Hydrothermal Treatments Affecting the Concentration of Neochlorogenic Acid in Dough of Tartary Buckwheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
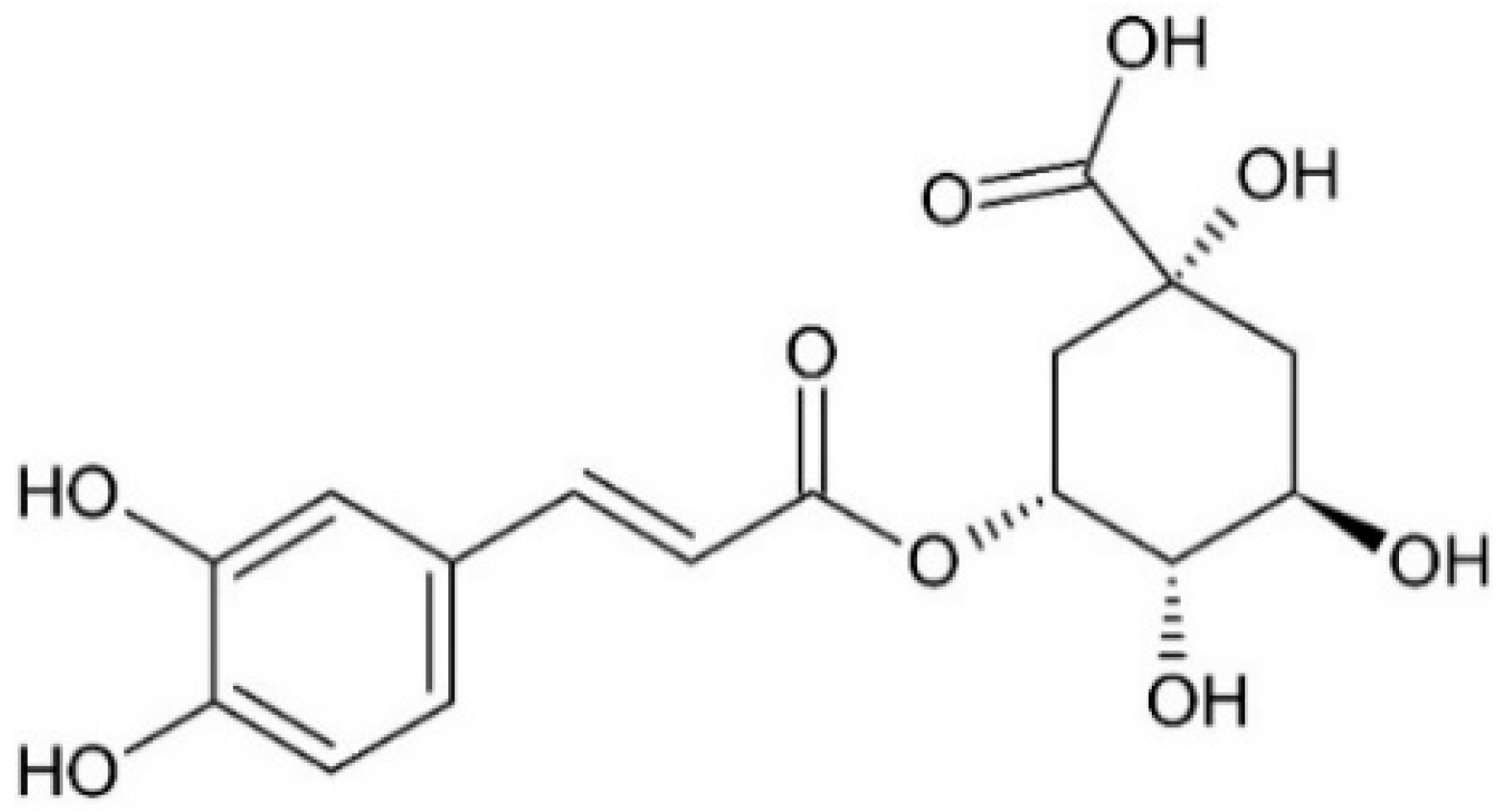
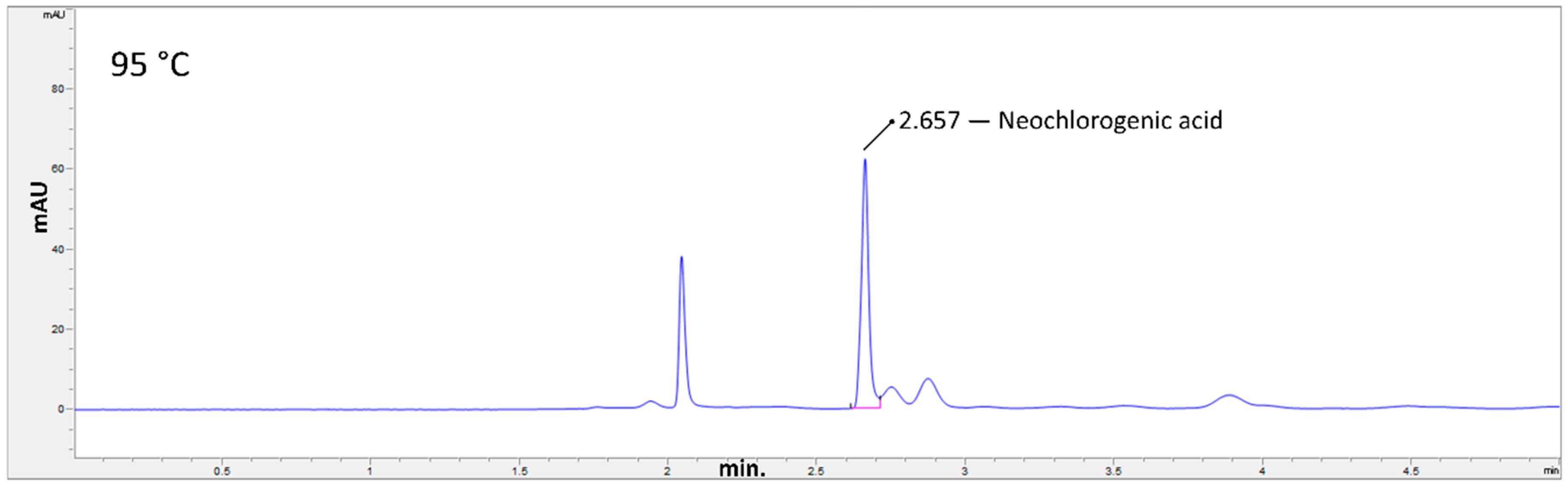
2.2. Determination of Neochlorogenic Acid
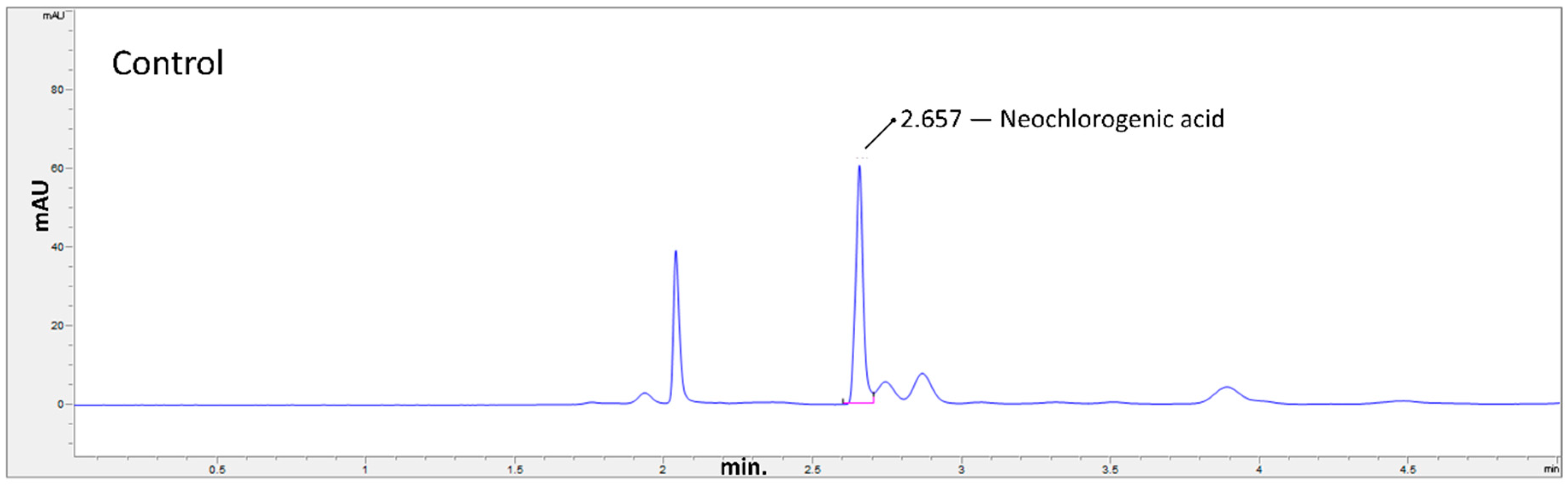
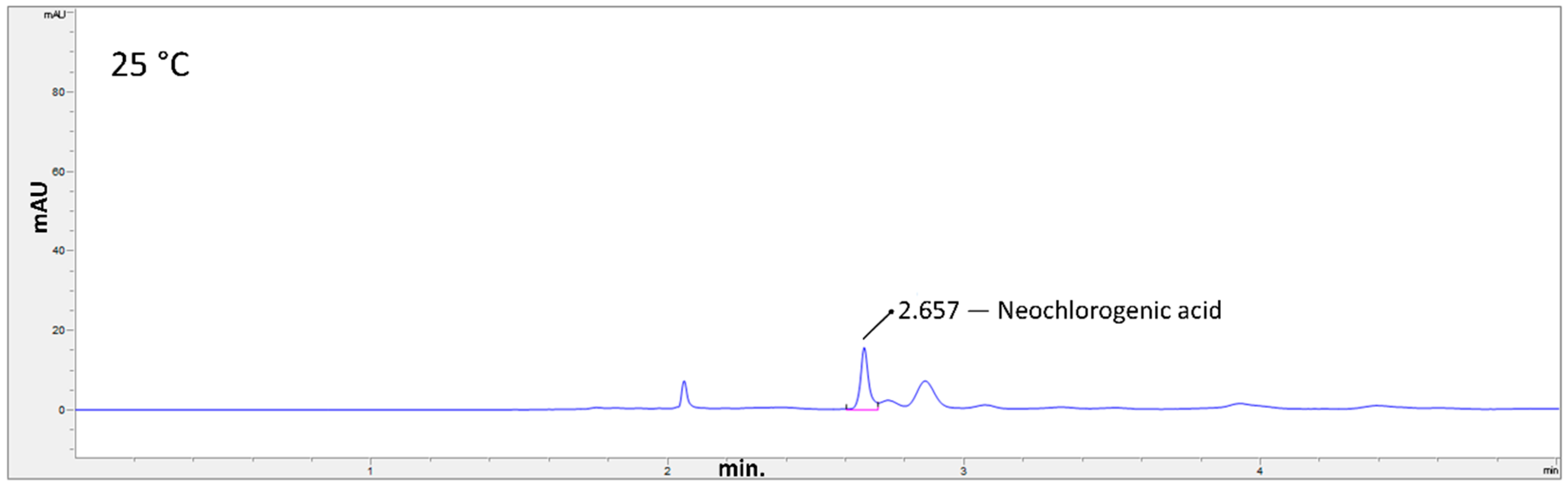
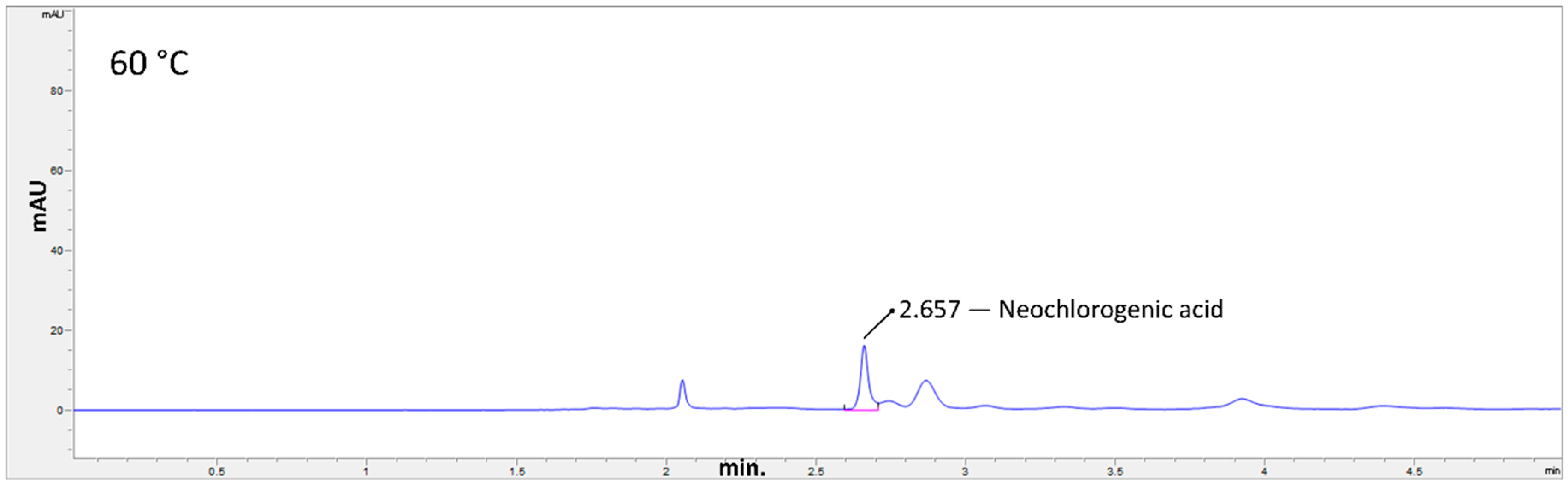
2.3. Hydrothermal Treatments
2.4. Statistical Analysis
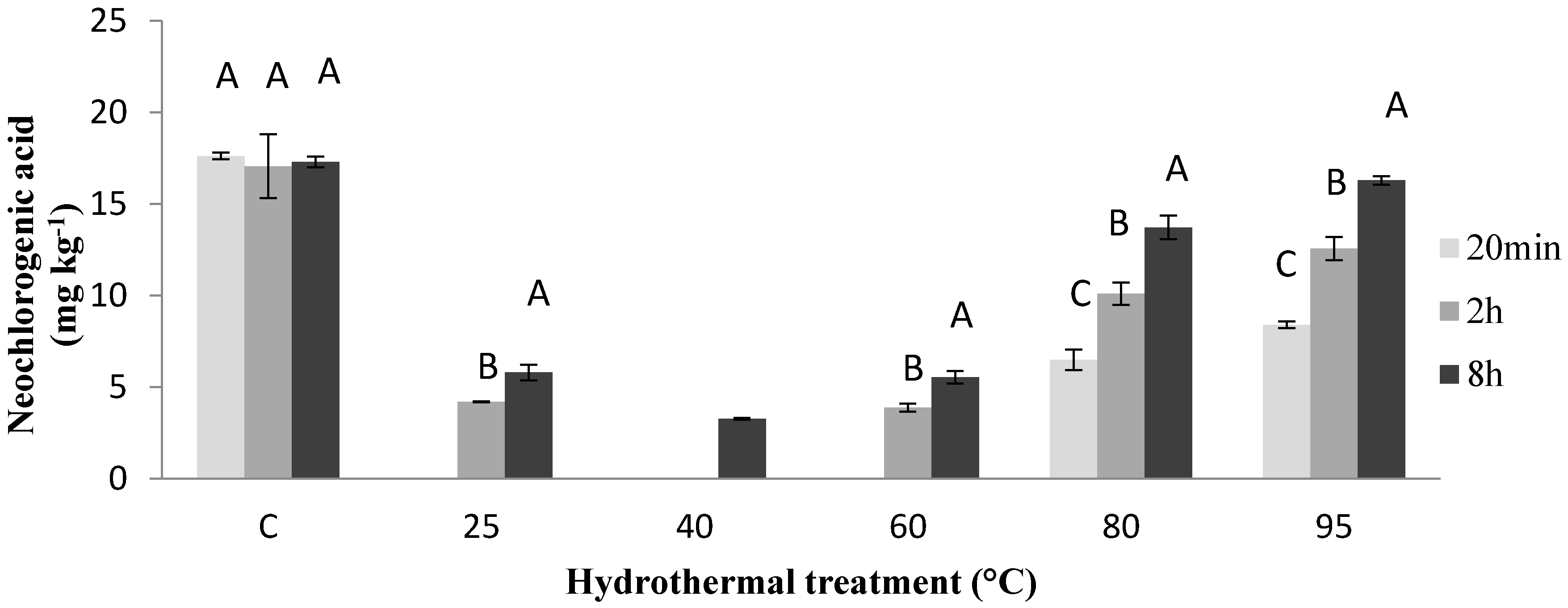
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonafaccia, G.; Gambelli, L.; Fabjan, N.; Kreft, I. Trace elements in flour and bran from common and Tartary buckwheat. Food Chem. 2003, 83, 1–5. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and Tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Capraro, J.; Magni, C.; Giorgi, A.; Duranti, M.; Scarafoni, A. Comparative 1d- and 2d-electrophoretic protein profiles of ancestral and modern buckwheat seeds grown in the Italian alpine region. Ital. J. Food Sci. 2018, 30, 497–503. [Google Scholar]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding buckwheat for nutritional quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Sulewska, H.; Kreft, I.; Gujska, E.; Walkowiak, J. The Content of Dietary Fibre and Polyphenols in Morphological Parts of Buckwheat (Fagopyrum tataricum). Plant Food Hum. Nutr. 2018, 73, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Žvikas, V.; Pukelevičienė, V.; Ivanauskas, L.; Romanovskaja, D.; Jakštas, V. Evaluation of phenolic antioxidant content in organically and conventionally grown buckwheat herb crop and its regrowth. J. Sci. Food Agric. 2017, 97, 3278–3283. [Google Scholar] [CrossRef]
- Inglett, G.E.; Rose, D.J.; Chen, D.; Stevenson, D.G.; Biswas, A. Phenolic content and antioxidant activity of extracts from whole buckwheat (Fagopyrum esculentum Moench) with or without microwave rradiation. Food Chem. 2010, 119, 1216–1219. [Google Scholar] [CrossRef]
- Costantini, L.; Lukšič, L.; Molinari, R.; Kreft, I.; Bonafaccia, G.; Manzi, L.; Merendino, N. Development of gluten-free bread using tartary buckwheat and chia flour rich in flavonoids and omega-3 fatty acids as ingredients. Food Chem. 2014, 165, 2–240. [Google Scholar] [CrossRef]
- Yildiz, O.; Bulut, B. Optimisation of gluten-free tulumba dessert with buckwheat flour and potato starch. Qual. Assur. Saf. Crop. 2016, 8, 117–128. [Google Scholar] [CrossRef]
- Unal, H.; Izli, G.; Izli, N.; Asik, B.B. Comparison of some physical and chemical characteristics of buckwheat (Fagopyrum esculentum Moench) grains. CYTA J. Food 2017, 15, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.R.; Yu, J.; Choi, S.J. Hydrothermal Treatment Enhances Antioxidant Activity and Intestinal Absorption of Rutin in Tartary Buckwheat Flour Extracts. Foods 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Li, W.; Hu, Y.; Zhou, X.; Ji, M.; Yu, D.; Fujita, K.; Tatsumi, E.; Luan, G. Comparison of pregelatinization methods on physicochemical, functional and structural properties of tartary buckwheat flour and noodle quality. J. Cereal Sci. 2018, 80, 63–71. [Google Scholar] [CrossRef]
- Azarbad, H.R.; Mostafa, M.T.; Rashidi, H. Optimization of Gluten-Free Bread Formulation Using Sorghum, Rice, and Millet Flour by D-Optimal Mixture Design Approach. J. Agric. Sci. Tech. 2019, 74, E140–E146. [Google Scholar] [CrossRef]
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Fabjan, N.; Rode, J.; Košir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef]
- Kim, S.J.; Zaidul, I.S.M.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and Tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef]
- Brunori, A.; Nobili, C.; Procacci, S. Toward the Use of Buckwheat as an Ingredient for the Preparation of Functional Food Molecular Breeding and Nutritional Aspects of Buckwheat. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.H., Chrungoo, N., Wislander, G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 219–225. [Google Scholar]
- Zhao, J.; Zhong, L.; Zou, L.; Zhang, C.; Peng, L.; Xiao, W.; Zhao, G. Efficient Promotion of the Sprout Growth and Rutin Production of Tartary Buckwheat by Associated Fungal Endophytes. Cereal Res. Commun. 2014, 42, 401–412. [Google Scholar] [CrossRef]
- Vollmer, M.; Schröter, D.; Esders, S.; Neugart, S.; Farquharson, F.M.; Duncan, S.H.; Schreiner, M.; Louis, P.; Maul, R.; Rohn, S. Chlorogenic acid versus amaranth’s caffeoylisocitric acid—Gut microbial degradation of caffeic acid derivatives. Food Res. Int. 2017, 100, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Food 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying peach and plum polyphenols with chemopreventive potential against estrogen-independent breast cancer cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef] [PubMed]
- Thurow, T. Effect of Chlorogenic Acid and Neochlorogenic Acid on Human Colon Cancer Cells. Food Science Undergraduate Honors Theses, University of Arkansas, Fayetteville, NC, USA, 2012. [Google Scholar]
- Vogrinčič, M.; Kreft, I.; Filipič, M.; Žegura, B. Antigenotoxic effect of Tartary (Fagopyrum tataricum) and common (Fagopyrum esculentum) buckwheat flour. J. Med. Food 2013, 16, 944–952. [Google Scholar] [CrossRef]
- Kurita, S.; Kashiwagi, T.; Ebisu, T.; Shimamura, T.; Ukeda, H. Identification of neochlorogenic acid as the predominant antioxidant in Polygonum cuspidatum leaves. Ital. J. Food Sci. 2016, 28, 25–31. [Google Scholar] [CrossRef]
- Kreft, I. Buckwheat in Slovenia. In Ethnobotany of Buckwheat; Kreft, I., Chang, K., Choi, Y.S., Park, C.H., Eds.; Jinsol Publishing Co: Seoul, Korea, 2003; pp. 71–79. [Google Scholar]
- Dawidowidz, A.; Typek, R. The influence of pH on the thermal stability of 5-O-caffeoylquinic acids in aqueous solutions. Eur. Food Res. Technol. 2011, 233, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Dawidowidz, A.L.; Typek, R. Transformation of 5 O caffeoylquinic acid in blueberries during high-temperature processing. J. Agric. Food Chem. 2014, 62, 10889–10895. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germ, M.; Árvay, J.; Vollmannová, A.; Tóth, T.; Kreft, I.; Golob, A. Hydrothermal Treatments Affecting the Concentration of Neochlorogenic Acid in Dough of Tartary Buckwheat. Agriculture 2020, 10, 601. https://doi.org/10.3390/agriculture10120601
Germ M, Árvay J, Vollmannová A, Tóth T, Kreft I, Golob A. Hydrothermal Treatments Affecting the Concentration of Neochlorogenic Acid in Dough of Tartary Buckwheat. Agriculture. 2020; 10(12):601. https://doi.org/10.3390/agriculture10120601
Chicago/Turabian StyleGerm, Mateja, Július Árvay, Alena Vollmannová, Tomáš Tóth, Ivan Kreft, and Aleksandra Golob. 2020. "Hydrothermal Treatments Affecting the Concentration of Neochlorogenic Acid in Dough of Tartary Buckwheat" Agriculture 10, no. 12: 601. https://doi.org/10.3390/agriculture10120601
APA StyleGerm, M., Árvay, J., Vollmannová, A., Tóth, T., Kreft, I., & Golob, A. (2020). Hydrothermal Treatments Affecting the Concentration of Neochlorogenic Acid in Dough of Tartary Buckwheat. Agriculture, 10(12), 601. https://doi.org/10.3390/agriculture10120601







