Native Perennial Plants Colonizing Abandoned Arable Fields in a Desert Area: Population Structure and Community Assembly
Abstract
1. Introduction
2. Materials and Methods
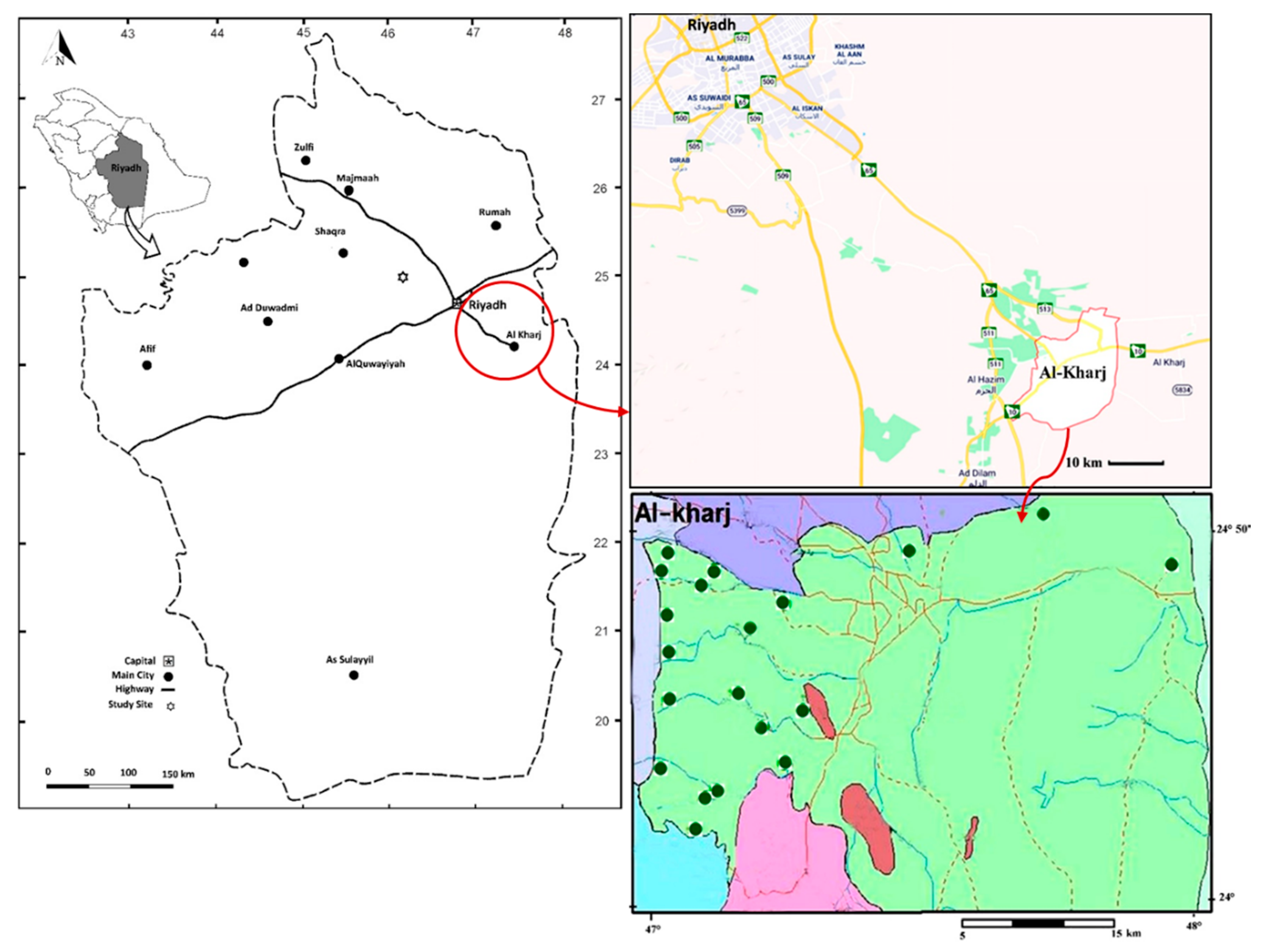
2.1. Study Area
2.2. Vegetation Sampling
2.3. Soil Analysis
2.4. Vegetation Data Analysis
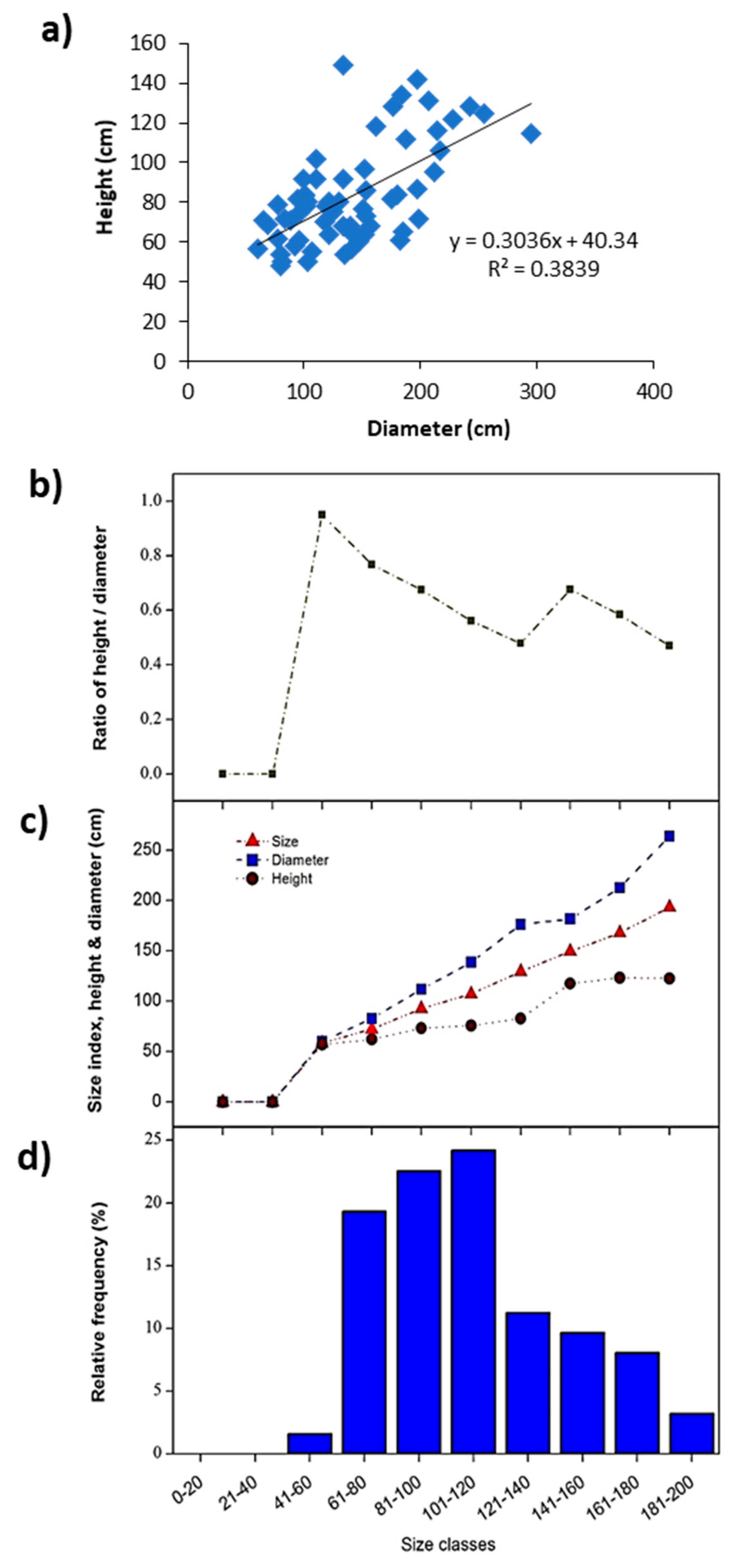
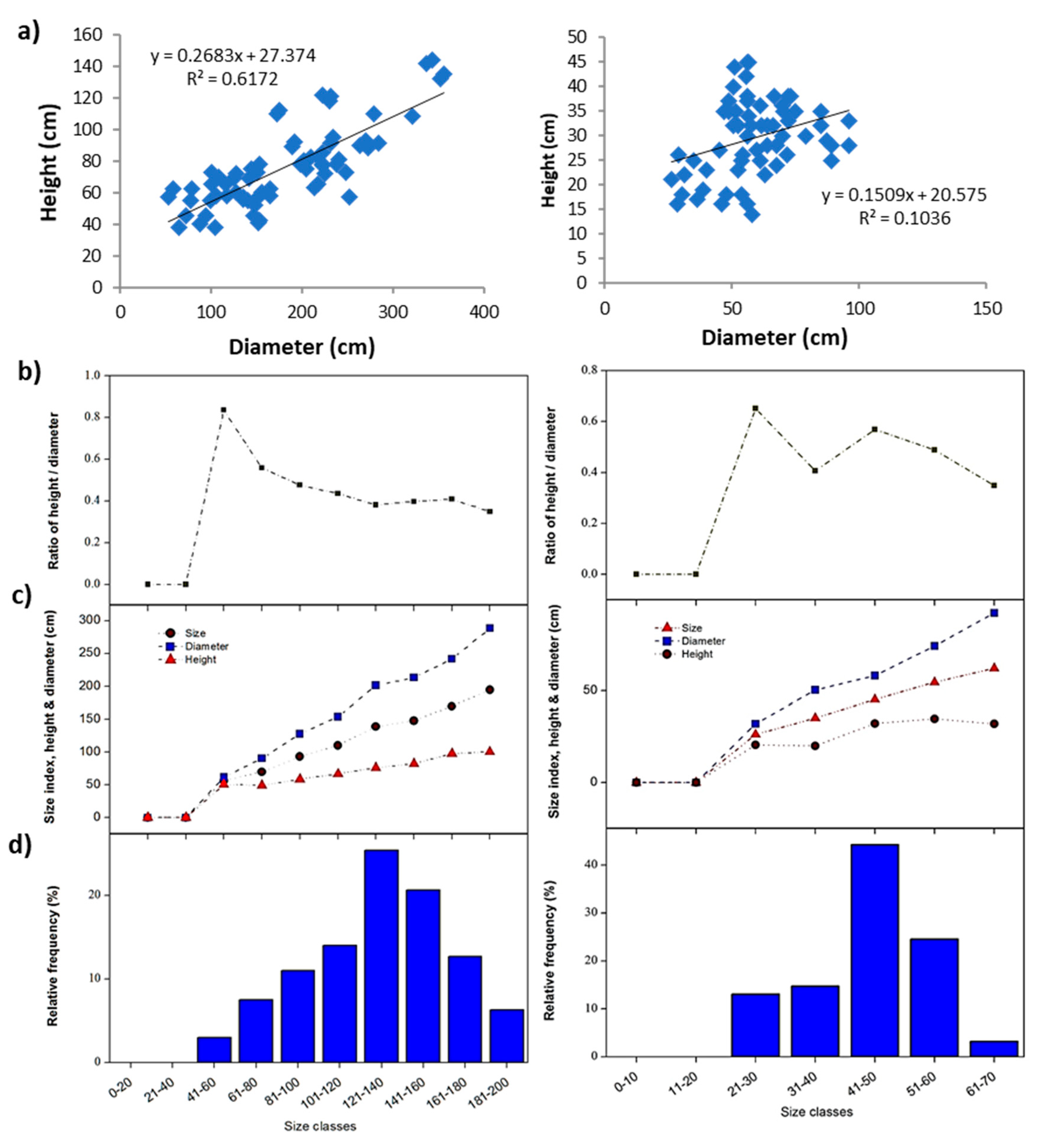
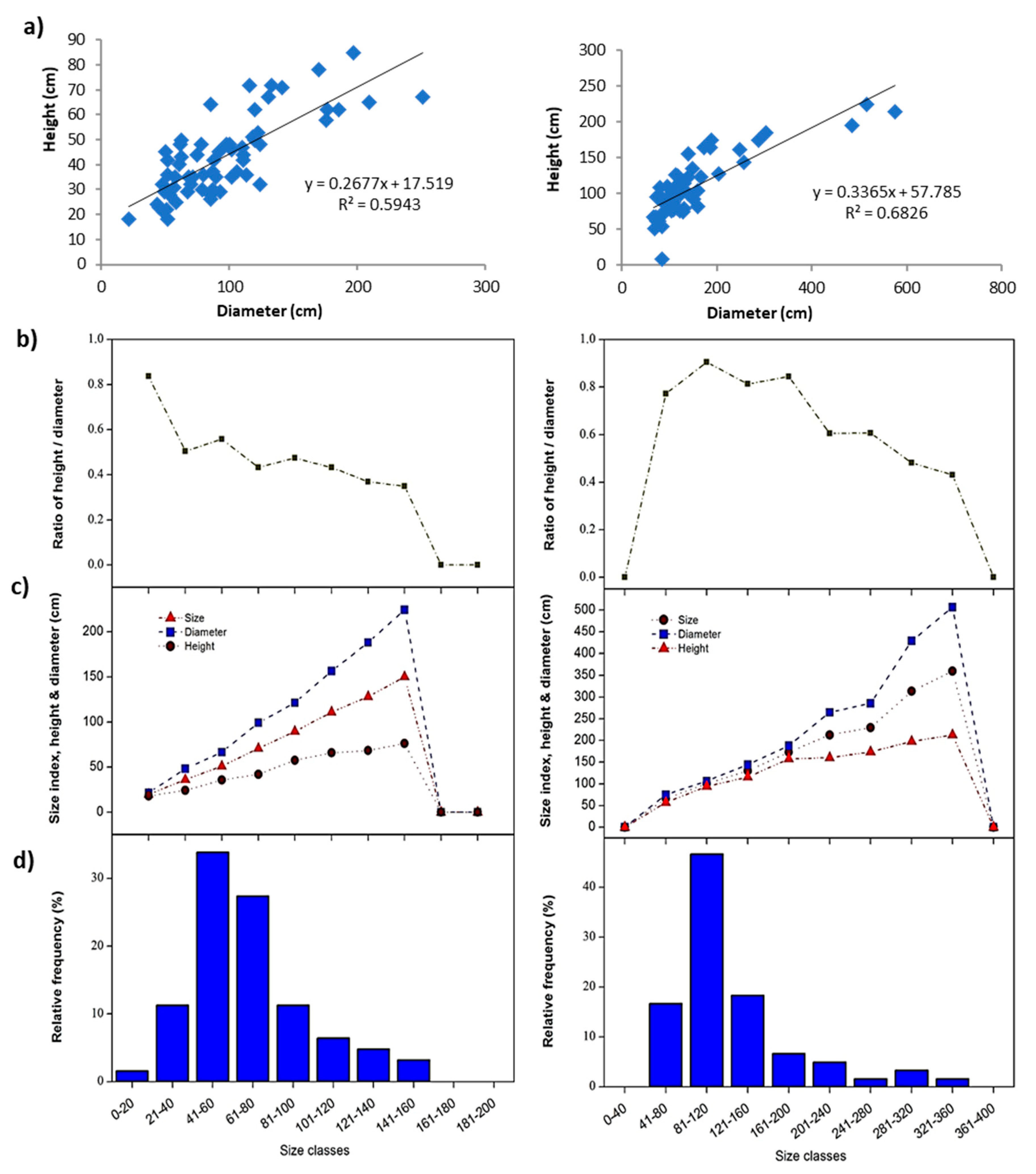
2.5. Species Size Structure Analysis
3. Results
3.1. Vegetation Composition
3.2. Vegetation–Soil Relationship
3.3. Demographic Analysis of the Dominant Plant Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vitousek, P.M. Beyond global warming: Ecology and global change. Ecology 1994, 75, 1861–1876. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Wong, N.K.; Morgan, J.W.; Dorrough, J. A conceptual model of plant community changes following cessation of cultivation in semi-arid grassland. Appl. Veg. Sci. 2010, 13, 389–402. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Latini, M.; Iberite, M.; Bonari, G.; Nicolella, G.; Rosati, L.; Salerno, G.; Abbate, G. The segetal flora of winter cereals and allied crops in Italy: Species inventory with chorological, structural and ecological features. Plant Biosyst. 2020, 154, 1–12. [Google Scholar] [CrossRef]
- Nowak, A.; Nowak, S.; Nobis, M.; Nobis, A. A report on the conservation status of segetal weeds in Tajikistan. Weed Res. 2014, 54, 635–648. [Google Scholar] [CrossRef]
- Storkey, J.; Neve, P. What good is weed diversity? Weed Res. 2018, 58, 239–243. [Google Scholar] [CrossRef]
- Fredh, E.D.; Lagerås, P.; Mazier, F.; Björkman, L.; Lindbladh, M.; Broström, A. Farm establishment, abandonment and agricultural practices during the last 1,300 years: A case study from southern Sweden based on pollen records and the LOVE model. Veg. Hist. Archaeobot. 2019, 28, 529–544. [Google Scholar] [CrossRef]
- Verstraete, M.M.; Scholes, R.J.; Smith, M.S. Climate and desertification: Looking at an old problem through new lenses. Front. Ecol. Environ. 2009, 7, 421–428. [Google Scholar] [CrossRef]
- Cramer, V.A.; Hobbs, R.J.; Standish, R.J. What’s new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol. 2008, 23, 104–112. [Google Scholar] [CrossRef]
- Benayas, J.R.; Martins, A.; Nicolau, J.M.; Schulz, J.J. Abandonment of agricultural land: An overview of drivers and consequences. CAB Rev. 2007, 2, 1–14. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of biodiversity and ecosystem services by ecological restoration: A meta-analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Armstrong, A.; Burney, J.; Ryan, G.; Moore-O’Leary, K.; Diédhiou, I.; Grodsky, S.M.; Saul-Gershenz, L.; Davis, R.; Macknick, J. Techno–ecological synergies of solar energy for global sustainability. Nat. Sustain. 2019, 2, 560–568. [Google Scholar] [CrossRef]
- Yang, Y.; Hobbie, S.E.; Hernandez, R.R.; Fargione, J.; Grodsky, S.M.; Tilman, D.; Zhu, Y.-G.; Luo, Y.; Smith, T.M.; Jungers, J.M. Restoring abandoned farmland to mitigate climate change on a full earth. One Earth 2020, 3, 176–186. [Google Scholar] [CrossRef]
- Isbell, F.; Tilman, D.; Reich, P.B.; Clark, A.T. Deficits of biodiversity and productivity linger a century after agricultural abandonment. Nat. Ecol. Evol. 2019, 3, 1533–1538. [Google Scholar] [CrossRef]
- Klopf, R.P.; Baer, S.G.; Bach, E.M.; Six, J. Restoration and management for plant diversity enhances the rate of belowground ecosystem recovery. Ecol. Appl. 2017, 27, 355–362. [Google Scholar] [CrossRef]
- Porensky, L.M.; Perryman, B.L.; Williamson, M.A.; Madsen, M.D.; Leger, E.A. Combining active restoration and targeted grazing to establish native plants and reduce fuel loads in invaded ecosystems. Ecol. Evol. 2018, 8, 12533–12546. [Google Scholar] [CrossRef]
- El-Sheikh, M.A. Plant succession on abandoned fields after 25 years of shifting cultivation in Assuit, Egypt. J. Arid Environ. 2005, 61, 461–481. [Google Scholar] [CrossRef]
- Ng, K.; Barton, P.S.; Blanchard, W.; Evans, M.J.; Lindenmayer, D.B.; Macfadyen, S.; McIntyre, S.; Driscoll, D.A. Disentangling the effects of farmland use, habitat edges, and vegetation structure on ground beetle morphological traits. Oecologia 2018, 188, 645–657. [Google Scholar] [CrossRef]
- Stoate, C.; Báldi, A.; Beja, P.; Boatman, N.; Herzon, I.; Van Doorn, A.; De Snoo, G.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe—A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef]
- Meyer, S.T.; Heuss, L.; Feldhaar, H.; Weisser, W.W.; Gossner, M.M. Land-use components, abundance of predatory arthropods, and vegetation height affect predation rates in grasslands. Agric. Ecosyst. Environ. 2019, 270, 84–92. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Liu, S.; Zhang, R.; Qi, W.; Zhang, R.; Knops, J.M.; Lu, J. Magnitude of species diversity effect on aboveground plant biomass increases through successional time of abandoned farmlands on the eastern Tibetan Plateau of China. Land Degrad. Dev. 2017, 28, 370–378. [Google Scholar] [CrossRef]
- Shiferaw, W.; Demissew, S.; Bekele, T. Ecology of soil seed banks: Implications for conservation and restoration of natural vegetation: A review. Int. J. Biodiv. Conserv. 2018, 10, 380–393. [Google Scholar]
- Gustavsson, E.; Lennartsson, T.; Emanuelsson, M. Land use more than 200 years ago explains current grassland plant diversity in a Swedish agricultural landscape. Biol. Conserv. 2007, 138, 47–59. [Google Scholar] [CrossRef]
- Römermann, C.; Bernhardt-Römermann, M.; Kleyer, M.; Poschlod, P. Substitutes for grazing in semi-natural grasslands—Do mowing or mulching represent valuable alternatives to maintain vegetation structure? J. Veg. Sci. 2009, 20, 1086–1098. [Google Scholar] [CrossRef]
- Stadler, J.; Trefflich, A.; Brandl, R.; Klotz, S. Spontaneous regeneration of dry grasslands on set-aside fields. Biodiv. Conserv. 2007, 16, 621–630. [Google Scholar] [CrossRef]
- Modaihsh, A.S.; Mahjoub, M.O.; Sallam, A.S.; Ghoneim, A.M. Evaluation of soil degradation in Al-kharj Centre, Saudi Arabia using remote sensing. Int. J. Remote Sens. Geosci. 2015, 4, 1–7. [Google Scholar]
- Chaudhary, S.A.; Le Houérou, H.N. The rangelands of the Arabian Peninsula. Sci. Chang. Planétaires Sécher. 2006, 17, 179–194. [Google Scholar]
- Vincent, P. Saudi Arabia: An Environmental Overview; Taylor & Francis: London, UK, 2008. [Google Scholar]
- Kent, M. Vegetation Description and Data Analysis: A Practical Approach; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Collenette, S. Wildflowers of Saudi Arabia; National Commission for Wildlife Conservation and Development (NCWCD): Riyadh, Saudi Arabia, 1999. [Google Scholar]
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia; Ministry of Agriculture and Water: Riyadh, Saudi Arabia, 1999–2011. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Interscience Publishers, Inc.: New York, NY, USA, 1947. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Carbonate and gypsum. In Methods of Soil Analysis: Chemical Methods; Bigham, J.M., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 437–474. [Google Scholar]
- Allen, S.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell: London, UK, 1989. [Google Scholar]
- Hill, M.O. DECORANA—A FORTRAN Program from Detrended Correspondence Analysis and Reciprocal Averaging; Cornell University: Ithaca, NY, USA, 1979. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. In Multivariate Analysis of Ecological Data; Version 6; MjM Software: Gleneden Beach, OR, USA, 2010. [Google Scholar]
- Crisp, M.D.; Lange, T.T. Age structure, distribution and survival under grazing of the arid zone shrub Acacia burkii. Oikos 1976, 27, 86–92. [Google Scholar] [CrossRef]
- El-Bana, M.I. Floristic composition of a threatened Mediterranean sabkhat of Sinai. In Sabkha Ecosystems; Khan, M.A., Böer, B., Kust, G.S., Barth, H., Eds.; Springer: Dordrecht, Germany, 2006; pp. 155–162. [Google Scholar]
- Allbed, A.; Kumar, L.; Sinha, P. Soil salinity and vegetation cover change detection from multi-temporal remotely sensed imagery in Al Hassa Oasis in Saudi Arabia. Geocarto Int. 2018, 33, 830–846. [Google Scholar] [CrossRef]
- Castellanos, A.; Martinez, M.; Llano, J.; Halvorson, W.; Espiricueta, M.; Espejel, I. Successional trends in Sonoran Desert abandoned agricultural fields in northern Mexico. J. Arid Environ. 2005, 60, 437–455. [Google Scholar] [CrossRef]
- Bonet, A. Secondary succession of semi-arid Mediterranean old-fields in south-eastern Spain: Insights for conservation and restoration of degraded lands. J. Arid Environ. 2004, 56, 213–233. [Google Scholar] [CrossRef]
- Alyemeni, N. Ecological studies on sand dunes vegetation in AI-Kharj region, Saudi Arabia. Saudi J. Biol. Sci. 2000, 7, 64–87. [Google Scholar]
- Hayati, A.A.; Al-shammary, F.H. Abiotic soil factors affecting plant distribution at Jubail Wildlife Sanctuary in the Eastern Region of Saudi Arabia. Sacha J. Environ. Stud. 2011, 1, 101–115. [Google Scholar]
- Beauchamp, V.B.; Shafroth, P.B. Floristic composition, beta diversity, and nestedness of reference sites for restoration of xeroriparian areas. Ecol. Appl. 2011, 21, 465–476. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Zhang, B.; Thomas, B.W.; Beck, R.; Willms, W.D.; Li, Y.; Hao, X. Short term recovery of vegetation and soil after abandoning cultivated mixedgrass prairies in Alberta, Canada. Catena 2019, 173, 321–329. [Google Scholar] [CrossRef]
- Knops, J.M.; Tilman, D. Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology 2000, 81, 88–98. [Google Scholar] [CrossRef]
- Zeller, V.; Bahn, M.; Aichner, M.; Tappeiner, U. Impact of land-use change on nitrogen mineralization in subalpine grasslands in the Southern Alps. Biol. Fertil. Soils 2000, 31, 441–448. [Google Scholar] [CrossRef]
- Dunjó, G.; Pardini, G.; Gispert, M. Land use change effects on abandoned terraced soils in a Mediterranean catchment, NE Spain. Catena 2003, 52, 23–37. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Tan, S.; Wang, Z.; Zhang, Q. Vegetation community and soil characteristics of abandoned agricultural land and pine plantation in the Qinling Mountains, China. For. Ecol. Manag. 2010, 259, 2036–2047. [Google Scholar] [CrossRef]
- McLauchlan, K.K. Effects of soil texture on soil carbon and nitrogen dynamics after cessation of agriculture. Geoderma 2006, 136, 289–299. [Google Scholar] [CrossRef]
- Du, F.; Shao, H.-B.; Shan, L.; Liang, Z.-S.; Shao, M.-A. Secondary succession and its effects on soil moisture and nutrition in abandoned old-fields of hilly region of Loess Plateau, China. Colloids Surf. B Biointerfaces 2007, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.W.; Pokorny, M.L.; Sheley, R.L.; James, J.J. Influence of plant functional group removal on inorganic soil nitrogen concentrations in native grasslands. Rangel. Ecol. Manag. 2007, 60, 304–310. [Google Scholar] [CrossRef]
- Carrera, A.L.; Mazzarino, M.J.; Bertiller, M.B.; del Valle, H.F.; Carretero, E.M. Plant impacts on nitrogen and carbon cycling in the Monte Phytogeographical Province, Argentina. J. Arid Environ. 2009, 73, 192–201. [Google Scholar] [CrossRef]
- Lesschen, J.; Cammeraat, L.; Kooijman, A.; van Wesemael, B. Development of spatial heterogeneity in vegetation and soil properties after land abandonment in a semi-arid ecosystem. J. Arid Environ. 2008, 72, 2082–2092. [Google Scholar] [CrossRef]
- Al-Jaloud, A.A.; Hussain, G. Sabkha ecosystem and halophyte plant communities in Saudi Arabia. In Sabkha Ecosystems; Khan, M., Böer, B., Kust, G., Barth, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–7. [Google Scholar]
- El-Bana, M.; Shaltout, K.; Khalafallah, A.; Mosallam, H. Ecological status of the Mediterranean Juniperus phoenicea L. relicts in the desert mountains of North Sinai, Egypt. Flora 2010, 205, 171–178. [Google Scholar] [CrossRef]
- Shaltout, K.; Sheded, M.; El-Kady, H.; Al-Sodany, Y. Phytosociology and size structure of Nitraria retusa along the Egyptian Red Sea coast. J. Arid Environ. 2003, 53, 331–345. [Google Scholar] [CrossRef]

| Plant Species | Family | Density (Indiv./25 m2) | Cover (%) |
|---|---|---|---|
| Alhagi graecorum | Papilionaceae | 2.22 ± 0.86 | 4.44 ± 2.18 |
| Calligonum comosum | Polygonaceae | 11.64 ± 5.64 | 28.53 ± 18.5 |
| Convolvulus cephalopods | Convolvulaceae | 1.14 ± 0.73 | 1.53 ± 0.53 |
| Ephedra alata | Ephederaceae | 1.58 ± 0.64 | 2.12 ± 0.65 |
| Halothamnus lancifolius | Chenopodiaceae | 4.54 ± 1.73 | 11.45 ± 3.21 |
| Leptadenia pyrotechnica | Asclepiadaceae | 2.73 ± 1.04 | 4.21 ± 1.34 |
| Prosopis farcta | Mimosaceae | 28.34 ± 10.23 | 35.84 ± 12.3 |
| Pulicaria undulata | Asteraceae | 7.65 ± 2.31 | 9.68 ± 5.72 |
| Seidlitzia rosmarinus | Chenopodiaceae | 9.54 ± 3.43 | 55.38 ± 23.2 |
| Traganum nudatum | Chenopodiaceae | 12.73 ± 7.54 | 48.75 ± 13.3 |
| Zygophyllum hamiense | Zygophyllaceae | 18.57 ± 6.78 | 40.52 ± 9.87 |
| Soil Factors | Plant Communities | F-Value | |||
|---|---|---|---|---|---|
| VG-I | VG-II | VG-III | VG-IV | ||
| Sand (%) | 80.12 ± 1.41 | 85.12 ± 8.49 | 89.12 ± 2.83 | 90.12 ± 4.24 | 12.6 ** |
| Silt (%) | 9.00 ± 1.41 | 6.00 ± 5.66 | 3.00 ± 1.41 | 4.05 ± 2.76 | 5.78 ** |
| Clay (%) | 10.64 ± 0.34 | 8.55 ± 2.62 | 7.75 ± 1.48 | 5.89 ± 1.40 | 3.43 * |
| CaCO3 (%) | 17.83 ± 1.41 | 15.67 ± 3.18 | 16.50 ± 2.36 | 14.24 ± 4.53 | 2.52 * |
| Organic matter (%) | 0.45 ± 0.11 | 0.34 ± 0.26 | 0.48 ± 0.47 | 0.16 ± 0.02 | 13.21 * |
| pH | 8.64 ± 0.37 | 8.32 ± 0.83 | 7.52 ± 0.30 | 7.85 ± 0.11 | 2.09 * |
| EC (dSm−1) | 6.36 ± 1.27 | 2.91 ± 1.61 | 2.52 ± 1.06 | 2.45 ± 0.40 | 13.23 ** |
| Nitrogen (mg/kg) | 4.20 ± 1.19 | 6.44 ± 1.98 | 11.20 ± 4.75 | 9.61 ± 0.13 | 6.32 ** |
| Phosphorus (mg/kg) | 10.80 ± 0.71 | 9.14 ± 3.06 | 6.90 ± 0.11 | 7.24 ± 0.62 | 9.18 * |
| Cations (meq/L) | |||||
| Ca2+ | 15.15 ± 2.99 | 9.25 ± 0.35 | 6.50 ± 3.54 | 16.80 ± 3.36 | 18.12 ** |
| Mg2+ | 7.28 ± 4.99 | 3.63 ± 0.18 | 3.65 ± 0.21 | 1.84 ± 0.23 | 12.34 ** |
| Na+ | 46.40 ± 2.42 | 18.04 ± 8.69 | 4.20 ± 0.88 | 4.45 ± 2.71 | 16.54 ** |
| K+ | 5.16 ± 3.94 | 1.96 ± 0.59 | 1.94 ± 0.56 | 1.53 ± 0.07 | 9.76 * |
| Anions(meq/L) | |||||
| HCO3− | 12.50 ± 3.89 | 9.73 ± 7.81 | 5.03 ± 1.17 | 3.60 ± 0.21 | 7.54 ** |
| Cl− | 9.88 ± 3.54 | 3.90 ± 1.91 | 3.40 ± 1.20 | 2.88 ± 0.88 | 10.21 ** |
| SO42− | 37.80 ± 3.83 | 13.88 ± 3.01 | 17.90 ± 3.60 | 17.13 ± 4.24 | 14.31 ** |
| Variable | Density | Cover | Richness |
|---|---|---|---|
| Cover | −0.626 | 0.00 | −0.394 |
| Density | 0.00 | −0.626 | 0.534 |
| Sand | 0.482 | −0.322 | 0.473 |
| Silt | −0.243 | 0.342 | −0.032 |
| Clay | −0.268 | 0.253 | −0.432 |
| CaCO3 | −0.465 | −0.238 | −0.282 |
| Organic matter | 0.534 | −0.321 | 0.473 |
| pH | −0.485 | 0.427 | −0.315 |
| EC | −0.587 | 0.461 | −0.482 |
| Nitrogen | 0.163 | 0.253 | 0.334 |
| Phosphorus | 0.134 | −0.282 | 0.134 |
| Ca2+ | −0.532 | 0.474 | −0.458 |
| Mg2+ | −0.452 | 0.473 | −0.323 |
| Na+ | −0.571 | 0.562 | −0.532 |
| K+ | −0.385 | 0.421 | −0.243 |
| HCO3− | −0.351 | 0.434 | −0.254 |
| Cl− | −0.647 | 0.546 | −0.446 |
| SO42− | −0.355 | 0.454 | −0.312 |
| Species | Diameter (cm) | Height (cm) | Height/Diameter | Size Index (cm) | Volume (m3) | |
|---|---|---|---|---|---|---|
| Seidlitzia rosmarinus | Mean | 181.73 | 76.13 | 0.46 | 128.93 | 2.79 |
| Min. | 53.50 | 38.00 | 0.23 | 51.25 | 0.12 | |
| Max. | 355.50 | 144.00 | 1.07 | 245.25 | 13.41 | |
| ±SD | 75.18 | 25.67 | 0.16 | 48.33 | 3.16 | |
| Zygophyllum hamiense | Mean | 95.62 | 43.11 | 0.49 | 69.37 | 0.48 |
| Min. | 21.50 | 18.00 | 0.26 | 19.75 | 0.01 | |
| Max. | 251.00 | 85.00 | 0.90 | 159.00 | 3.32 | |
| ±SD | 45.53 | 15.81 | 0.15 | 29.29 | 0.66 | |
| Traganum nudatum | Mean | 140.93 | 83.13 | 0.63 | 112.03 | 1.66 |
| Min. | 60.00 | 48.00 | 0.33 | 58.50 | 0.16 | |
| Max. | 295.00 | 149.00 | 1.11 | 205.00 | 7.86 | |
| ±SD | 51.32 | 25.14 | 0.19 | 34.87 | 1.66 | |
| Prosopis farcta | Mean | 58.98 | 29.48 | 0.53 | 44.23 | 0.09 |
| Min. | 26.50 | 14.00 | 0.24 | 22.25 | 0.01 | |
| Max. | 96.00 | 45.00 | 0.90 | 64.50 | 0.24 | |
| ±SD | 16.53 | 7.75 | 0.16 | 10.20 | 0.05 | |
| Calligonum comosum | Mean | 145.91 | 106.88 | 0.82 | 126.40 | 4.06 |
| Min. | 66.50 | 68.00 | 0.10 | 76.00 | 1.04 | |
| Max. | 575.00 | 225.00 | 1.33 | 395.00 | 55.85 | |
| ±SD | 102.00 | 41.54 | 0.23 | 69.16 | 10.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rowaily, S.L.; Al-Dosari, D.H.; Assaeed, A.M.; Abd-ElGawad, A.M.; El-Sheikh, M.A.; El-Bana, M.I.; Al-Taisan, W.A. Native Perennial Plants Colonizing Abandoned Arable Fields in a Desert Area: Population Structure and Community Assembly. Agriculture 2020, 10, 550. https://doi.org/10.3390/agriculture10110550
Al-Rowaily SL, Al-Dosari DH, Assaeed AM, Abd-ElGawad AM, El-Sheikh MA, El-Bana MI, Al-Taisan WA. Native Perennial Plants Colonizing Abandoned Arable Fields in a Desert Area: Population Structure and Community Assembly. Agriculture. 2020; 10(11):550. https://doi.org/10.3390/agriculture10110550
Chicago/Turabian StyleAl-Rowaily, Saud L., Dekhil H. Al-Dosari, Abdulaziz M. Assaeed, Ahmed M. Abd-ElGawad, Mohamed A. El-Sheikh, Magdy I. El-Bana, and Wafa’a A. Al-Taisan. 2020. "Native Perennial Plants Colonizing Abandoned Arable Fields in a Desert Area: Population Structure and Community Assembly" Agriculture 10, no. 11: 550. https://doi.org/10.3390/agriculture10110550
APA StyleAl-Rowaily, S. L., Al-Dosari, D. H., Assaeed, A. M., Abd-ElGawad, A. M., El-Sheikh, M. A., El-Bana, M. I., & Al-Taisan, W. A. (2020). Native Perennial Plants Colonizing Abandoned Arable Fields in a Desert Area: Population Structure and Community Assembly. Agriculture, 10(11), 550. https://doi.org/10.3390/agriculture10110550







