Seed of nine hemp cultivars was obtained from those entered in Cornell University (Ithaca, NY, USA) 2018 field trials and grown under pilot program research authorization from NYS Dept of Agriculture and Markets. Of these nine cultivars, five were dual-purpose (D) and four were for dedicated grain cultivars (G). Nine cultivars were purchased from UNISeeds (Cobden, ON, Canada), Assocanapa USA (Lexington, KY, USA), HGI (Saskatoon, SK, Canada), Legacy Hemp (Hastings, MN, USA) and Parkland (Dauphin, MB, Canada) (
Table 1). Prior to our experiments, there was no available information on flavor, aroma or other quality attributes of baby leaf hemp. Therefore, in consultation with commercial growers, the initial selection of these nine cultivars was based on cost (including seed price and shipping) as well as availability. At the time of our experiment, the lowest cost cultivar was ‘Anka’ (
$5.83/kg) and the most expensive cultivars were ‘Canda’ and ‘Joey’ (each at
$22.11/kg). For all experiments the following common methods were used. Plants were grown in a single layer glass greenhouse located at Cornell University in Ithaca, NY (42° N latitude) under ambient light and placed on a bench made by galvanized steel elevated 85 cm from the floor. Each experimental unit was planted in a polystyrene cell (8 × 8 × 6 cm; Dillen-ITML Greenhouse, Twinsburg, OH, USA) placed on the bench. For all experiments, the substrate mix was a custom seeding mix (Jiffy Group, Zwijndrecht, Zuid-Holland, The Netherlands), which was a blend of OMRI (Organic Materials Review Institute) approved coconut coir, peat moss from Jiffy Canada (Lorain, OH, USA) and dolomitic limestone. Substrate nutrient analysis was conducted (J.R. Peter’s Inc., Allentown, PA, USA) with the following values: 0.34 ppm nitrate (NO
3-N), 2 ppm of ammonium (NH
4-N), 2.31 ppm phosphorus (P), 82 potassium (K), 6 ppm calcium (Ca), 10 ppm magnesium (Mg), 4 ppm sulfur (S), 0.04 ppm boron (B), 0.24 ppm iron (Fe), 0.01 ppm manganese (Mn), 0.03 ppm copper (Cu), 0.01 ppm zinc (Zn), 0.08 ppm molybdenum (Mo), 0.12 ppm aluminum (Al), 35 ppm sodium (Na) and 179 ppm chloride (Cl). The initial substrate pH was 5.58 and EC (Electrical Conductivity) was 0.63 dS·m
−1. The substrate mix was prepared by mixing it with RO (reverse osmosis) water in a 2:1 ratio by volume to achieve adequate moisture. Cells were filled to a 5 cm substrate depth, seeds were sown and an additional 1 cm of the same substrate was covered on the top of the seeds. All treatments received the same access to irrigation water with water soluble fertilizer by sub-irrigation by filling a flat to a 3 cm level with a 150 mg·L
−1 N nutrient solution (21 N-2.2 P-16.6 K Jack’s All-Purpose Liquid Feed, J.R. Peter’s Inc., Allentown, PA, USA) and allowing each cell to take up water for 90 s before removing. The germination period (time to seedling emergence) usually took 48 to 96 h depending on the temperature and lighting conditions. Plants were harvested when half of the seedlings reached the stage of emergence of the third true leaf. The time period from seed to harvest was around 13 to 18 d depending on the temperature and lighting conditions. Temperature and relative humidity during each experiment and crop cycle are listed in
Table 2. In the experiments, an experimental unit was considered to be one 8 × 8 cm cell. Measurements were collected for germination percentage (number of seedlings emerged divided by total sown seeds), height (from the surface of the substrate to the tallest part of representative seedlings), and fresh weight (FW, using only the epicotyl, i.e., part of the plant above the cotyledons, based on commercial practice). After harvesting for FW, epicotyls from all plants in an experimental unit were bagged and placed in a 70 °C oven for 72 h to determine dry weight (DW). The fresh weight per plant (FWPP) was calculated as FW divided by number of seedling emergence for each treatment.
2.3. Seed Size Experiment
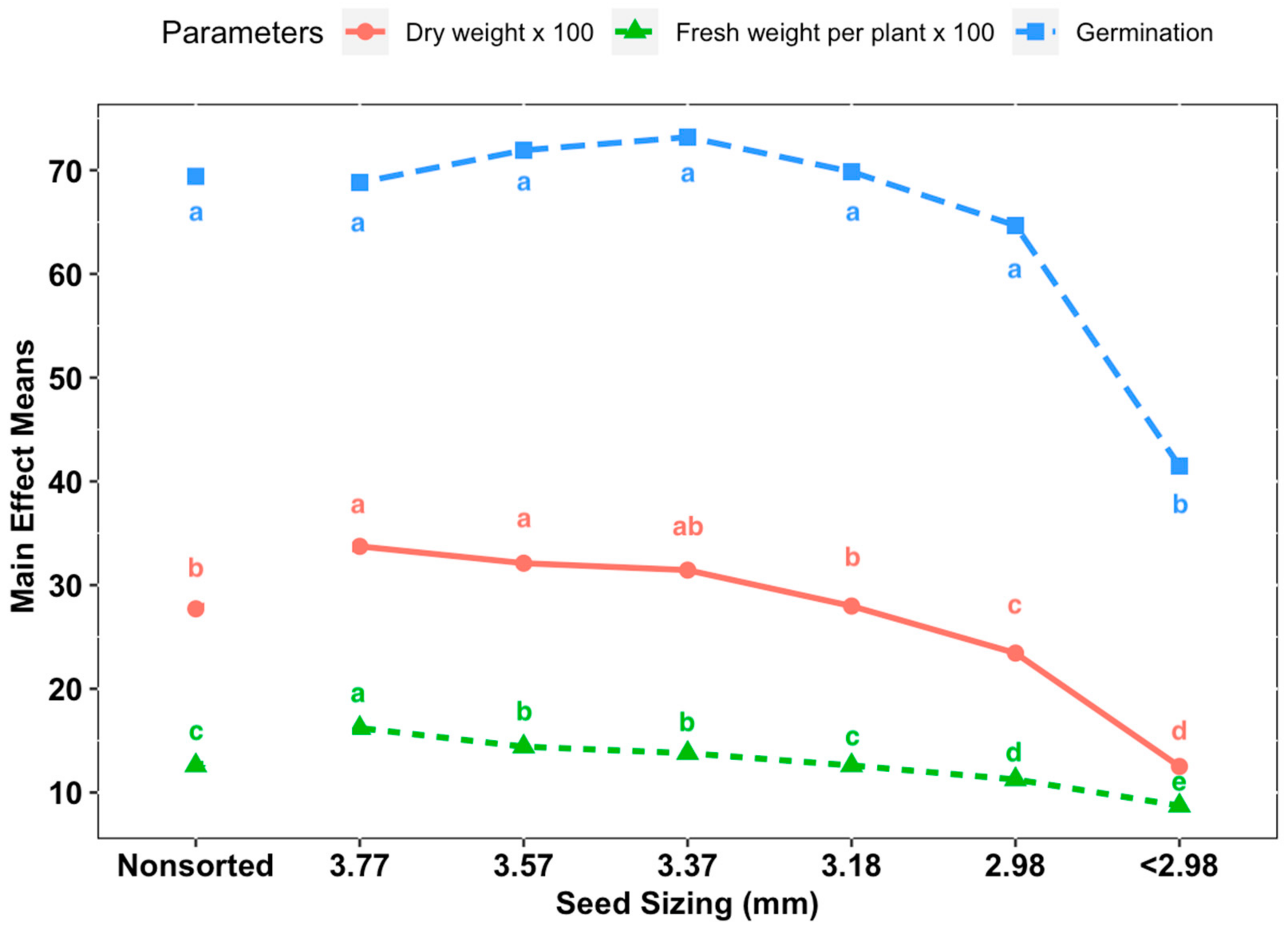
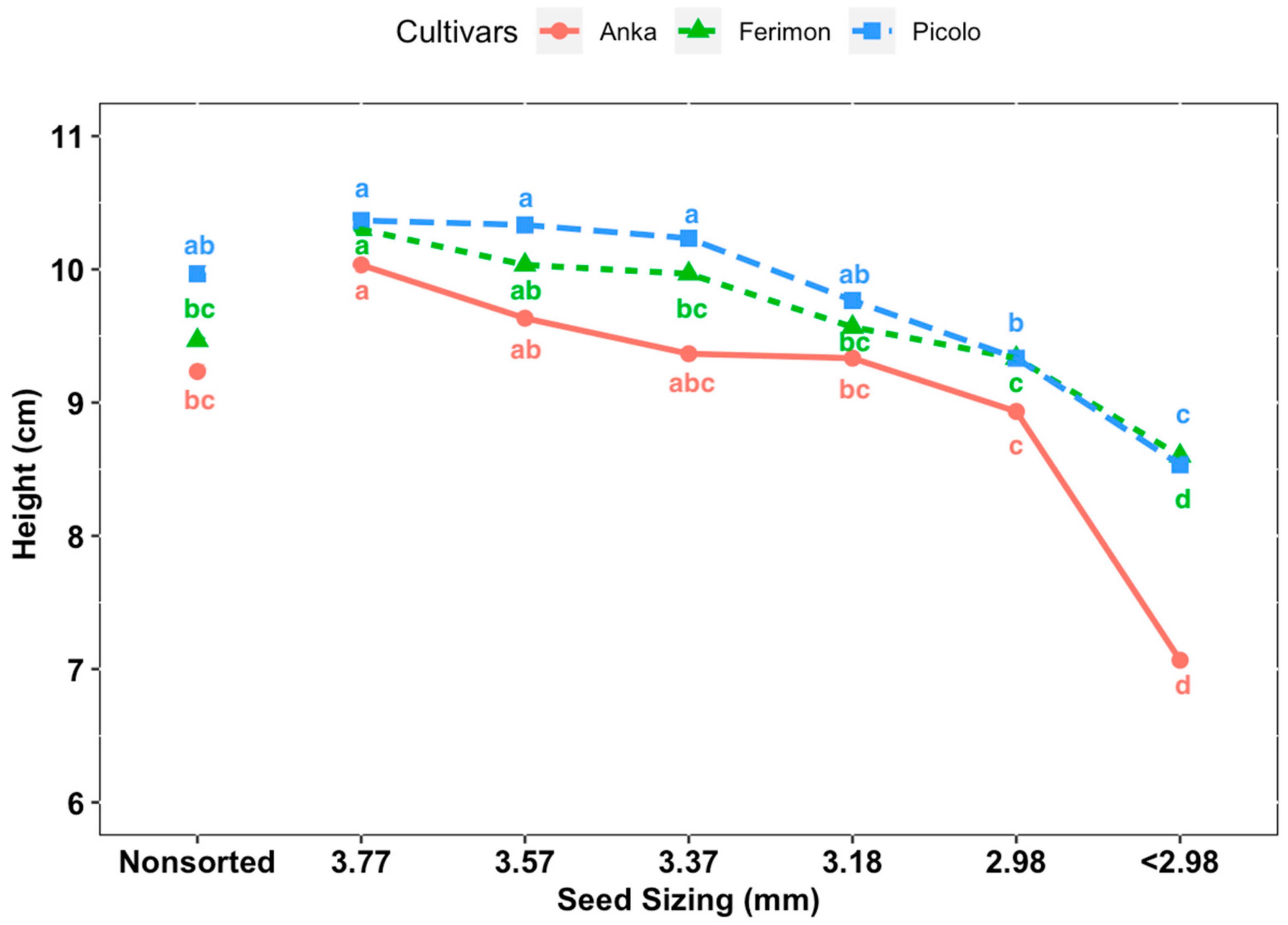
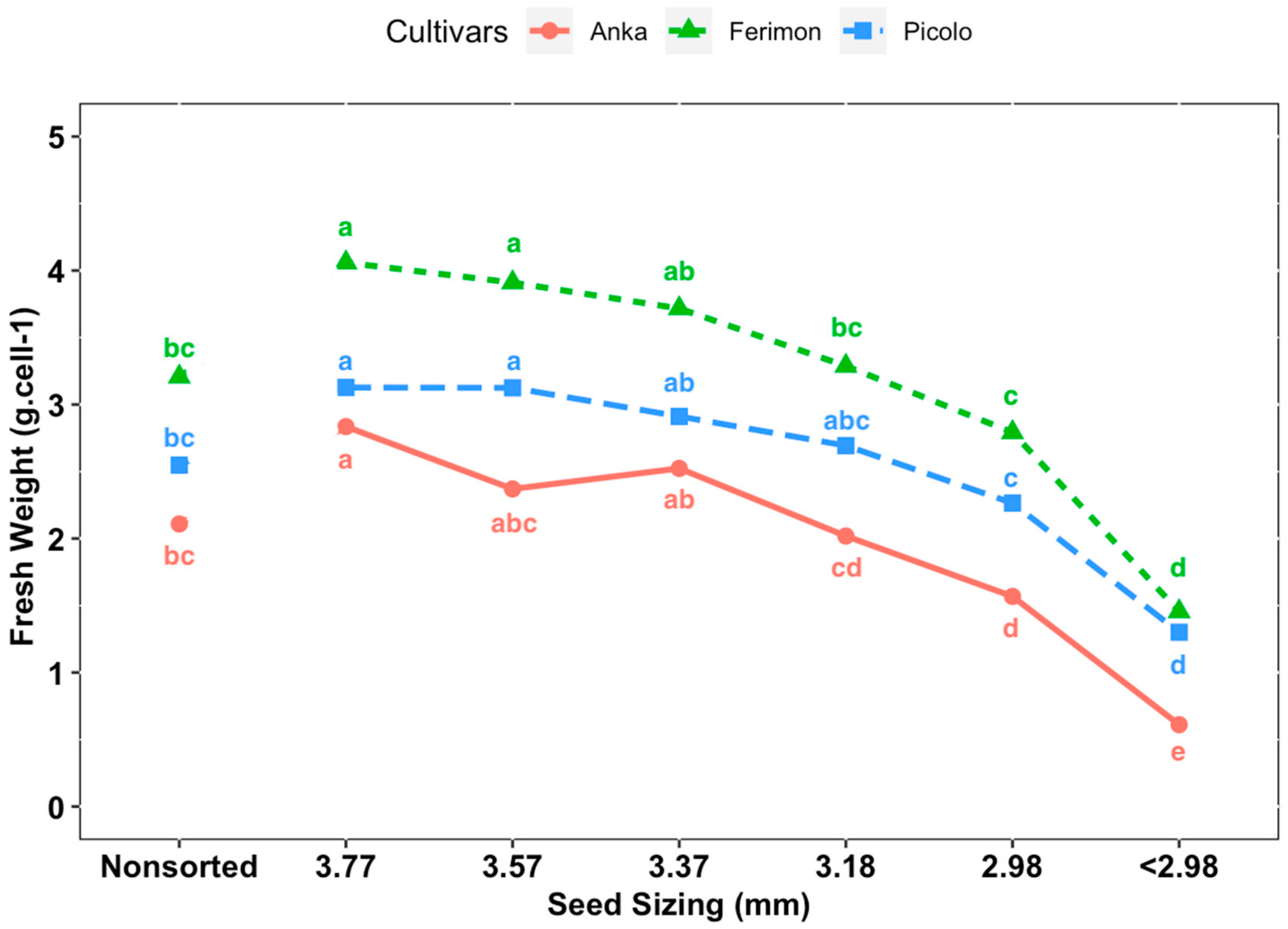
Cultivars ‘Anka’, ‘Picolo’ and ‘Ferimon’ were selected to evaluate the seed-size distribution and the effect of seed size on measured parameters. Seeds were sorted by size using eight different 23 cm × 23 cm hand screen sieves (Seedburo Equipment Company, Des Plaines, IL, USA) with round perforations for sorting by seed width. Seeds were sized through a series of stacked sieves from largest to smallest and seeds retained on a particular sieve were grouped: 3.77 mm (i.e., sieve size 9.5/64 inches), 3.57 mm (9/64 inches), 3.37 mm (8.5/64 inches), 3.18 mm (8/64 inches), 2.98 mm (7.5/64 inches), 2.78 mm (7/64 inches), 2.58 mm (6.5/64 inches) and 2.83 mm (6/64 inches). The distribution of 100 g of seed from each cultivar according to the sieve sizes was collected and analyzed (in terms of percent of seeds in each class by weight) with three replicates. In the evaluation of the effect of seed size on biomass/yield of baby leaf hemp, there were seven treatments according to seed width size: control (non-sorted seeds), sieve size 3.77, 3.57, 3.18, 2.98 and <2.98 mm. Sieved seeds of three entries from each treatment were sown evenly at a density 0.47 seeds·cm−2. The experiment was repeated three times with seeding date and harvesting dates of: 8 December to 26 December 2019; 30 December 2019, to 17 January 2020; and 21 January to 8 February 2020, respectively. Plants were harvested at the third true leaf stage and all parameters mentioned above were recorded for each experimental unit.
2.4. Statistical Analysis
In cultivar selection experiment, for each crop cycle there were six blocks placed in a randomized complete block design where each block contained one experimental unit from each of the nine different cultivars. In the sowing density experiment, there were five experimental units per cultivar and sowing density treatments arranged in a randomized complete block design where each block consisted of one experimental unit from each cultivar at each of the five seed densities. Within a block, the 10 cells were completely randomized. For seed size experiment, each crop cycle had five blocks where each block consisted of one experimental unit per cultivar per seed size treatment. The experiment was also arranged in a randomized complete block design. The block was based on location in the greenhouse bench. All three experiments were replicated over time for a total of three crop cycles. Data were analyzed with R studio (Version 1.2.1335, RStudio, Inc., Boston, MA, USA), using a mixed model including linear and quadratic regression (when treatments followed a quantitative independent variable, i.e., sowing density), Analysis of Variance (ANOVA), and mean separation comparison by Tukey’s honestly significant difference test (alpha = 0.05) with the following packages in R studio: library(ggplot2), library(multcomp), library(emmeans), library(lsmeans), library(lme4).