Effects of Irrigation on N2O Emissions in a Maize Crop Grown on Different Soil Types in Two Contrasting Seasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Cropping System, and Management
2.2. Soil N2O Flux Measurement, Nitrogen Content, and Above-Ground Biomass
2.3. Statistical Analysis
3. Results and Discussion
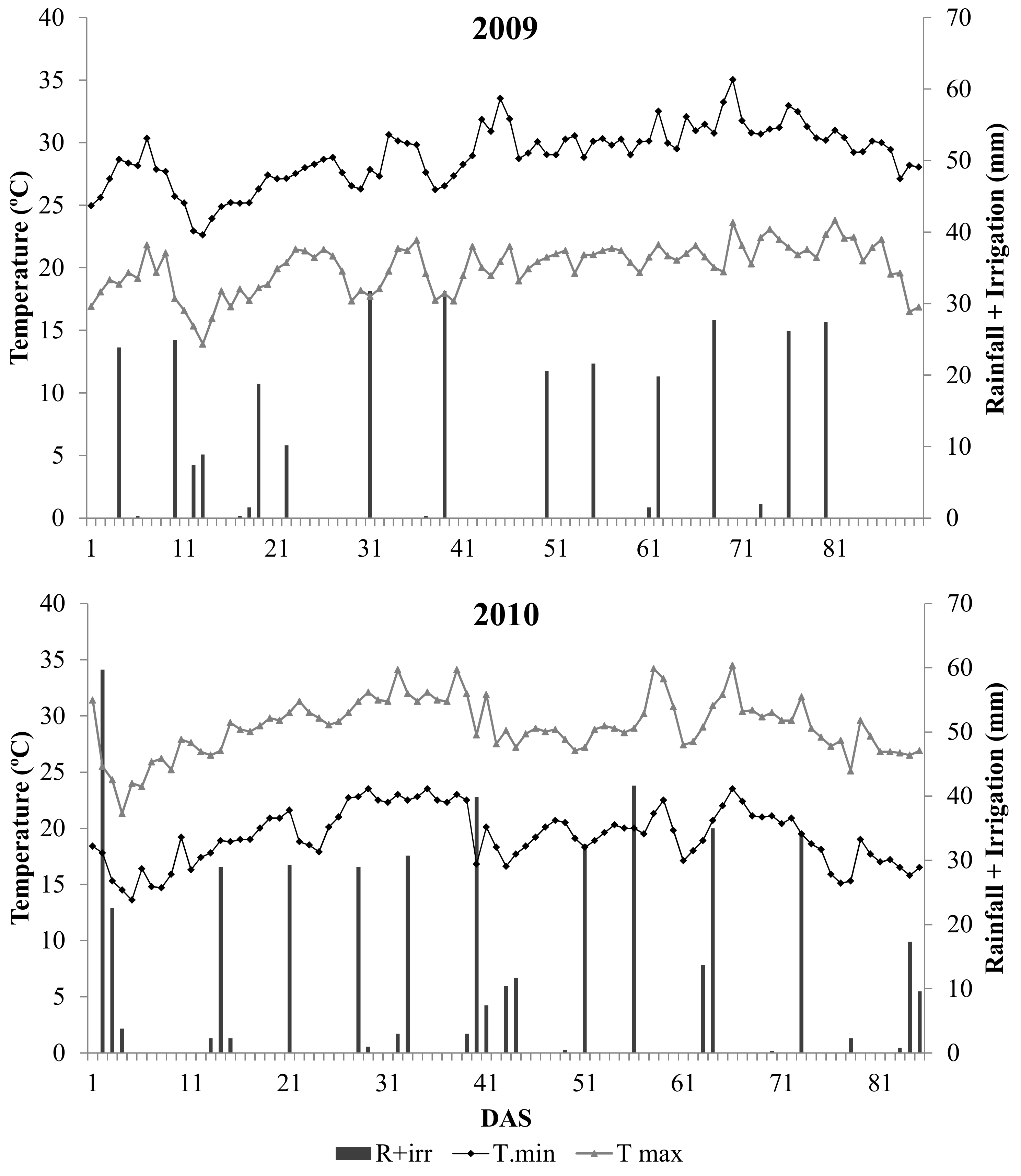
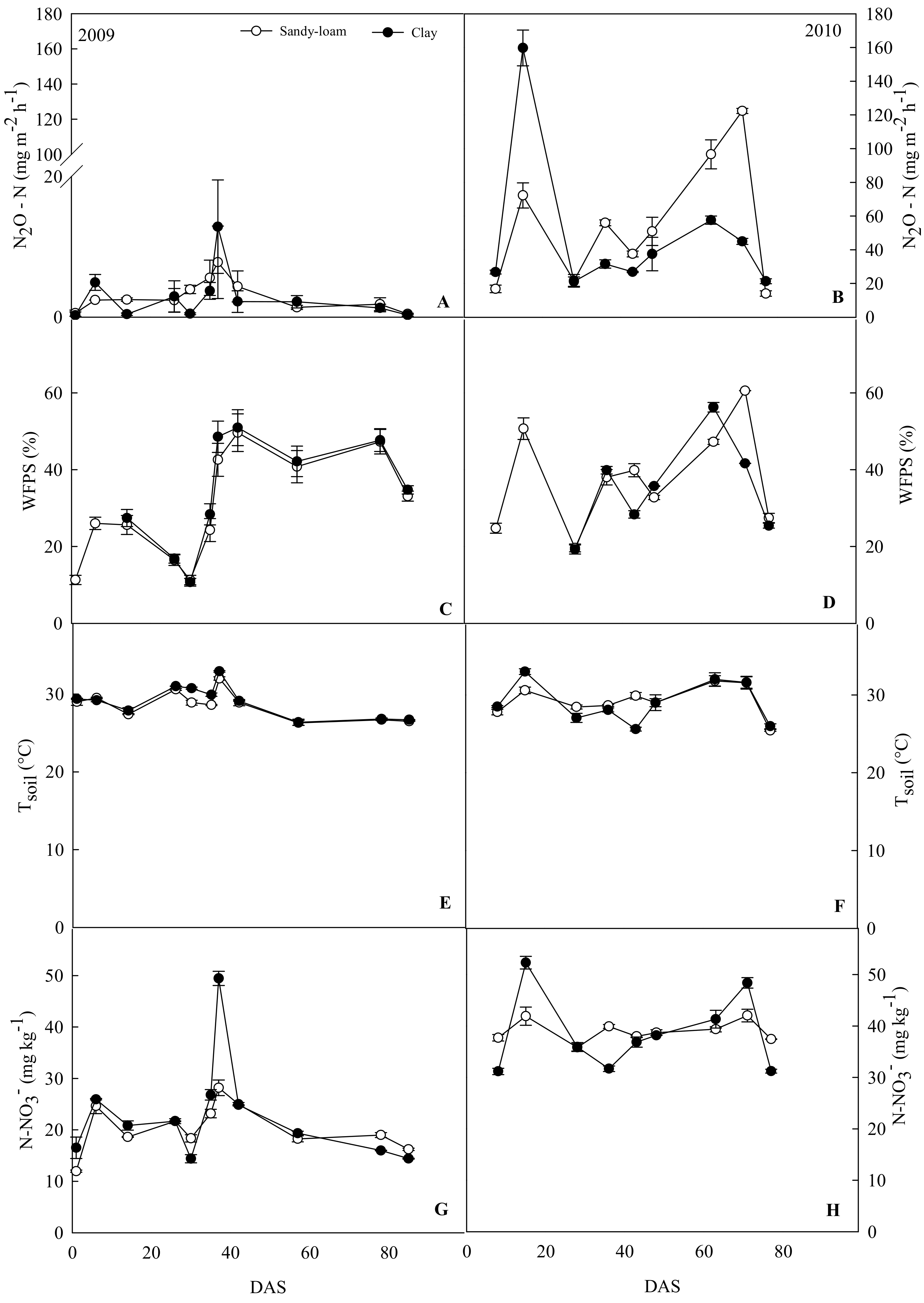
3.1. Soil Related Measurements and N2O Fluxes in Clay and Sandy-Loam Sites
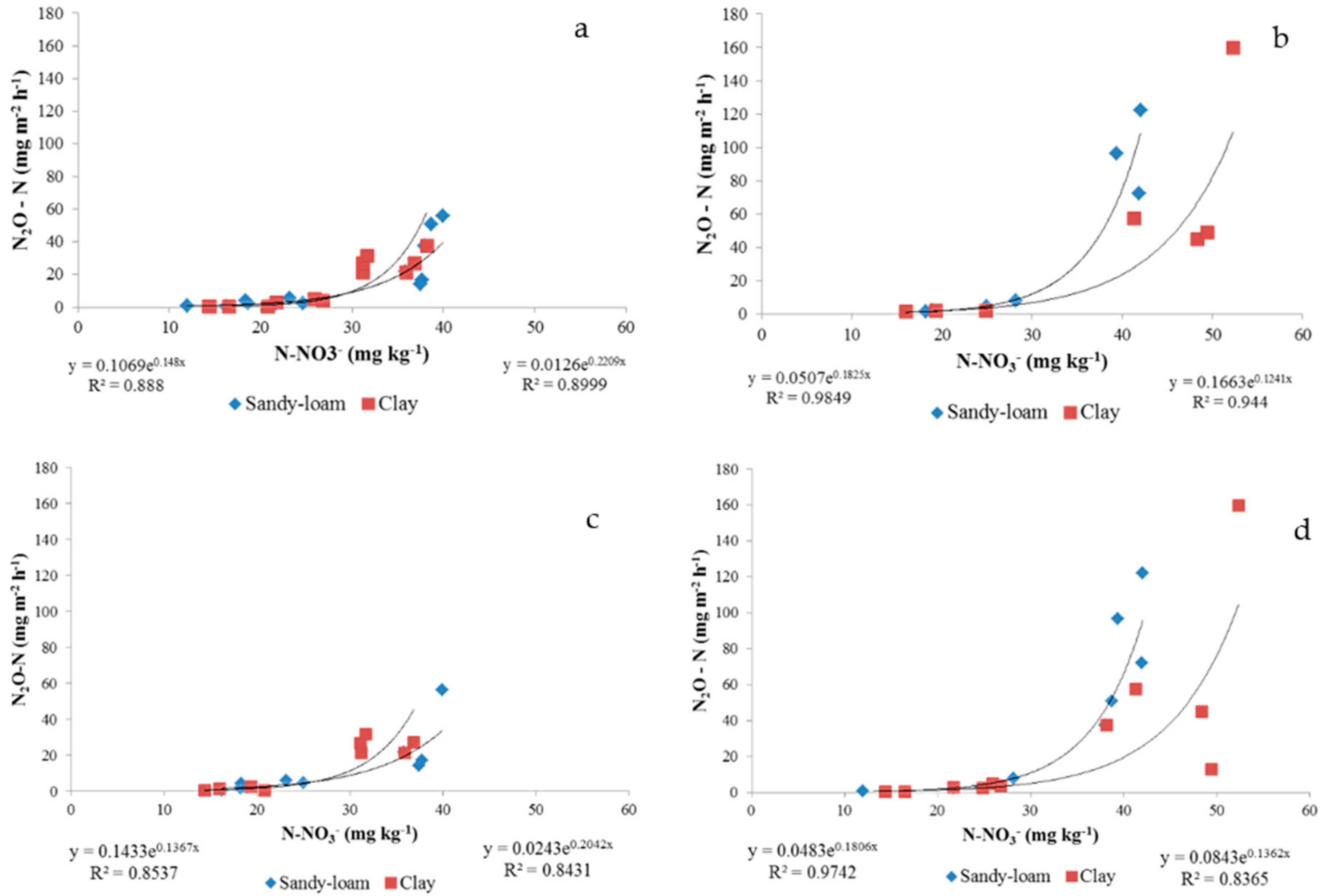
3.2. Regression Analysis on the Whole Dataset
3.3. Above-Ground Biomass on Clay and Sandy-Loam Sites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Netz, B.; Davidson, O.R.; Bosch, P.R.; Dave, R.; Meyer, L.A. Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Summary for Policymakers; Netz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2007. [Google Scholar]
- Xu, X.F.; Tian, H.Q.; Chen, G.S.; Liu, M.L.; Ren, W.; Lu, C.Q.; Zhang, C. Multifactor controls on terrestrial N2O flux over North America from 1979 through 2010. Biogeosciences 2012, 9, 1351–1366. [Google Scholar] [CrossRef]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Cayuela, M.; Aguilera, E.; Sanz-Cobena, A.; Adams, D.; Abalos, D.; Barton, L.; Ryals, R.; Silver, W.L.; Alfaro, M.A.; Pappa, V.A.; et al. Direct nitrous oxide emissions in Mediterranean climate cropping systems: Emission factors based on a meta-analysis of available measurement data. Agric. Ecosyst. Environ. 2017, 238, 25–35. [Google Scholar] [CrossRef]
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; Van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Shakoor, A.; Xu, Y.; Wang, Q.; Chen, N.; He, F.; Zuo, H.; Yin, H.; Yan, X.; Ma, Y.; Yang, S. Effects of fertilizer application schemes and soil environmental factors on nitrous oxide emission fluxes in a rice-wheat cropping system, east China. PLoS ONE 2018, 13, e0202016. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Medhi, K.; Fagodiya, R.K.; Subrahmanyam, G.; Mondal, R.; Raja, P.; Malyan, S.K.; Gupta, D.K.; Gupta, C.K.; Pathak, H. Molecular and ecological perspectives of nitrous oxide producing microbial communities in agro-ecosystems. Rev. Environ. Sci. Biotechnol. 2020, 19, 717–750. [Google Scholar] [CrossRef]
- Fagodiya, R.K.; Pathak, H.; Bhatia, A.; Jain, N.; Gupta, D.K.; Kumar, A.; Tomer, R. Nitrous oxide emission and mitigation from maize–wheat rotation in the upper Indo-Gangetic Plains. Carbon Manag. 2019, 10, 489–499. [Google Scholar] [CrossRef]
- Davidson, E.A.; Keller, M.; Erickson, H.E.; Verchot, L.V.; Veldkamp, E. Testing a conceptual model of soil emissions of nitrous and nitric oxides: Using two functions based on soil nitrogen availability and soil water content, the hole-in-the-pipe model characterizes a large fraction of the observed variation of nitric oxide and nitrous oxide emissions from soils. Bioscience 2000, 50, 667–680. [Google Scholar]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Ranjan, R.; Yadav, R. Targeting nitrogen use efficiency for sustained production of cereal crops. J. Plant Nutr. 2019, 42, 1086–1113. [Google Scholar] [CrossRef]
- Khalil, M.I.; Hossain, M.B.; Schmidhalter, U. Carbon andnitrogen mineralization in different upland soils of the sub-tropics treated with organic materials. Soil Biol. Biochem. 2005, 37, 1507–1518. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Forge, T.A.; Goyer, C.; Brin, L.D. Effect ofsoil acidification on nitrification in soil. Can. J. Soil Sci. 2015, 95, 359–363. [Google Scholar] [CrossRef]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Eichner, M.J. Nitrous oxide emissions from fertilized soils: Summary of available data. J. Environ. Qual. 1990, 19, 272–280. [Google Scholar] [CrossRef]
- Gebremichael, A.W.; Osborne, B.; Orr, P. Flooding-related increases in CO2 and N2O emissions from a temperate coastal grassland ecosystem. Biogeosciences 2017, 14, 2611–2626. [Google Scholar] [CrossRef]
- Shakoor, A.; Shahbaz, M.; Hassan, T.; Sahar, N.E.; Muhammad, S.; Mohsin, M.; Ashraf, M. A global meta-analysis of greenhouse gases emission and crop yield under no-tillage as compared to conventional tillage. Sci. Total Environ. 2021, 750, 142299. [Google Scholar] [CrossRef]
- Liu, H.S.; Li, L.H.; Han, X.G.; Huang, J.H.; Sun, J.X.; Wang, H.Y. Respiratory substrate availability plays a crucial role in the response of soil respiration to environmental factors. Appl. Soil Ecol. 2006, 29, 284–293. [Google Scholar] [CrossRef]
- Merbold, L.; Eugster, W.; Stieger, J.; Zahniser, M.; Nelson, D.; Buchmann, N. Greenhouse gas budget (CO2, CH4 and N2O) of intensively managed grassland following restoration. Glob. Chang. Biol. 2014, 20, 1913–1928. [Google Scholar] [CrossRef]
- Tian, Y.; Luo, C.; Lu, Y.; Tang, C.; Ouyang, Q. Cell cycle synchronization by nutrient modulation. Integr. Biol. 2012, 4, 328–334. [Google Scholar] [CrossRef]
- Davidson, E.A.; Hart, S.C.; Shanks, C.A.; Firestone, M.K. Measuring gross nitrogen mineralization, immobilization, and nitrifi cation by 15 N isotopic pool dilution intact soil cores. J. Soil Sci. 1991, 42, 335–349. [Google Scholar] [CrossRef]
- Bollmann, A.; Conrad, R. Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Glob. Chang. Biol. 1998, 4, 387–396. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.; Romberger, J.S.; Papendick, R.I. Estimating generalized soil-water characteristics from texture. Soil Sci. Soc. Am. J. 1986, 50, 1031–1036. [Google Scholar] [CrossRef]
- Smith, K.A. Changing views of nitrous oxide emissions from agricultural soil: Key controlling processes and assessment at different spatial scales. Eur. J. Soil Sci. 2017, 68, 137–155. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garniere, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Castaldi, S.; Alberti, G.; Bertolini, T.; Forte, A.; Miglietta, F.; Valentini, R.; Fierro, A. N2O Emission Factors for Italian Crops. In The Greenhouse Gas Balance of Italy; Environmental Science and Engineering; Valentini, R., Miglietta, E.F., Eds.; Springer: Berlin, Germany, 2015; Chapter 9; pp. 135–144. [Google Scholar]
- Vitale, L.; Di Tommasi, P.; Arena, C.; Riondino, M.; Forte, A.; Verlotta, A.; Fierro, A.; Virzo De Santo, A.; Fuggi, A.; Magliulo, V. Growth and gas exchange response to water shortage of a maize crop on different soil types. Acta Physiol. Plant. 2009, 31, 331–341. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Mosier, A.R. Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- Smith, K.A.; Clayton, H.; McTaggart, I.P.; Thomson, P.E.; Arah, J.R.M.; Scott, A. The measurement of nitrous oxide emissions from soil by using chambers. Philos. Trans. R. Soc. 1995, 351, 27–38. [Google Scholar]
- Ranucci, S.; Bertolini, T.; Vitale, L.; Di Tommasi, P.; Ottaiano, L.; Oliva, M.; Magliulo, V. The influence of management and environmental variables on soil N2O emissions in a crop system in Southern Italy. Plant Soil 2001, 343, 83–96. [Google Scholar] [CrossRef]
- Rowell, D.L. Soil Science: Methods and Applications; Longman Ltd.: Harlow, UK, 1994; p. 61. [Google Scholar]
- Castaldi, S. Microbial Processes Contributing to N2O Production in Two Sandy Scottish Soils. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1997. [Google Scholar]
- Bart, G.; von Tucher, S.; Schmidhalter, U. Effectiveness of 3,4-dimethylpyrazole phosphate as nitrification inhibitor in soil as influenced by inhibitor concentration, application form, and soil matric potential. Pedosphere 2008, 18, 378–385. [Google Scholar] [CrossRef]
- Duan, Y.F.; Kong, X.W.; Schramm, A.; Labouriau, R.; Eriksen, J.; Petersen, S.O. Microbial N Transformations and N2O Emission after Simulated Grassland Cultivation: Effects of the Nitrification Inhibitor3,4-Dimethylpyrazole Phosphate (DMPP). Appl. Environ. Microbiol. 2017. [Google Scholar] [CrossRef]
- Vinzent, B.; Fuß, R.; Maidl, F.-X.; Hülsbergen, K.-J. N2O emissions and nitrogen dynamics of winter rapeseed fertilized with different N forms and a nitrification inhibitor. Agric. Ecosyst. Environ. 2018, 259, 86–97. [Google Scholar] [CrossRef]
- Dobbie, K.E.; Smith, K.A. Nitrous oxide emission factors for agricultural soils in Great Britain: The impact of soil water-filled pore space and other controlling variables. Glob. Chang. Biol. 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Forte, A. Denitrification and Nitrification Activities and N2O Emissions of Fine and Coarse Texture Soils of a Mediterranean Irrigated Cropland in Southern Italy. Ph.D. Thesis, University of Naples Federico II, Naples, Italy, 2006. [Google Scholar]
- Tan, I.Y.S.; Van Es, H.M.; Duxbury, J.M.; Melkonian, J.J.; Schindelbeck, R.R.; Geohring, L.D.; Hively, W.D.; Moebius, B.N. Single-eventnitrous oxide losses under maize production as affected by soil type, tillage, rotation, and fertilization. Soil Tillage 2009, 102, 19–26. [Google Scholar] [CrossRef]
- Gaillard, R.; Duval, B.D.; Osterholz, W.R.; Kucharik, C.J. Simulated effects of soil texture on nitrous oxide emissionfactors from corn and soybean agroecosystems in Wis-consin. J. Environ. Qual. 2016, 45, 1540–1548. [Google Scholar] [CrossRef]
- Phan, T.; Farrell, R.; Kate, C. The effect of soil moisture onnitrous oxide flux and production pathway in different soiltypes. In Soils and Crops Workshop; University of Saskatchewan Canada: Saskatoon, SK, Canada, 2019. [Google Scholar]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Dobbie, K.E.; McTaggart, I.P.; Smith, K.A. Nitrous oxide emissions from intensive agricultural systems: Variations between crops and seasons, key driving variables, and mean emission factors. J. Geophys. Res. Atmos. 1999, 104, 26891–26899. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2fromsoil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Yuttitham, M.; Chidthaisong, A.; Ruangchu, U. N2O fluxes and direct N2O emission factors from maize cultivation on Oxisols in Thailand. Geoderma Reg. 2020, 20, e00244. [Google Scholar] [CrossRef]
- Forte, A.; Fierro, A. Denitrification Rate and Its Potential to Predict Biogenic N2O Field Emissions in a Mediterranean Maize-Cropped Soil in Southern Italy. Land 2019, 8, 97. [Google Scholar] [CrossRef]
- Li, D.; Watson, C.J.; Yan, M.J.; Lalor, S.; Rafique, R.; Hyde, B.; Humphreys, J. A review of nitrous oxide mitigation by farm nitrogen management in temperate grassland-based agriculture. J. Environ. Manag. 2013, 128, 893–903. [Google Scholar] [CrossRef]
- Arah, J.R.M.; Smith, K.A.; Cricthon, I.J.; Li, H.S. Nitrous oxide production and denitrification in Scottish arable soils. J. Soil Sci. 1991, 42, 351–367. [Google Scholar] [CrossRef]

| Profile | Sandy (%) | Silt (%) | Clay (%) | pH | OM (%) | Bulk Density (g cm−3) | FC (gwater g−1 dw) | WFPS at FC (%) | USDA |
|---|---|---|---|---|---|---|---|---|---|
| Est | 29.8 | 22.1 | 48.1 | 7.63 | 3.7 | 1.15 | 0.206 | 45.4 | Clay |
| West | 75.0 | 11.0 | 14.0 | 7.65 | 7.8 | 1.01 | 0.391 | 78.3 | Sandy-Loam |
| Year | Sowing | 1° Fertilization | 2° Fertilization | Harvesting | Total Water Supplied (mm) |
|---|---|---|---|---|---|
| 2009 | 12–13 June | 12–13 June 68 kg N ha−1 | 10 July 190 kg N ha−1 | 8–9 September | 389 |
| 2010 | 18–19 June | 18–19 June 65 kg N ha−1 | 20 July 187 kg N ha−1 | 21–22 September | 476 |
| Years | Soil Texture | N2O Kg CO2 Equiv |
|---|---|---|
| 2009 | SL | 23.15 ± 0.44 |
| C | 25.32 ± 0.45 | |
| 2010 | SL | 461.18 ± 14.31 |
| C | 520.39 ± 17.88 |
| Soil Type | 2009 | 2010 |
|---|---|---|
| Clay | 19.72 ± 2.75 c | 28.70 ± 1.83 a |
| Sandy–Loam | 18.59 ± 1.15 c | 24.00 ± 1.58 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottaiano, L.; Di Mola, I.; Di Tommasi, P.; Mori, M.; Magliulo, V.; Vitale, L. Effects of Irrigation on N2O Emissions in a Maize Crop Grown on Different Soil Types in Two Contrasting Seasons. Agriculture 2020, 10, 623. https://doi.org/10.3390/agriculture10120623
Ottaiano L, Di Mola I, Di Tommasi P, Mori M, Magliulo V, Vitale L. Effects of Irrigation on N2O Emissions in a Maize Crop Grown on Different Soil Types in Two Contrasting Seasons. Agriculture. 2020; 10(12):623. https://doi.org/10.3390/agriculture10120623
Chicago/Turabian StyleOttaiano, Lucia, Ida Di Mola, Paul Di Tommasi, Mauro Mori, Vincenzo Magliulo, and Luca Vitale. 2020. "Effects of Irrigation on N2O Emissions in a Maize Crop Grown on Different Soil Types in Two Contrasting Seasons" Agriculture 10, no. 12: 623. https://doi.org/10.3390/agriculture10120623
APA StyleOttaiano, L., Di Mola, I., Di Tommasi, P., Mori, M., Magliulo, V., & Vitale, L. (2020). Effects of Irrigation on N2O Emissions in a Maize Crop Grown on Different Soil Types in Two Contrasting Seasons. Agriculture, 10(12), 623. https://doi.org/10.3390/agriculture10120623







