Factors Governing Total and Permanganate Oxidizable C Pools in Agricultural Soils from Southern Italy
Abstract
1. Introduction
2. Materials and Methods
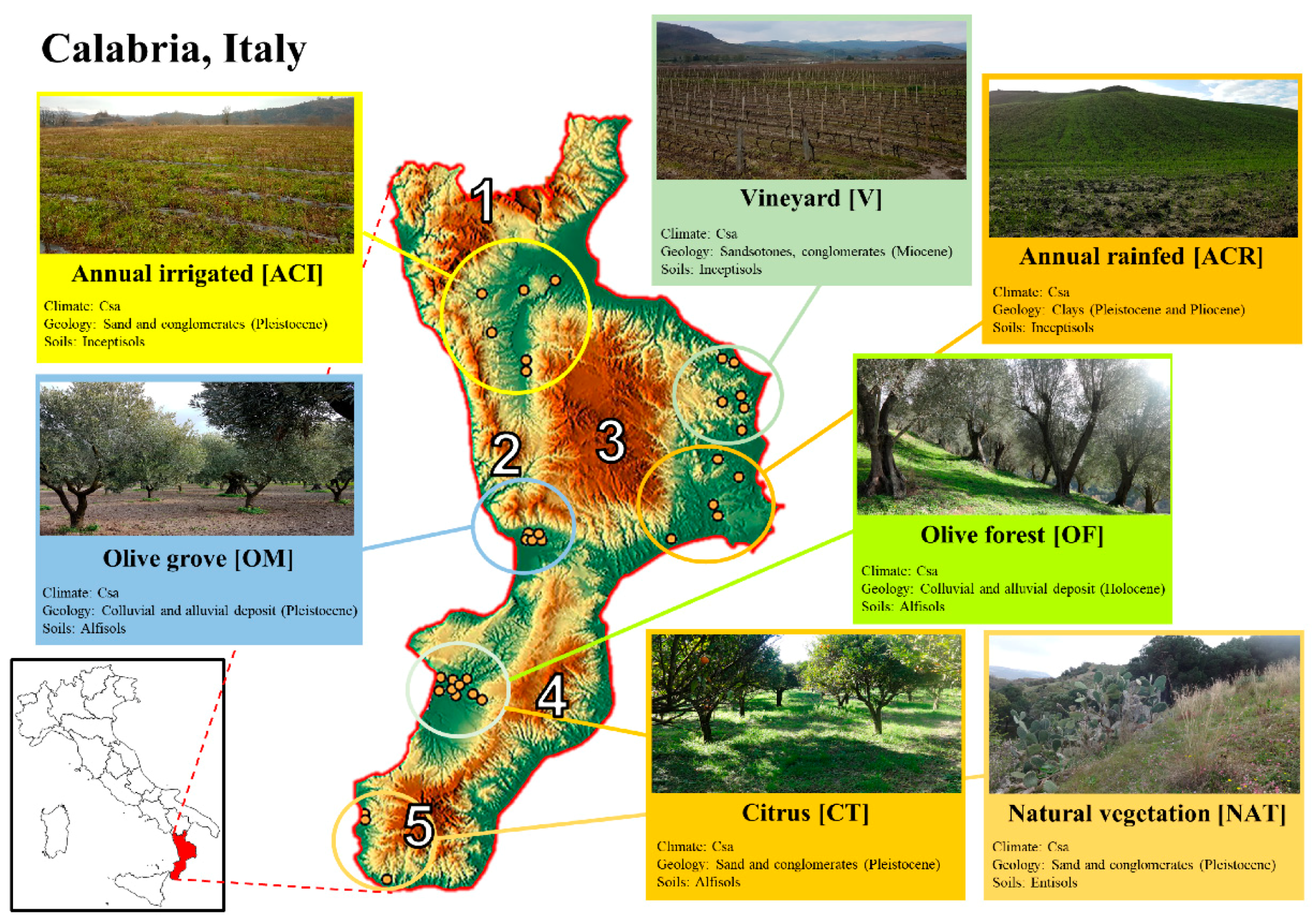
2.1. Study Area
2.2. Sampling Sites
2.3. Soil Sampling
2.4. Sample Preparation and Analysis
2.5. Climatic Data
2.6. Data Calculation and Statistical Analyses
3. Results
3.1. Soil Properties
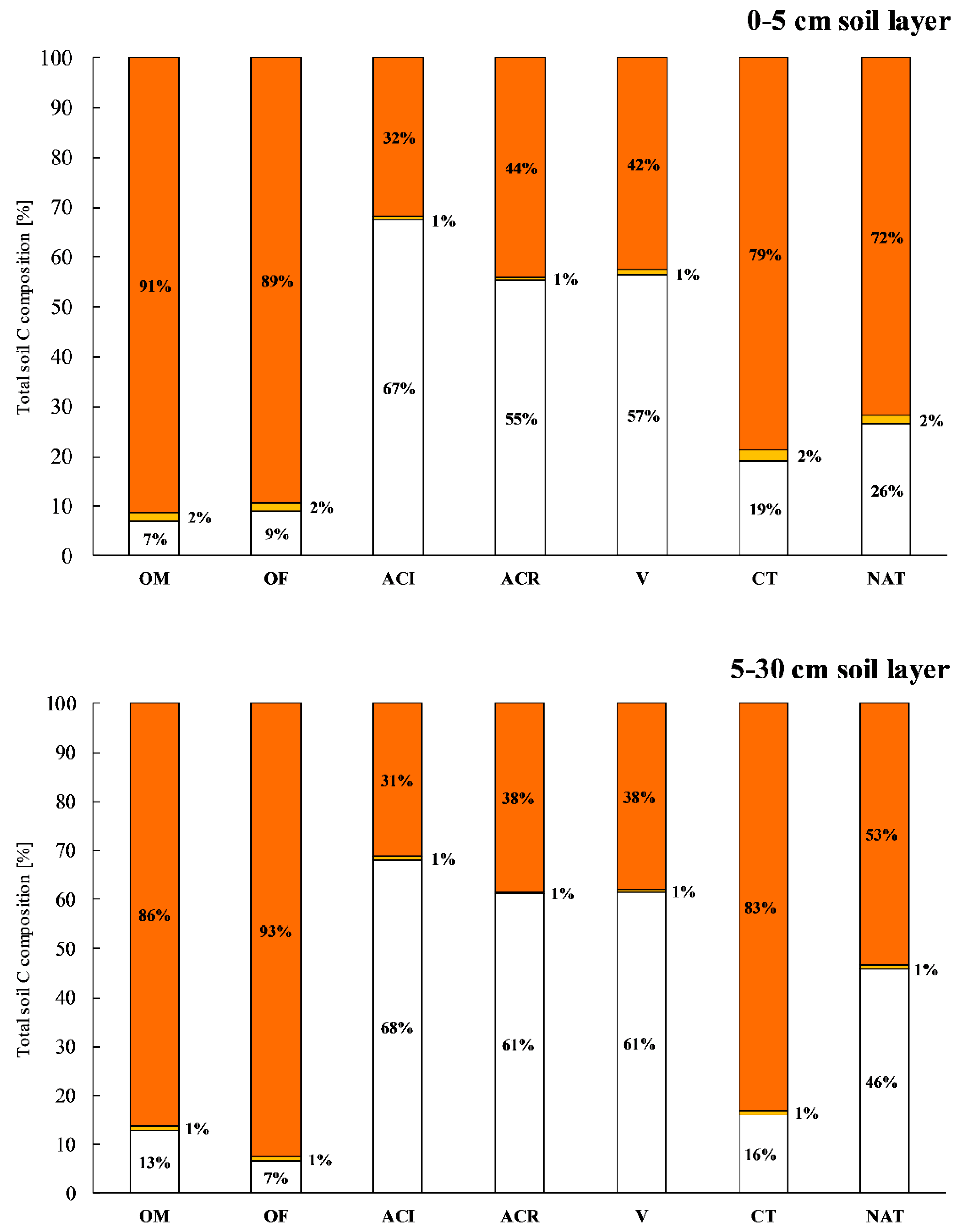
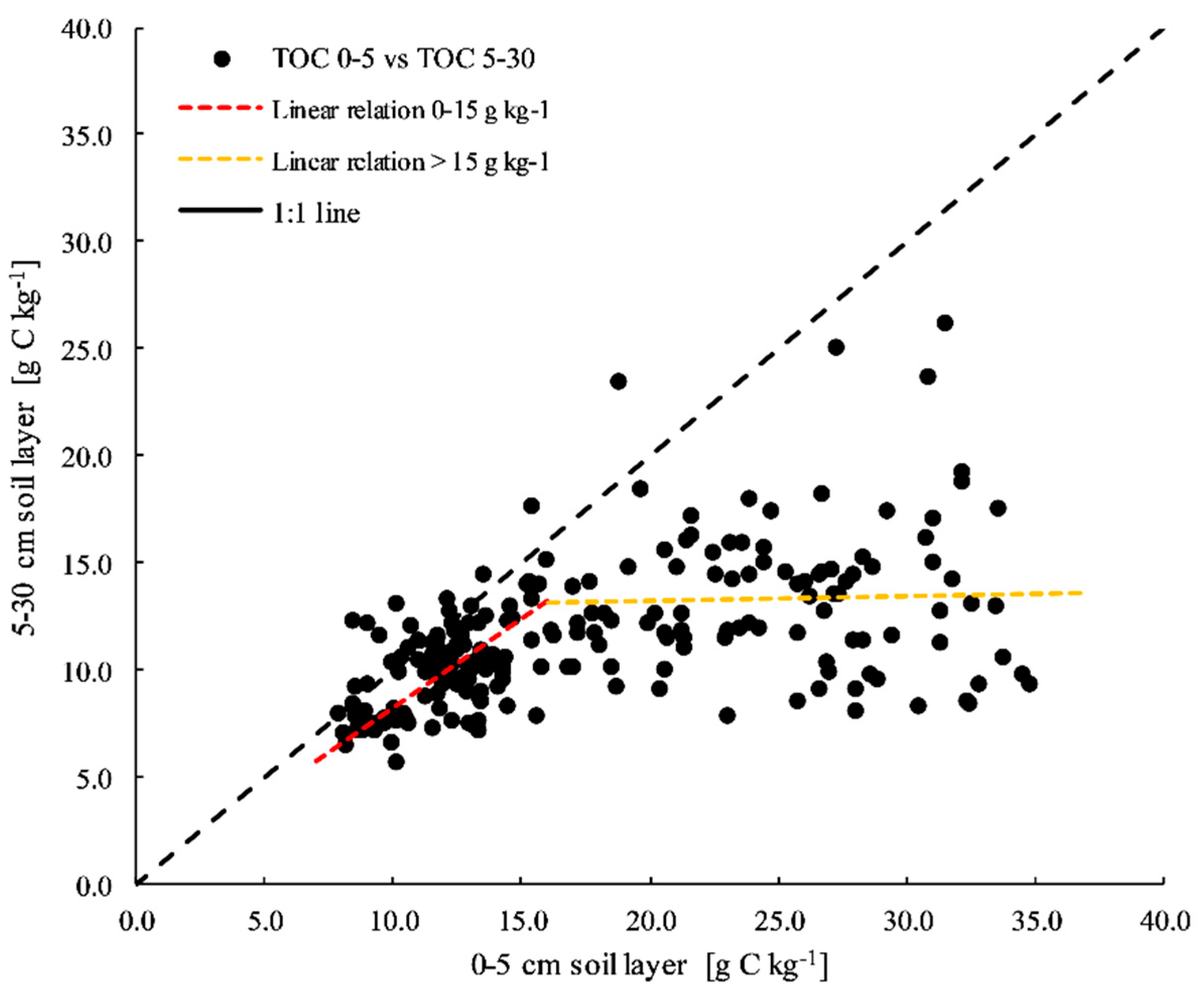
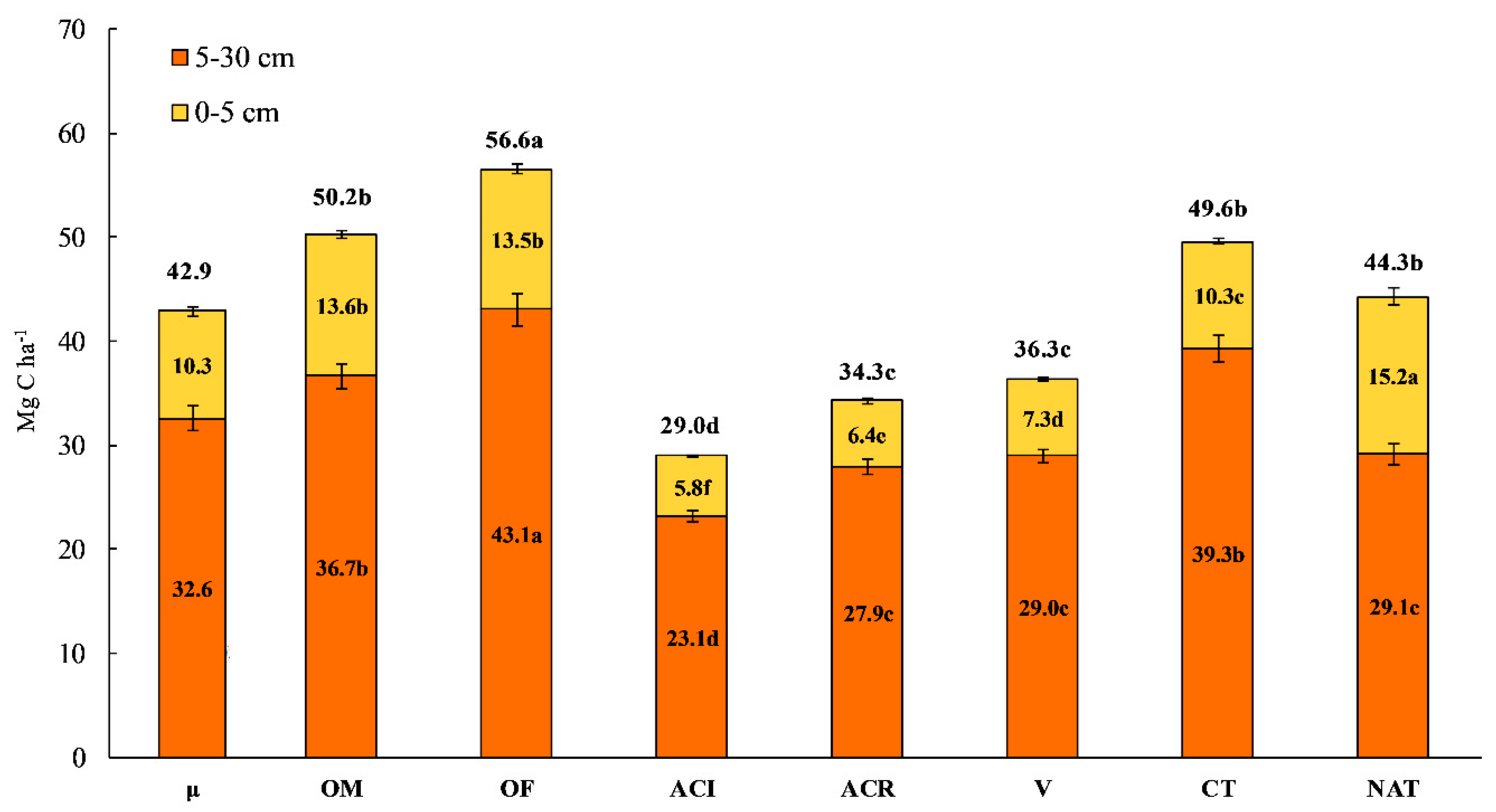
3.2. Soil C
3.3. Pearson’s Correlations
3.4. Multiple Linear Regressions
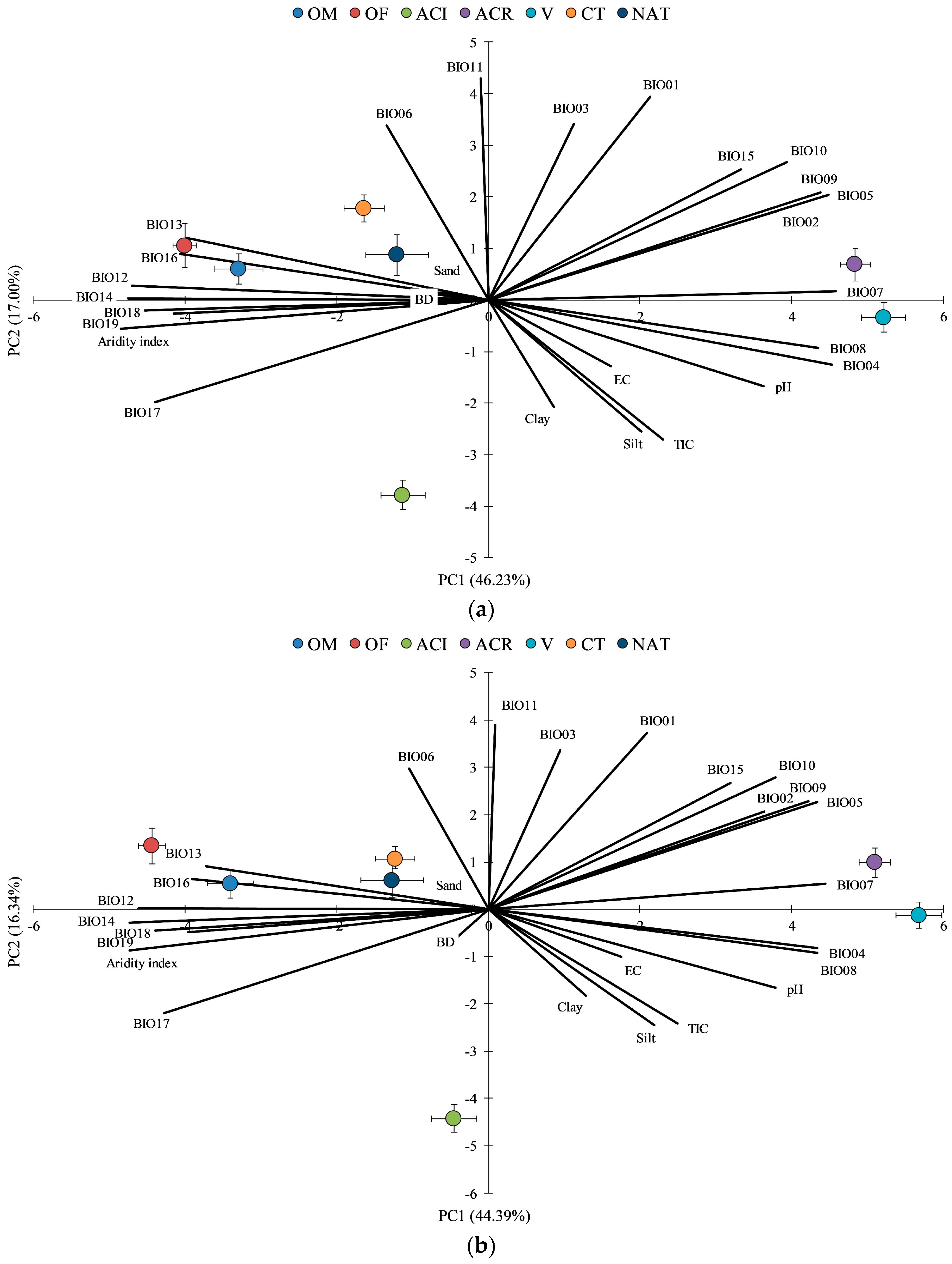
3.5. Principal Component Analysis
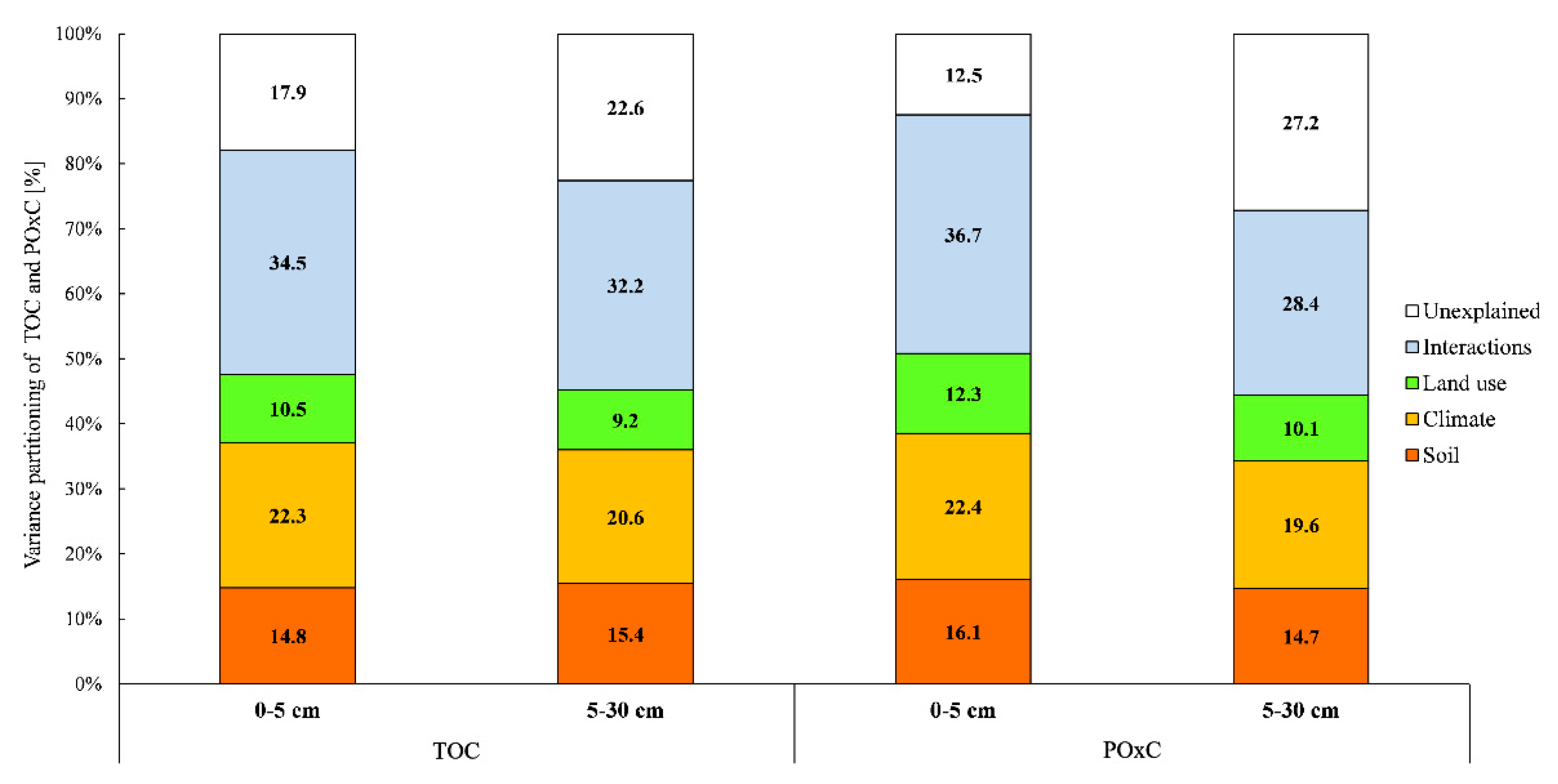
3.6. Variance Partitioning
4. Discussion
4.1. Soil TOC, C Stock and POxC Concentration
4.2. Main Factors Affecting C Accumulation
4.3. Contribution of Each Group of Edaphic Factor to C Soil Stocks
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- FAO. International Year of Soil; FAO: Rome, Italy, 2015. [Google Scholar]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Parras-Alcántara, L.; Díaz-Jaimes, L.; Lozano-García, B. Management effects on soil organic carbon stock in mediterranean open rangelands-treeless grasslands. L. Degrad. Dev. 2015, 26, 22–34. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Wang, K.; Pan, F.; Yang, S.; Shu, S. Factors controlling accumulation of soil organic carbon along vegetation succession in a typical karst region in Southwest China. Sci. Total Environ. 2015, 521–522, 52–58. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Jordán, A.; Zavala, L.M.; De la Rosa, D.; Abd-Elmabod, S.K.; Anaya-Romero, M. Impact of Land Use and Land Cover Changes on Organic Carbon Stocks in Mediterranean Soils (1956-2007). L. Degrad. Dev. 2015, 26, 168–179. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Francaviglia, R.; Benedetti, A.; Doro, L.; Madrau, S.; Ledda, L. Influence of land use on soil quality and stratification ratios under agro-silvo-pastoral Mediterranean management systems. Agric. Ecosyst. Environ. 2014, 183, 86–92. [Google Scholar] [CrossRef]
- Marschner, B.; Brodowski, S.; Dreves, A.; Gleixner, G.; Gude, A.; Grootes, P.M.; Hamer, U.; Heim, A.; Jandl, G.; Ji, R.; et al. How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 2008, 171, 91–110. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Jordán, A.; Zavala, L.M.; De la Rosa, D.; Abd-Elmabod, S.K.; Anaya-Romero, M. Organic carbon stocks in Mediterranean soil types under different land uses (Southern Spain). Solid Earth 2012, 3, 375–386. [Google Scholar] [CrossRef]
- Zdruli, P.; Jones, R.J.A.; Montanarella, L. Organic Matter in the Soils of Southern Europe; Office for Official Publications of the European Communities: Luxembourg, 2004.
- IPCC Climate change 2007: The physical science basis. Intergov. Panel Clim. Chang. 2007, 446, 727–728.
- Wang, Z.P.; Han, X.G.; Chang, S.X.; Wang, B.; Yu, Q.; Hou, L.Y.; Li, L.H. Soil organic and inorganic carbon contents under various land uses across a transect of continental steppes in Inner Mongolia. Catena 2013, 109, 110–117. [Google Scholar] [CrossRef]
- Lal, R.; Kimble, J.M. Pedogenic carbonates and the global carbon cycle. In Global Climate Change Pedogenic Carbonates; CRC Lewis: Boca Raton, FL, USA, 2000; pp. 1–14. [Google Scholar]
- Yaalon, D.H. Soils in the Mediterranean region: What makes them different? Catena 1997, 28, 157–169. [Google Scholar] [CrossRef]
- Escolano, J.J.; Pedreño, J.N.; Lucas, I.G.; Almendro Candel, M.B.; Zorpas, A.A. Decreased organic carbon associated with land management in Mediterranean environments. In Soil Management Climate Change; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–13. [Google Scholar]
- Bhattacharjya, S.; Bhaduri, D.; Chauhan, S.; Chandra, R.; Raverkar, K.P.P.; Pareek, N. Comparative evaluation of three contrasting land use systems for soil carbon, microbial and biochemical indicators in North-Western Himalaya. Ecol. Eng. 2017, 103, 21–30. [Google Scholar] [CrossRef]
- da Silva Oliveira, D.M.; Paustian, K.; Cotrufo, M.F.; Fiallos, A.R.; Cerqueira, A.G.; Cerri, C.E.P. Assessing labile organic carbon in soils undergoing land use change in Brazil: A comparison of approaches. Ecol. Indic. 2017, 72, 411–419. [Google Scholar] [CrossRef]
- ISTAT. 6 Censimento Generale Dell’ Agricoltura in Calabria; ISTAT: Roma, Italy, 2012.
- Iaquinta, P.; Terranova, O. Lineamenti climatici e termometrici della Calabria: Classificazione climatica di Köppen mediante sistemi GIS e Geoprocessing. In Relazione Finale “Pericolosità Legata ai Fenomeni di Intensa Erosione Idrica Areale e Lineare”; Terranova, O., Ed.; CNR IRPI: Cosenza, Italy, 2010; pp. 1–18. [Google Scholar]
- Soil Survey Staff Soil Taxonomy: A basic system of soil classification for making and interpreting soil surveys. In Natural Resources Conservation Service, Agriculture; USDA: Washington, DC, USA, 1999.
- ARSSA. I Suoli Della Calabria—Carta dei Suoli in Scala 1:250.000; Rubbettino Industrie Grafiche: Catanzaro Italy, 2003. [Google Scholar]
- Grossman, R.B.; Reinsch, T.G. Core method. In Methods of Soil Analysis. Part 4 Physical Methods; Topp, G.C., Dane, J.H., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 2002; Volume 4, pp. 207–210. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis, Part 3. Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 2015, 237–238, 149–158. [Google Scholar] [CrossRef]
- Walling, D.E.; Collins, A.L. Integrated Assessment of Catchment Suspended Sediment Budgets: A Technical Manual; University of Exeter: Exeter, UK, 2000. [Google Scholar]
- Rittl, T.F.; Oliveira, D.; Cerri, C.E.P. Soil carbon stock changes under different land uses in the Amazon. Geoderma Reg. 2017, 10, 138–143. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Zomer, R.J.; Bossio, D.A.; Trabucco, A.; Yuanjie, L.; Gupta, D.C.; Singh, V.P. Trees and Water: Smallholder Agroforestry on Irrigated Lands in Northern India; IWMI: Colombo, Sri Lanka, 2007; Volume 122, ISBN 9290906855. [Google Scholar]
- Zomer, R.J.; Trabucco, A.; Bossio, D.A. Climate change mitigation: A spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Agric. Ecosyst. Environ. 2008, 126, 67–80. [Google Scholar] [CrossRef]
- SAS. SAS/STAT 9.3 User’s Guide; User’s Guid; SAS Inst. Inc.: Cary, NC, USA, 2011. [Google Scholar]
- R Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013.
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package. R Package Version 2.5-2, 2018.
- Chiti, T.; Gardin, L.; Perugini, L.; Quaratino, R.; Vaccari, F.P.; Miglietta, F.; Valentini, R. Soil organic carbon stock assessment for the different cropland land uses in Italy. Biol. Fertil. Soils 2012, 48, 9–17. [Google Scholar] [CrossRef]
- Chiti, T.; Blasi, E.; Pellis, G.; Perugini, L.; Chiriacò, M.V.; Valentini, R. Soil organic carbon pool’s contribution to climate change mitigation on marginal land of a Mediterranean montane area in Italy. J. Environ. Manag. 2018, 218, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Willaarts, B.A.; Oyonarte, C.; Muñoz-Rojas, M.; Ibáñez, J.J.; Aguilera, P.A. Environmental factors controlling soil organic carbon stocks in two contrasting mediterranean climatic areas of southern Spain. L. Degrad. Dev. 2016, 27, 603–611. [Google Scholar] [CrossRef]
- Farina, R.; Marchetti, A.; Francaviglia, R.; Napoli, R.; Di Bene, C.; Bene, C. Di Modeling regional soil C stocks and CO2 emissions under Mediterranean cropping systems and soil types. Agric. Ecosyst. Environ. 2017, 238, 128–141. [Google Scholar] [CrossRef]
- Francaviglia, R.; Renzi, G.; Doro, L.; Parras-Alcántara, L.; Lozano-García, B.; Ledda, L. Soil sampling approaches in Mediterranean agro-ecosystems. Influence on soil organic carbon stocks. Catena 2017, 158, 113–120. [Google Scholar] [CrossRef]
- Francaviglia, R.; Renzi, G.; Ledda, L.; Benedetti, A. Organic carbon pools and soil biological fertility are affected by land use intensity in Mediterranean ecosystems of Sardinia, Italy. Sci. Total Environ. 2017, 599–600, 789–796. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Novara, A.; La Mantia, T.; Barbera, V.; Gristina, L. Paired-site approach for studying soil organic carbon dynamics in a Mediterranean semiarid environment. Catena 2012, 89, 1–7. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Abd-Elmabod, S.K.; Zavala, L.M.; De la Rosa, D.; Jordán, A. Climate change impacts on soil organic carbon stocks of Mediterranean agricultural areas: A case study in Northern Egypt. Agric. Ecosyst. Environ. 2017, 238, 142–152. [Google Scholar] [CrossRef]
- Martin, M.P.; Wattenbach, M.; Smith, P.; Meersmans, J.; Jolivet, C.; Boulonne, L.; Arrouays, D. Spatial distribution of soil organic carbon stocks in France. Biogeosciences 2011, 8, 1053–1065. [Google Scholar] [CrossRef]
- Meersmans, J.; Martin, M.P.; De Ridder, F.; Lacarce, E.; Wetterlind, J.; De Baets, S.; Le Bas, C.; Louis, B.P.; Orton, T.G.; Bispo, A.; et al. A novel soil organic C model using climate, soil type and management data at the national scale in France. Agron. Sustain. Dev. 2012, 32, 873–888. [Google Scholar] [CrossRef]
- Rodríguez-Murillo, J.C. Organic carbon content under different types of land use and soil in peninsular Spain. Biol. Fertil. Soils 2001, 33, 53–61. [Google Scholar] [CrossRef]
- Mudge, P.L.; Kelliher, F.M.; Knight, T.L.; O’Connell, D.; Fraser, S.; Schipper, L.A. Irrigating grazed pasture decreases soil carbon and nitrogen stocks. Glob. Chang. Biol. 2017, 23, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Leronni, V.; Piazzi, M.; Petrella, F.; Mairota, P.; Peressotti, A.; Piussi, P.; Valentini, R.; Gristina, L.; La Mantia, T.; et al. Impact of woody encroachment on soil organic carbon and nitrogen in abandoned agricultural lands along a rainfall gradient in Italy. Reg. Environ. Chang. 2011, 11, 917–924. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Liu, L.; Luo, G.; Li, Y.; Chen, X. Effect of land use history and pattern on soil carbon storage in arid region of central asia. PLoS ONE 2013, 8, e68372. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar]
- Wang, T.; Kang, F.; Cheng, X.; Han, H.; Ji, W. Soil organic carbon and total nitrogen stocks under different land uses in a hilly ecological restoration area of North China. Soil Tillage Res. 2016, 163, 176–184. [Google Scholar] [CrossRef]
- Tortorella, D.; Gelsomino, A. Influence of compost amendment and maize root system on soil CO2 efflux: A mesocosm approach. Agrochimica 2011, 55, 161–177. [Google Scholar]
- Badagliacca, G.; Benítez, E.; Amato, G.; Badalucco, L.; Giambalvo, D.; Laudicina, V.A.; Ruisi, P. Long-term effects of contrasting tillage on soil organic carbon, nitrous oxide and ammonia emissions in a Mediterranean Vertisol under different crop sequences. Sci. Total Environ. 2018, 619–620, 18–27. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Sun, Y.; Xu, H.; Yang, Z.; Liu, S.; Jia, X.; Zheng, H. The effects of different water and nitrogen managements on yield and nitrogen use efficiency in hybrid rice of China. F. Crops Res. 2012, 127, 85–98. [Google Scholar] [CrossRef]
- Wu, H.B.; Guo, Z.T.; Peng, C.H. Changes in terrestrial carbon storage with global climate changes since the last interglacial. Quat. Sci. 2001, 21, 366–1373. [Google Scholar]
- Tan, W.F.; Zhang, R.; Cao, H.; Huang, C.Q.; Yang, Q.K.; Wang, M.; Koopal, L.K. Soil inorganic carbon stock under different soil types and land uses on the Loess Plateau region of China. Catena 2014, 121, 22–30. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Culman, S.W.; Horwath, W.R.; Wade, J.; Cass, D.; Beniston, J.W.; Bowles, T.M.; Grandy, A.S.; Franzluebbers, A.J.; Schipanski, M.E.; et al. Comparison of permanganate-oxidizable carbon and mineralizable carbon for assessment of organic matter stabilization and mineralization. Soil Sci. Soc. Am. J. 2016, 80, 1352–1364. [Google Scholar] [CrossRef]
- Srivastava, P.; Sharma, Y.K.; Singh, N. Soil carbon sequestration potential of Jatropha curcas L. growing in varying soil conditions. Ecol. Eng. 2014, 68, 155–166. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Palazzolo, E.; Catania, P.; Vallone, M.; García, A.D.; Badalucco, L. Soil quality indicators as affected by shallow tillage in a vineyard grown in a semiarid Mediterranean environment. L. Degrad. Dev. 2017, 28, 1038–1046. [Google Scholar] [CrossRef]
- Hiederer, R. Distribution of Organic Carbon in Soil Profile Data; Office for Official Publications of the European Communities: Luxembourg, 2009; 126p.
- Badagliacca, G.; Benítez, E.; Amato, G.; Badalucco, L.; Giambalvo, D.; Laudicina, V.A.; Ruisi, P. Long-term no-tillage application increases soil organic carbon, nitrous oxide emissions and faba bean (Vicia faba L.) yields under rain-fed Mediterranean conditions. Sci. Total Environ. 2018, 639, 350–359. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Parras-Alcántara, L.; Lozano-García, B. Conventional tillage versus organic farming in relation to soil organic carbon stock in olive groves in Mediterranean rangelands (southern Spain). Solid Earth 2014, 5, 299–311. [Google Scholar] [CrossRef]
- Franzluebbers, A. Soil organic matter stratification ratio as an indicator of soil quality. Soil Tillage Res. 2002, 66, 95–106. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, J.F.; Zhang, X.Q.; Kong, F.L.; Chen, F.; Lal, R.; Zhang, H.L. stratification and storage of soil organic carbon and nitrogen as affected by tillage practices in the North China plain. PLoS ONE 2015, 10, e0128873. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, A.; Azzellino, A. Multivariate analysis of soils: Microbial biomass, metabolic activity, and bacterial-community structure and their relationships with soil depth and type. J. Plant Nutr. Soil Sci. 2011, 174, 381–394. [Google Scholar] [CrossRef]
- Alvarez, R. Predicting average regional yield and production of wheat in the Argentine Pampas by an artificial neural network approach. Eur. J. Agron. 2009, 30, 70–77. [Google Scholar] [CrossRef]
- Kiem, R.; Knicker, H.; Kögel-Knabner, I. Refractory organic carbon in particle-size fractions of arable soils I: Distribution of refractory carbon between the size fractions. Org. Geochem. 2002, 33, 1683–1697. [Google Scholar] [CrossRef]
- Conforti, M.; Lucà, F.; Scarciglia, F.; Matteucci, G.; Buttafuoco, G. Soil carbon stock in relation to soil properties and landscape position in a forest ecosystem of southern Italy (Calabria region). Catena 2016, 144, 23–33. [Google Scholar] [CrossRef]
- Tonon, G.; Sohi, S.; Francioso, O.; Ferrari, E.; Montecchio, D.; Gioacchini, P.; Ciavatta, C.; Panzacchi, P.; Powlson, D. Effect of soil pH on the chemical composition of organic matter in physically separated soil fractions in two broadleaf woodland sites at Rothamsted, UK. Eur. J. Soil Sci. 2010, 61, 970–979. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Thomson, B.C.; Plassart, P.; Gweon, H.S.; Stone, D.; Creamer, R.E.; Bailey, M.J. Mapping and validating predictions of soil bacterial biodiversity using European and national scale datasets. Appl. Soil Ecol. 2016, 97, 61–68. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Wright, D.; Goulding, K.W.T.; Jones, D.L. pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol. Biochem. 2006, 38, 898–911. [Google Scholar] [CrossRef]
- Hu, P.L.; Liu, S.J.; Ye, Y.Y.; Zhang, W.; Wang, K.L.; Su, Y.R. Effects of environmental factors on soil organic carbon under natural or managed vegetation restoration. L. Degrad. Dev. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, G.-y.; Tang, Z.-s.; Shangguan, Z.-p. Global patterns of the effects of land-use changes on soil carbon stocks. Glob. Ecol. Conserv. 2016, 5, 127–138. [Google Scholar] [CrossRef]
- Del Grosso, S.; Parton, W.; Stohlgren, T.; Zheng, D.; Bachelet, D.; Prince, S.; Hibbard, K.; Olson, R. Global potential net primary production predicted from vegetation class, precipitation, and temperature. Ecology 2008, 89, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wan, S.; Hui, D.; Wallace, L.L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 2001, 413, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; García-Gómez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Rial, M.; Martínez Cortizas, A.; Rodríguez-Lado, L. Understanding the spatial distribution of factors controlling topsoil organic carbon content in European soils. Sci. Total Environ. 2017, 609, 1411–1422. [Google Scholar] [CrossRef]
- Terranova, O.G. Caratteristiche degli eventi pluviometrici a scala giornaliera. In Proceedings of the XXIX Convegno di Idraulica e Costruzioni Idrauliche, Trento, Italy, 7–10 September 2004; EdiBios: Cosenza, Italy, 2004. [Google Scholar]
- Ciais, P.; Wattenbach, M.; Vuichard, N.; Smith, P.C.; Piao, S.L.; Don, A.; Luyssaert, S.; Janssens, I.A.; Bondeau, A.; Dechow, R.; et al. The European carbon balance. Part 2: Croplands. Glob. Chang. Biol. 2010, 16, 1409–1428. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M.; Kitayama, K.; Knicker, H. Climate and parent material controls on organic matter storage in surface soils: A three-pool, density-separation approach. Geoderma 2008, 147, 23–33. [Google Scholar] [CrossRef]
- Versace, P.; Ferrari, E.; Gabriele, S.; Rossi, F. Valutazione Delle Piene in Calabria; CNR IRPI: Cosenza, Italy, 1989. [Google Scholar]
- Capra, A.; Consoli, S.; Scicolone, B. Long-term climatic variability in calabria and effects on drought and agrometeorological parameters. Water Resour. Manag. 2013, 27, 601–617. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Tighe, M.; Cowie, A.; Wilson, B.R.; Schwenke, G.; Mcleod, M.; Badgery, W.; Baldock, J. The relationships between land uses, soil management practices, and soil carbon fractions in South Eastern Australia. Agric. Ecosyst. Environ. 2014, 197, 41–52. [Google Scholar] [CrossRef]

| 0–5 cm Soil Layer | ||||||||
|---|---|---|---|---|---|---|---|---|
| Land Use | TN (g kg−1) | pH (-) | EC (μS cm−1) | BD (g cm−3) | ||||
| µ | σ | µ | σ | µ | σ | µ | σ | |
| OM | 2.3 | 0.4 | 5.7 | 0.6 | 293 | 174 | 1.01 | 0.05 |
| OF | 2.5 | 0.4 | 5.3 | 0.5 | 151 | 72 | 0.92 | 0.02 |
| ACI | 1.0 | 0.1 | 7.2 | 0.4 | 255 | 61 | 1.13 | 0.08 |
| ACR | 1.2 | 0.2 | 7.5 | 0.1 | 229 | 54 | 1.02 | 0.07 |
| V | 1.2 | 0.1 | 7.4 | 0.1 | 272 | 59 | 1.08 | 0.06 |
| CT | 1.5 | 0.2 | 6.8 | 0.7 | 236 | 81 | 1.17 | 0.08 |
| NAT | 2.5 | 0.7 | 6.3 | 0.8 | 279 | 128 | 1.01 | 0.10 |
| 5–30 cm Soil Layer | ||||||||
| OM | 1.4 | 0.3 | 5.7 | 0.7 | 166 | 91 | 1.11 | 0.03 |
| OF | 1.7 | 0.4 | 5.0 | 0.5 | 105 | 77 | 1.03 | 0.02 |
| ACI | 0.8 | 0.1 | 7.2 | 0.5 | 191 | 51 | 1.16 | 0.09 |
| ACR | 1.1 | 0.1 | 7.5 | 0.1 | 223 | 38 | 1.04 | 0.05 |
| V | 1.0 | 0.1 | 7.5 | 0.1 | 230 | 37 | 1.13 | 0.05 |
| CT | 1.2 | 0.2 | 6.9 | 0.6 | 204 | 77 | 1.11 | 0.08 |
| NAT | 1.0 | 0.2 | 6.2 | 1.1 | 186 | 99 | 1.14 | 0.09 |
| Land Use | Sand (%) | Silt (%) | Clay (%) | |||
|---|---|---|---|---|---|---|
| µ | σ | µ | σ | µ | σ | |
| OM | 17.7 | 17.5 | 63.4 | 16.6 | 18.9 | 5.7 |
| OF | 27.8 | 20.2 | 51.7 | 14.3 | 20.5 | 6.2 |
| ACI | 2.0 | 4.5 | 75.9 | 4.0 | 22.1 | 2.7 |
| ACR | 9.5 | 11.1 | 71.4 | 9.9 | 19.1 | 1.8 |
| V | 2.0 | 4.5 | 75.6 | 4.6 | 22.4 | 3.8 |
| CT | 10.5 | 14.4 | 72.9 | 12.5 | 16.6 | 3.4 |
| NAT | 28.9 | 19.1 | 58.4 | 13.2 | 12.6 | 8.2 |
| TOC | POxC | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–5 cm | 5–30 cm | 0–5 cm | 5–30 cm | |||||
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| TIC | −0.46 | <0.0001 | −0.33 | <0.0001 | −0.42 | <0.0001 | −0.09 | 0.205 |
| pH | −0.70 | <0.0001 | −0.48 | <0.0001 | −0.57 | <0.0001 | −0.09 | 0.196 |
| EC | −0.01 | 0.870 | −0.27 | <0.0001 | 0.09 | 0.200 | 0.12 | 0.083 |
| BD | −0.22 | 0.002 | −0.26 | <0.0001 | −0.16 | 0.021 | −0.21 | 0.003 |
| Sand | −0.48 | <0.0001 | −0.23 | 0.001 | −0.45 | <0.0001 | −0.15 | 0.029 |
| Silt | 0.50 | <0.0001 | 0.29 | <0.0001 | 0.41 | <0.0001 | 0.14 | 0.048 |
| Clay | 0.26 | <0.0001 | 0.22 | 0.002 | 0.40 | <0.0001 | 0.13 | 0.046 |
| BIO01 | −0.12 | 0.094 | −0.01 | 0.901 | −0.02 | 0.804 | 0.01 | 0.914 |
| BIO02 | −0.22 | 0.001 | −0.07 | 0.339 | −0.32 | <0.0001 | 0.25 | <0.0001 |
| BIO03 | 0.13 | 0.053 | 0.21 | 0.751 | 0.06 | 0.357 | 0.15 | 0.026 |
| BIO04 | −0.53 | <0.0001 | −0.40 | <0.0001 | −0.53 | <0.0001 | 0.13 | 0.067 |
| BIO05 | −0.40 | <0.0001 | −0.19 | 0.007 | −0.39 | <0.0001 | 0.13 | 0.059 |
| BIO06 | 0.13 | 0.057 | −0.17 | 0.015 | 0.27 | <0.0001 | −0.18 | 0.007 |
| BIO07 | −0.43 | <0.0001 | −0.25 | 0.001 | −0.49 | <0.0001 | 0.21 | 0.002 |
| BIO08 | −0.68 | <0.0001 | −0.52 | <0.0001 | −0.64 | <0.0001 | −0.05 | 0.484 |
| BIO09 | −0.42 | <0.0001 | −0.24 | 0.001 | −0.35 | <0.0001 | 0.06 | 0.378 |
| BIO10 | −0.34 | <0.0001 | −0.19 | 0.007 | −0.26 | <0.0001 | 0.06 | 0.427 |
| BIO11 | 0.11 | 0.120 | 0.17 | 0.011 | 0.19 | 0.005 | −0.06 | 0.395 |
| BIO12 | 0.51 | <0.0001 | 0.40 | <0.0001 | 0.48 | <0.0001 | −0.10 | 0.143 |
| BIO13 | 0.44 | <0.0001 | 0.51 | <0.0001 | 0.35 | <0.0001 | −0.06 | 0.409 |
| BIO14 | 0.57 | <0.0001 | 0.48 | <0.0001 | 0.54 | <0.0001 | −0.09 | 0.186 |
| BIO15 | −0.16 | 0.024 | 0.09 | 0.173 | −0.28 | <0.0001 | 0.22 | 0.001 |
| BIO16 | 0.44 | <0.0001 | 0.47 | <0.0001 | 0.36 | <0.0001 | −0.06 | 0.386 |
| BIO17 | 0.32 | <0.0001 | 0.14 | 0.047 | 0.37 | <0.0001 | −0.20 | 0.003 |
| BIO18 | 0.46 | <0.0001 | 0.25 | 0.001 | 0.49 | <0.0001 | −0.14 | 0.041 |
| BIO19 | 0.40 | <0.0001 | 0.27 | <0.0001 | 0.32 | <0.0001 | −0.06 | 0.389 |
| Ai | 0.46 | <0.0001 | 0.33 | <0.0001 | 0.44 | <0.0001 | −0.15 | 0.301 |
| TOC (g kg−1) | POxC (g kg−1) | ||||
|---|---|---|---|---|---|
| y | x | m | y | x | m |
| 0–5 cm | BD (g cm−3) | −31.03 | 0–5 cm | pH (-) | −0.030 |
| Clay (%) | 2.46 | BD (g cm−3) | 0.304 | ||
| BIO03 | −2.37 | PSD (0.03 mm) (%) | 0.011 | ||
| BIO04 | −0.02 | BIO01 | 0.031 | ||
| BIO08 | −0.48 | BIO13 | 0.002 | ||
| BIO17 | −0.57 | BIO14 | 0.073 | ||
| BIO19 | −0.03 | BIO17 | −0.008 | ||
| Constant | 340.72 | Constant | −2.737 | ||
| R2 = 0.86 | F = 100.0 | p < 0.0001 | R2 = 0.86 | F = 107.1 | p < 0.0001 |
| 5–30 cm | pH (-) | 0.61 | 5–30 cm | TIC (mg kg−1) | −0.091 |
| BD (g cm−3) | −15.51 | Silt (%) | 0.002 | ||
| PSD (1.4 μm) (%) | 4.60 | Clay (%) | 0.009 | ||
| BIO08 | −0.14 | BIO07 | 0.010 | ||
| BIO14 | −1.38 | BIO09 | −0.025 | ||
| BIO18 | −0.30 | BIO10 | 0.023 | ||
| BIO19 | −0.02 | BIO14 | 0.032 | ||
| Constant | 37.58 | Constant | −2.25 | ||
| R2 = 0.76 | F = 59.66 | p < 0.0001 | R2 = 0.70 | F = 50.6 | p < 0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badagliacca, G.; Romeo, M.; Lo Presti, E.; Gelsomino, A.; Monti, M. Factors Governing Total and Permanganate Oxidizable C Pools in Agricultural Soils from Southern Italy. Agriculture 2020, 10, 99. https://doi.org/10.3390/agriculture10040099
Badagliacca G, Romeo M, Lo Presti E, Gelsomino A, Monti M. Factors Governing Total and Permanganate Oxidizable C Pools in Agricultural Soils from Southern Italy. Agriculture. 2020; 10(4):99. https://doi.org/10.3390/agriculture10040099
Chicago/Turabian StyleBadagliacca, Giuseppe, Maurizio Romeo, Emilio Lo Presti, Antonio Gelsomino, and Michele Monti. 2020. "Factors Governing Total and Permanganate Oxidizable C Pools in Agricultural Soils from Southern Italy" Agriculture 10, no. 4: 99. https://doi.org/10.3390/agriculture10040099
APA StyleBadagliacca, G., Romeo, M., Lo Presti, E., Gelsomino, A., & Monti, M. (2020). Factors Governing Total and Permanganate Oxidizable C Pools in Agricultural Soils from Southern Italy. Agriculture, 10(4), 99. https://doi.org/10.3390/agriculture10040099








