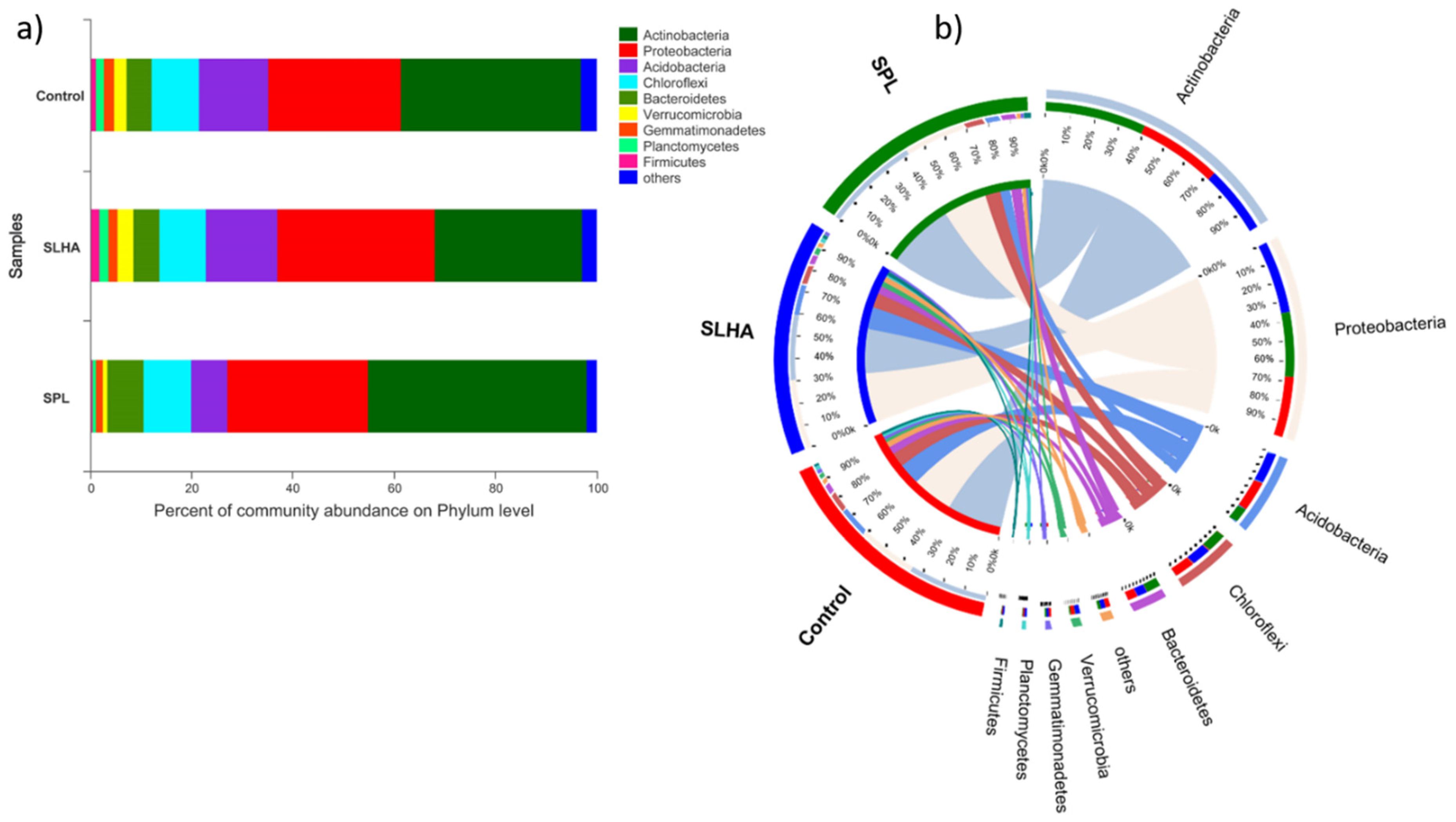
The microbial community composition of SLHA contained predominantly
Proteobacteria, which could possess plant growth-promoting properties, providing nutrients that are easy to uptake by the plant [
64]. The domination of
Proteobacteria in the SLHA samples may also be associated with humic substances’ depolymerization, which proceeds humic acid degradation reactions [
65,
66].
The observed increase in the tuber yield in response to the LHA and PL treatments can be obviously deduced to the rise in the relative number of stems and tubers. Our findings on the SLHA stimulative effects are in good agreement with the results reported by Z. Ekin and earlier by R. Selladurai et al. [
67,
68], who revealed that humic acid treatment significantly increased the yield of potato compared to the control under both greenhouse and field conditions.
Soil supplementation with PL had a less significant effect on plant growth and tuber yield. However, due to the complex nature of leonardite, it is difficult to characterize all the reactions involved in coal conversion in soil and microbial degradation of coal organic matter (making it available for uptake by plants). Noteworthy, a high abundance of
Actinobacteria was observed in the SPL samples. Due to the filamentous nature, the
Actinobacteria can penetrate the smaller pores within the coal matrix, taking full advantage of growth. In addition, many members of
Actinobacteria produce biosurfactants that contribute to the solubilization of hydrocarbons and facilitate the uptake of difficult-to-access carbon sources [
69,
70]. The reaction of these bacterial communities indicates the good leonardite biodegradation potential in the soil, provided enough time is allowed. Recent studies by S.J. Robbins et al. [
71] and A. Detman et al. [
72] also suggested that techniques like bioaugmentation (inoculation of exogenous degrading microorganisms to the soil) [
73] and biostimulation (stimulation of the degrading capacity of the indigenous community by adding nutrients to avoid metabolic limitations) [
73] can be very interesting options for the facilitation of leonardite degradation. As visible from the given examples, consideration of the issues related to microbial dynamics is important for interpreting long-term soil quality changes when leonardite is directly introduced into the soil.
5. Conclusions
In summary, the present study suggests beneficial impacts of leonardite-derived amendments on potato plant growth and soil microbial community structure. According to our metagenomic analysis, the soil samples amended with coal-based humic acids displayed high microbial diversity and richness compared to the control. The greenhouse trials demonstrated that both the plant growth and tuber yield were affected by the supplementation of the soil with leonardite-based amendments.
Humic acids, being the most important component of any soil, may represent an enzymatically active complex, which can trigger various reactions that are usually assigned to the microbial metabolic activity. The observed effects of the supplementation may presumably be attributed to (a) lowering of the pH soil samples; (b) higher concentration and availability of nitrogen-containing functional groups; (c) better ion-exchange capacity; (d) better water retention capacity; (e) facilitation (heterophase catalysis) of certain biochemical reactions; and (f) hypothetic adaptogenic mechanisms, etc.
Our findings indicated stimulating effects of leonardite-derived humic substances on plant growth and tuber yield. The humic acid compounds from leonardite may provide useful options in developing sustainable agricultural technologies for soil amendments and organic fertilizers in an ecologically responsible manner.
However, some limitations and other issues should be addressed in the future in order to successfully implement the positive effects of leonardite-based amendments on plant growth and yield. The impact of humic-based coal residues on phylogenetic distinct and abundant groups of microorganisms still lacks an adequate understanding. The important aspects for future studies include the heterogeneity, variability, and complexity of coal-derived humic substances; lack of valid experimental studies on an amendment dosage depending on the soil type, exact definitions of dose–response relationships; necessity for a better understanding of the underlying mechanism of LHA in plant growth promotion and development; etc.