Water-Soluble Carbohydrate Recovery in Pastures of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.) Under Two Defoliation Frequencies Determined by Thermal Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Evaluated Variables and Sampling
2.4. Statistical Analysis
3. Results
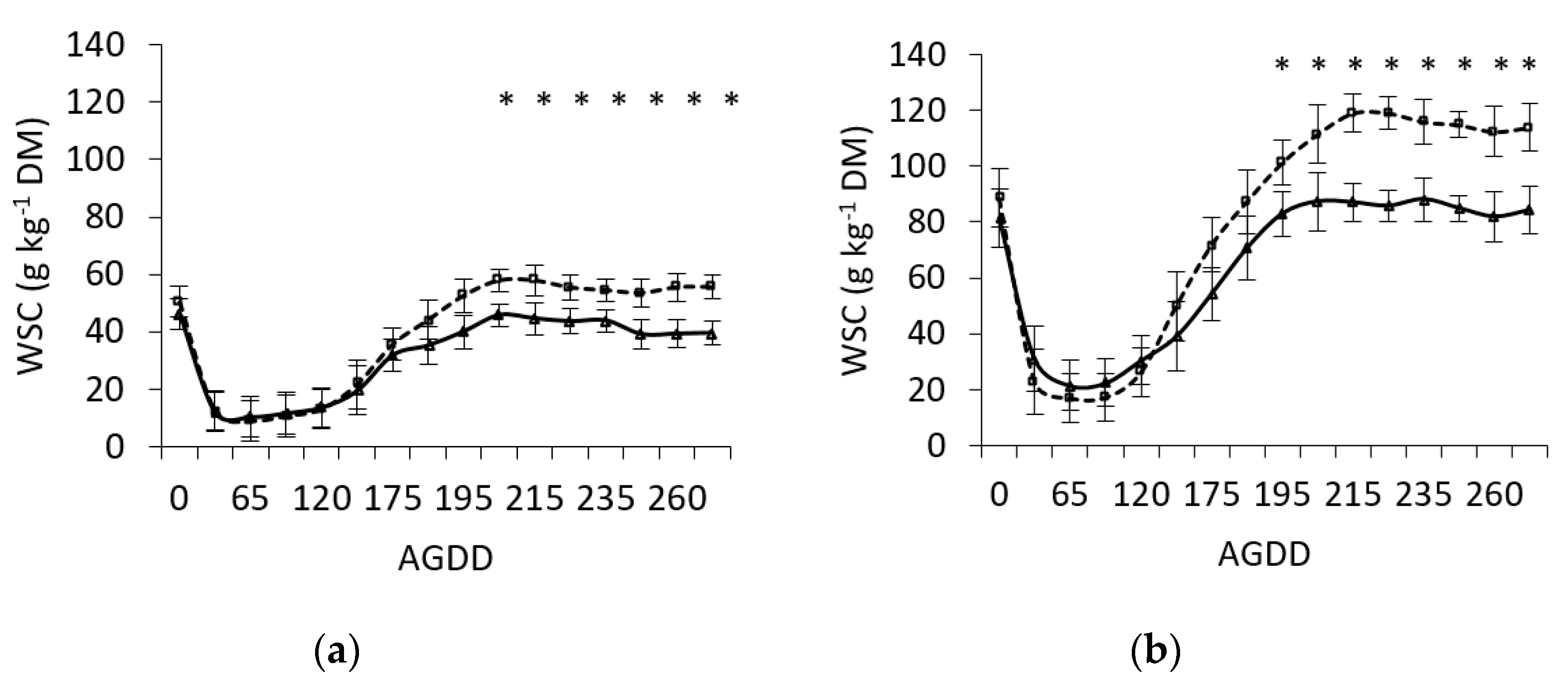
3.1. Effect of Defoliation Frequency on WSC Concentration in Leaf Sheaths and Blades
3.2. Water-soluble Carbohydrates and Pasture Regrowth
3.3. Defoliation Frequency and Growth
4. Discussion
4.1. Defoliation Frequency and WSCs
4.2. Defoliation Frequency and AHM
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clerget, B.; Dingkuhn, M.; Goze, E.; Rattunde, H.F.; Ney, B. Variability of phyllochron, plastochron and rate of increase in height in photoperiod-sensitive sorghum varieties. Ann. Bot. 2008, 101, 579–594. [Google Scholar] [CrossRef]
- Chaves, G.G.; Cargnelutti Filho, A.; Alves, B.M.; Lavezo, A.; Wartha, C.A.; Uliana, D.B.; Pezzini, R.V.; Kleinpaul, J.A.; Neu, I.M.M. Phyllochron and leaf appearance rate in oat. Bragantia 2017, 76, 73–81. [Google Scholar] [CrossRef]
- Wilhelm, W.; McMaster, G.S. Symposium on the phyllochron: Importance of the phyllochron in studying development and growth in grasses. Crop Sci. 1995, 35, 1–3. [Google Scholar] [CrossRef]
- Calvache, I.; Balocchi, O.; Alonso, M.; Keim, J.P.; López, I.F. Thermal Time as a Parameter to Determine Optimal Defoliation Frequency of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.). Agronomy 2020, 10, 620. [Google Scholar] [CrossRef]
- Donaghy, D.; Fulkerson, W. Priority for allocation of water-soluble carbohydrate reserves during regrowth of Lolium perenne. Grass Forage Sci. 1998, 53, 211–218. [Google Scholar] [CrossRef]
- Turner, L.; Donaghy, D.; Lane, P.; Rawnsley, R. Effect of defoliation management, based on leaf stage, on perennial ryegrass (Lolium perenne L.), prairie grass (Bromus willdenowii Kunth.) and cocksfoot (Dactylis glomerata L.) under dryland conditions. 1. Regrowth, tillering and water-soluble carbohydrate concentration. Grass Forage Sci. 2006, 61, 164–174. [Google Scholar]
- Pelletier, S.; Tremblay, G.F.; Bélanger, G.; Bertrand, A.; Castonguay, Y.; Pageau, D.; Drapeau, R. Forage nonstructural carbohydrates and nutritive value as affected by time of cutting and species. Agron. J. 2010, 102, 1388–1398. [Google Scholar] [CrossRef]
- Smith, K.; Simpson, R.; Culvenor, R.; Humphreys, M.O.; Prud’Homme, M.; Oram, R. The effects of ploidy and a phenotype conferring a high water-soluble carbohydrate concentration on carbohydrate accumulation, nutritive value and morphology of perennial ryegrass (Lolium perenne L.). J. Agric. Sci. 2001, 136, 65–74. [Google Scholar] [CrossRef]
- Cajarville, C.; Britos, A.; Errandonea, N.; Gutiérrez, L.; Cozzolino, D.; Repetto, J.L. Diurnal changes in water-soluble carbohydrate concentration in lucerne and tall fescue in autumn and the effects on in vitro fermentation. N. Z. J. Agric. Res. 2015, 58, 281–291. [Google Scholar] [CrossRef]
- Donaghy, D.J.; Turner, L.R.; Adamczewski, K.A. Effect of defoliation management on water-soluble carbohydrate energy reserves, dry matter yields, and herbage quality of tall fescue. Agron. J. 2008, 100, 122. [Google Scholar] [CrossRef]
- Zhao, D.; MacKown, C.T.; Starks, P.J.; Kindiger, B.K. Interspecies variation of forage nutritive value and nonstructural carbohydrates in perennial cool-season grasses. Agron. J. 2008, 100, 837–844. [Google Scholar] [CrossRef]
- Savitch, L.V.; Gray, G.R.; Huner, N.P. Feedback-limited photosynthesis and regulation of sucrose-starch accumulation during cold acclimation and low-temperature stress in a spring and winter wheat. Planta 1997, 201, 18–26. [Google Scholar] [CrossRef]
- Xue, G.P.; McIntyre, C.L.; Jenkins, C.L.; Glassop, D.; van Herwaarden, A.F.; Shorter, R. Molecular dissection of variation in carbohydrate metabolism related to water-soluble carbohydrate accumulation in stems of wheat. Plant Physiol. 2008, 146, 441–454. [Google Scholar] [CrossRef]
- Ciavarella, T.A.; Simpson, R.J.; Dove, H.; Leury, B.J.; Sims, I.M. Diurnal changes in the concentration of water soluble carbohydrates in Phalaris aquatica L. pasture in spring, and the effect of short-term shading. Aust. J. Agric. Res. 2000, 51, 749–756. [Google Scholar] [CrossRef]
- Liu, Q.; Rasmussen, S.; Johnson, L.J.; Xue, H.; Parsons, A.J.; Jones, C.S. Molecular Mechanisms Regulating Carbohydrate Metabolism During Lolium perenne Regrowth Vary in Response to Nitrogen and Gibberellin Supply. J. Plant Growth Regul. 2020, 39, 1342–1355. [Google Scholar] [CrossRef]
- Cairns, A.J.; Begley, P.; Sims, I.M. The structure of starch from seeds and leaves of the fructan-accumulating ryegrass, Lolium temulentum L. J. Plant Physiol. 2002, 159, 221–230. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C.; Thorneycroft, D.; Smith, S.M. Starch mobilization in leaves. J. Exp. Bot. 2003, 54, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Amiard, V.; Morvan-Bertrand, A.; Cliquet, J.B.; Billard, J.P.; Huault, C.; Sandström, J.P.; Prud’homme, M.P. Carbohydrate and amino acid composition in phloem sap of Lolium perenne L. before and after defoliation. Can. J. Bot. 2004, 82, 1594–1601. [Google Scholar] [CrossRef]
- Delagarde, R.; Peyraud, J.; Delaby, L.; Faverdin, P. Vertical distribution of biomass, chemical composition and pepsin––cellulase digestibility in a perennial ryegrass sward: Interaction with month of year, regrowth age and time of day. Anim. Feed Sci. Technol. 2000, 84, 49–68. [Google Scholar] [CrossRef]
- Griggs, T.C.; MacAdam, J.W.; Mayland, H.F.; Burns, J.C. Nonstructural carbohydrate and digestibility patterns in orchardgrass swards during daily defoliation sequences initiated in evening and morning. Crop Sci. 2005, 45, 1295–1304. [Google Scholar] [CrossRef]
- CIREN. Centro de Información de Recursos Naturales. Agrological Study X Region, Descriptions of Soils, Materials and Symbols; Publicación CIREN N° 123: Santiago, Chile, 2003. [Google Scholar]
- Biligetu, B.; Coulman, B. Responses of Three Bromegrass (Bromus) Species to Defoliation under Different Growth Conditions. Int. J. Agron. 2010, 20, 1–5. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Canseco, C.; Demanet, R.; Balocchi, O.; Parga, J.; Anwandter, V.; Abarzúa, A.; Teuber, N.; Lopetegui, J. Determinación de la disponibilidad de materia seca de praderas en pastoreo. Manejo Del Pastor. Imprenta Am. 2007, 10, 23–50. [Google Scholar]
- Littell, R.; Henry, P.; Ammerman, C. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.G.; Chatterton, N.J.; Harrison, P.A.; Filgueiras, T.S.; Figueiredo-Ribeiro, R.C.L. Diversity of non-structural carbohydrates in grasses (Poaceae) from Brazil. Grass Forage Sci. 2012, 68, 165–177. [Google Scholar] [CrossRef]
- White, L.M. Carbohydrate reserves of grasses: A review. J. Range Manag. 1973, 26, 13–18. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Gramig, G.G.; Stoltenberg, D.E. Leaf appearance base temperature and phyllochron for common grass and broadleaf weed species. Weed Technol. 2017, 21, 249–254. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.; Trentmann, O.; Klemens, P.A.W.; Neuhaus, H.E. In concert: Orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physiol. 2018, 59, 1290–1299. [Google Scholar] [CrossRef]
- Berone, G.D.; Lattanzi, F.A.; Agnusdei, M.G.; Bertolotti, N. Growth of individual tillers and tillering rate of Lolium perenne and Bromus stamineus subjected to two defoliation frequencies in winter in Argentina. Grass Forage Sci. 2008, 63, 504–512. [Google Scholar] [CrossRef]
- Loaiza, P.A.; Balocchi, O.; Bertrand, A. Carbohydrate and crude protein fractions in perennial ryegrass as affected by defoliation frequency and nitrogen application rate. Grass Forage Sci. 2017, 72, 556–567. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2015. [Google Scholar]
- Zeeman, S.C.; Smith, S.M.; Smith, A.M. The diurnal metabolism of leaf starch. Biochem. J. 2007, 401, 13–28. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Jagadish, K.S.V. Temperature regulation of plant phenological development. Environ. Exp. Bot. 2015, 111, 83–90. [Google Scholar] [CrossRef]
- McClung, C.R.; Davis, S.J. Ambient thermometers in plants: From physiological outputs towards mechanisms of thermal sensing. Curr. Biol. 2010, 20, R1086–R1092. [Google Scholar] [CrossRef]
- Lee, J.M.; Donaghy, D.J.; Roche, J.R. Effect of defoliation severity on regrowth and nutritive value of perennial ryegrass dominant swards. Agron. J. 2008, 100, 308–314. [Google Scholar] [CrossRef]
- Herz, K.; Dietz, S.; Haider, S.; Jandt, U.; Scheel, D.; Bruelheide, H. Predicting individual plant performance in grasslands. Ecol. Evol. 2017, 7, 8958–8965. [Google Scholar] [CrossRef]
- Hoogmoed, M.; Sadras, V.O. The importance of water-soluble carbohydrates in the theoretical framework for nitrogen dilution in shoot biomass of wheat. Field Crops Res. 2016, 193, 196–200. [Google Scholar] [CrossRef]
- Beltran, I.E.; Morales, A.; Balocchi, O.; Pulido, P. Interaction between herbage mass and time of herbage allocation modify milk production, grazing behaviour and nitrogen partitioning of dairy cows. Anim. Prod. Sci. 2019, 59, 1837–1846. [Google Scholar] [CrossRef]




| Species | AGDDs | Blade | Sheath | Hectare |
|---|---|---|---|---|
| B. valdivianus | 135 | 37.95 b | 53.70 c | 193 c |
| 270 | 39.63 b | 84.28 b | 238 b | |
| L. perenne | 135 | 40.72 b | 54.65 c | 192 c |
| 270 | 55.61 a | 113.93 a | 348 a | |
| SEM | 1.481 | 1.923 | 36.7 | |
| p-Value | 0.001 | 0.001 | 0.001 |
| Species | AGDDs | Growth |
|---|---|---|
| B. valdivianus | 135 | 906 x |
| 270 | 723 | |
| L. perenne | 135 | 816 x |
| 270 | 854 | |
| SEM | 17.319 | |
| p-Value | 0.415 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvache, I.; Balocchi, O.; Alonso, M.; Keim, J.P.; López, I. Water-Soluble Carbohydrate Recovery in Pastures of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.) Under Two Defoliation Frequencies Determined by Thermal Time. Agriculture 2020, 10, 563. https://doi.org/10.3390/agriculture10110563
Calvache I, Balocchi O, Alonso M, Keim JP, López I. Water-Soluble Carbohydrate Recovery in Pastures of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.) Under Two Defoliation Frequencies Determined by Thermal Time. Agriculture. 2020; 10(11):563. https://doi.org/10.3390/agriculture10110563
Chicago/Turabian StyleCalvache, Iván, Oscar Balocchi, Máximo Alonso, Juan Pablo Keim, and Ignacio López. 2020. "Water-Soluble Carbohydrate Recovery in Pastures of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.) Under Two Defoliation Frequencies Determined by Thermal Time" Agriculture 10, no. 11: 563. https://doi.org/10.3390/agriculture10110563
APA StyleCalvache, I., Balocchi, O., Alonso, M., Keim, J. P., & López, I. (2020). Water-Soluble Carbohydrate Recovery in Pastures of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.) Under Two Defoliation Frequencies Determined by Thermal Time. Agriculture, 10(11), 563. https://doi.org/10.3390/agriculture10110563






