Genotype and Sowing Time Effects on Soybean Yield and Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Details of the Field Trial
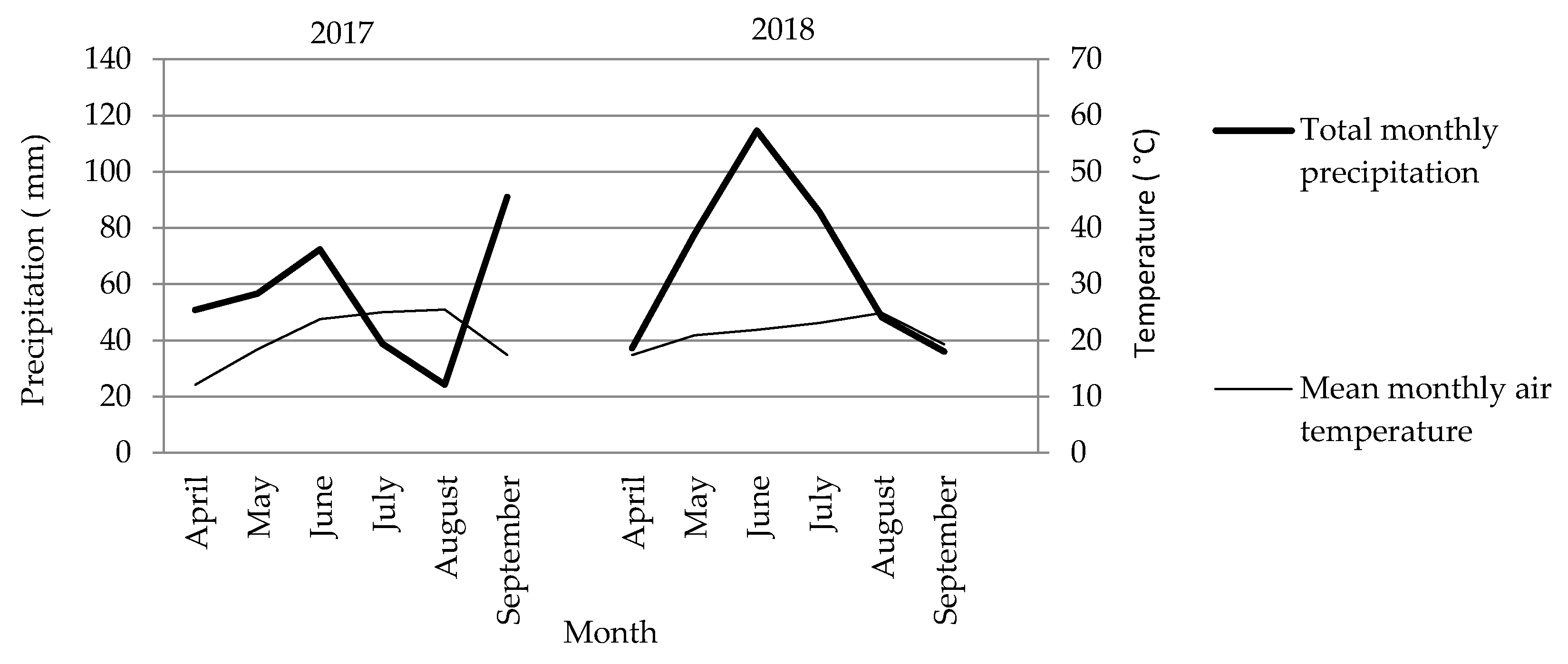
2.2. Soil Properties and Meteorological Conditions
2.3. Data Collection
2.4. Statistical Data Analysis
3. Results
3.1. Analyses of Variance for Effects of Year, Genotype, and Sowing Time, and their Interactions on Quantitative and Quality Soybean Traits
3.2. Interaction of Factors Affected Quantitative and Quality Traits
3.3. Pearson’s Correlation Coefficient (r) among Studied Traits
4. Discussion
4.1. Year and Genotype Affected Quantitative and Quality Traits
4.2. Sowing Time Affected Quantitative and Quality Traits
4.3. Interaction of Genotype and Sowing Time and Correlation between the Studied Traits
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sudarić, A.; Kočar, M.M.; Duvnjak, T.; Zdunić, Z.; Kulundžić, A.M. Improving seed quality of soybean suitable for growing in Europe. In Soybean for Human Consumption and Animal Feed; 2019; Available online: https://www.intechopen.com/online-first/improving-seed-quality-of-soybean-suitable-for-growing-in-europe (accessed on 15 July 2020).
- Ngalamu, T.; Ashraf, M.; Meseka, S. Soybean (Glycine max L.) genotype and environment interaction effect on yield and other related traits. Am. J. Exp. Agric. 2013, 3, 977–987. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). 2020. Available online: http://www.fao.org/faostat/en/#data (accessed on 15 July 2020).
- Mandić, V.; Bijelić, Z.; Krnjaja, V.; Simić, A.; Ružić-Muslić, D.; Dragičević, V.; Petričević, V. The rainfall use efficiency and soybean grain yield under rainfed conditions in Vojvodina. Biotechnol. Anim. Husb. 2017, 33, 475–486. [Google Scholar] [CrossRef]
- Vidić, M.; Hrustić, M.; Miladinović, J.; Đukić, V.; Đorđević, V.; Popović, V. Latest NS varieties of soybean. Field Veg. Crop. Res. 2010, 47, 347–355. [Google Scholar]
- Marcos Filho, J. Produção de Sementes de Soja; Fundação Cargill: Campinas, Brazil, 1986; p. 86. [Google Scholar]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2018, 24, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Egli, D.B.; Cornelius, P.L. A regional analysis of the response of soybean yield to planting date. Agron. J. 2009, 101, 330–335. [Google Scholar] [CrossRef]
- Purcell, L.C.; Salmeron, M.; Ashlock, L. Growth and Development; Chapter 2; Arkansas soybean handbook; Ark. Coop. Ext. Ser.: Little Rock, AR, USA, 2013; pp. 1–8. [Google Scholar]
- Kumagai, E.; Takahashi, T. Soybean (Glycine max (L.) Merr.) Yield reduction due to late sowing as a function of radiation interception and use in a cool region of Northern Japan. Agronomy 2020, 10, 66. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Yamazaki, R.; Katayama, K. Effects of late sowing on soybean yields and yield components in southwestern Japan. Plant Prod. Sci. 2018, 21, 339–348. [Google Scholar] [CrossRef]
- Avilla, M.; Braccini, A.; Motta, I.; Scapini, C.; Braccini, L. Sowing season and quality of soybean seeds. Sci. Agricola 2003, 60, 1–13. [Google Scholar]
- Soliman, M.M.; Rabie, E.M.; Ragheb, S.B. Response of soybean yield to late sowing date. J. Agric. Sci. 2007, 15, 51–59. [Google Scholar] [CrossRef]
- EL-Harty, E.H.; Rizk, A.M.A.; Gendy, E.K.; Abd El-Aal, H.T. Performance of soybean genotypes under four sowing dates at middle Egypt. Egypt J. Plant Breed 2010, 14, 283–293. [Google Scholar]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 2015, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Ojo, D.K.; Adebisi, M.A.; Tijani, B.O. Influence of environment on protein and oil contents of soybeans seed (Glycine Max (L.) Merril). Global J. Agric. Sci. 2002, 1, 27–32. [Google Scholar] [CrossRef]
- Walter, H.; Lieth, H. Klimadiagram–Weltatlas; VEB Gustav Fischer Verlag: Jena, Germany, 1967. [Google Scholar]
- Rehman, M.; Khaliq, T.; Ahmad, A.; Wajid, S.A.; Rasul, F.; Hussain, J.; Hussain, S. Effect of planting time and cultivar and soybean performance in semi–arid Punjab, Pakistan. Global J. Sci. Front. Res. Agric. Vet. 2014, 14, 41–45. [Google Scholar]
- Kron, A.P.; Souza, G.M.; Ribeiro, R.V. Water deficiency at different developmental stages of Glycine max can improve drought tolerance. Bragantia 2008, 67, 43–49. [Google Scholar] [CrossRef]
- Mandić, V.; Đorđević, S.; Bijelić, Z.; Krnjaja, V.; Pantelić, V.; Simić, A.; Dragičević, V. Agronomic responses of soybean genotypes to starter nitrogen fertilizer rate. Agronomy 2020, 10, 535. [Google Scholar] [CrossRef]
- De Castro, J.N.; Müller, C.; Almeida, G.M.; Costa, A.C. Physiological tolerance to drought under high temperature in soybean cultivars. Aust. J. Crop Sci. 2019, 13, 976–987. [Google Scholar] [CrossRef]
- Zaytsev, N.I.; Bochkaryov, N.I.; Zelentsov, S.V. Prospects and directions for soybean breeding in Russia under implementation conditions of the national strategy of import substitution. Oil Crop. Sci. Tech. Bull. 2016, 2, 3–11. [Google Scholar]
- Arnold, B.H. Planting date, rate, and twin–row vs. single–row soybean. Midsouth. Agron. J. 2011, 103, 1313. [Google Scholar]
- Morsy, A.R. Evaluation of performance in top–cross of soybean yield through different planting dates. Ann. Agric. Sci. Moshtohor 2010, 48, 1–21. [Google Scholar]
- Bastidas, A.M.; Setiyono, T.D.; Dobermann, A.; Cassman, K.G.; Elmore, R.W.; Graef, G.L.; Specht, J.E. Soybean sowing date: The vegetative, reproductive, and agronomic impacts. Crop Sci. 2008, 48, 727–740. [Google Scholar] [CrossRef]
- Kandil, A.A.; Sharief, A.E.; Morsy, A.R.; El–Sayed, A.I.M. Influence of planting date on some genotypes of soybean growth, yield and seed quality. J. Biol. Sci. 2012, 13, 146–151. [Google Scholar]
- Kang, B.K.; Kim, H.T.; Choi, M.S.; Koo, S.C.; Seo, J.H.; Kim, H.S.; Shin, S.O.; Yun, H.T.; Oh, I.S.; Kulkarni, K.P.; et al. Genetic and environmental variation of first pod height in soybean [Glycine max (L.) Merr.]. Plant Breed. Biotechnol. 2017, 5, 36–44. [Google Scholar] [CrossRef]
- Lima, M.D.D.; Peluzio, J.M.; Mandarino, J.M.G.; Lima, D. Potassium fertilization and sowing seasons on protein yield in soybean cultivars. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 392–397. [Google Scholar] [CrossRef]
- Pípolo, A.E.; Hungria, M.; Franchini, J.C.; Balbinot, A.A., Jr.; Debiasi, H.; Mandarino, J.M.G. Teores de Óleo e Proteínaem Soja: Fatores Envolvidos e Qualidade Para a Indústria; Embrapa Soybean: Londrina, Brazil, 2015; pp. 1–15. [Google Scholar]
- Benzain, B.; Lane, P.W. Protein concentration of grains in relation to some weather and soil factors during 17 years of English winter wheat experiments. J. Sci. Food Agric. 1986, 37, 435–444. [Google Scholar] [CrossRef]
- Setiyono, T.D.; Weiss, A.; Specht, J.; Bastidas, A.M.; Cassman, K.G.; Dobermann, A. Understanding and modeling the effect of temperature and day length on soybean phenology under high-yield conditions. Field Crop. Res. 2006, 100, 257–271. [Google Scholar] [CrossRef]
- Hu, M.; Wiatrak, P. Effect of planting date on soybean growth yield and grain quality: Review. Agron. J. 2012, 104, 785–790. [Google Scholar] [CrossRef]
- Ohyama, T.; Tewari, K.; Ishikawa, S.; Tanaka, K.; Kamiyama, S.; Ono, Y.; Hatano, S.; Ohtake, N.; Sueyoshi, K.; Hasegawa, H.; et al. Role of nitrogen on growth and seed yield of soybean and a new fertilization technique to promote nitrogen fixation and seed yield, soybean. In Soybean—The Basis of Yield, Biomass and Productivity; Kasai, M., Ed.; IntechOpen Limited: London, UK, 2017; pp. 154–185. [Google Scholar]
- Carvalho, C.G.P.; Arias, C.A.A.; Toledo, J.F.F.; Oliveira, M.F.; Vello, N.A. Correlation and path analyses in soybean lines sowed at different sowing dates. Braz. J. Agric. Res. 2002, 37, 311–320. [Google Scholar]
- Carrera, C.; Martínez, M.J.; Dardanelli, J.; Balzarini, M. Environmental variation and correlation of seed components in non–transgenic soybeans: Protein, oil, unsaturated fatty acids, tocopherols and isoflavones. Crop Sci. 2011, 51, 800–809. [Google Scholar] [CrossRef]
| Factor | PH | FPH | NN | NP | SWP | TSW | PC | OC | SY |
|---|---|---|---|---|---|---|---|---|---|
| p Value | |||||||||
| Year (Y) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.078 | 0.461 | 0.000 |
| Genotype (G) | 0.000 | 0.000 | 0.084 | 0.000 | 0.000 | 0.151 | 0.021 | 0.015 | 0.002 |
| Sowing time (ST) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.822 | 0.000 |
| Y × G | 0.000 | 0.093 | 0.000 | 0.000 | 0.000 | 0.000 | 0.537 | 0.000 | 0.000 |
| Y × ST | 0.816 | 0.036 | 0.221 | 0.031 | 0.000 | 0.034 | 0.011 | 0.238 | 0.000 |
| G × ST | 0.000 | 0.830 | 0.005 | 0.000 | 0.000 | 0.211 | 0.920 | 0.000 | 0.004 |
| Y × G × ST | 0.000 | 0.034 | 0.065 | 0.281 | 0.003 | 0.047 | 0.000 | 0.000 | 0.000 |
| Factor | PH | FPH | NN | NP | SWP | TSW | PC | OC | SY | |
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype (G) | Galina | 87.6 c | 10.9 c | 13.7 a | 34.0 a | 10.1 b | 135.3 ab | 40.8 c | 16.7 a | 1658.1 ab |
| Sava | 108.5 a | 12.6 a | 13.9 a | 35.0 a | 11.9 a | 141.7 a | 41.6 a | 15.0 c | 1726.8 a | |
| Rubin | 97.9 b | 12.4 b | 12.8 b | 30.2 b | 9.2 c | 130.7 b | 41.2 b | 15.8 b | 1576.2 b | |
| F test | G | ** | ** | ** | ** | ** | * | ** | ** | ** |
| Sowing time (ST) | Standard | 105.7 a | 13.0 a | 14.9 a | 34.9 a | 11.1 a | 146.3 a | 41.8 a | 15.9 a | 1819.3 a |
| Late | 90.2 b | 11.0 b | 12.0 b | 31.2 b | 9.7 b | 125.5 b | 40.6 b | 15.7 b | 1488.1 b | |
| F test | ST | ** | ** | ** | ** | ** | ** | ** | * | ** |
| G × ST | ** | ** | * | * | * | * | ** | ** | ** | |
| Mean | 98.0 | 12.0 | 13.4 | 33.0 | 10.4 | 135.9 | 41.2 | 15.8 | 1653.7 | |
| Factor | PH | FPH | NN | NP | SWP | TSW | PC | OC | SY | |
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype (G) | Galina | 107.3 c | 12.5 | 15.7 a | 33.3 c | 11.9 a | 145.3 a | 40.7 | 15.5 | 3790.6 b |
| Sava | 117.9 b | 13.3 | 15.1 b | 41.0 b | 11.1 b | 136.6 b | 41.1 | 16.2 | 3700.0 c | |
| Rubin | 128.5 a | 13.4 | 16.0 a | 46.1 a | 12.0 a | 142.3 a | 41.0 | 16.0 | 3971.0 a | |
| F test | G | ** | ns | ** | ** | ** | ** | ns | ns | ** |
| Sowing time (ST) | Standard | 125.7 a | 13.7 a | 16.9 a | 43.3 a | 13.2 a | 148.3 a | 41.1 | 15.9 | 4220.8 a |
| Late | 110.0 b | 12.4 b | 14.3 b | 36.9 b | 10.1 b | 134.5 b | 40.8 | 16.0 | 3420.3 b | |
| F test | ST | ** | ** | ** | ** | ** | ** | ns | ns | ** |
| G × ST | ** | ns | * | ** | ** | ** | ns | * | ** | |
| Mean | 117.9 | 13.0 | 15.6 | 40.1 | 11.7 | 141.4 | 40.9 | 15.9 | 3820.5 | |
| PH | FPH | NN | NP | SWP | TSW | PC | OC | |
|---|---|---|---|---|---|---|---|---|
| FPH | 0.79 ** | |||||||
| NN | 0.79 ** | 0.67 ** | ||||||
| NP | 0.83 ** | 0.53 ** | 0.67 ** | |||||
| SWP | 0.72 ** | 0.53 ** | 0.79 ** | 0.65 ** | ||||
| TSW | 0.60 ** | 0.59 ** | 0.78 ** | 0.47 ** | 0.79 ** | |||
| PC | 0.26 | 0.30 * | 0.26 | 0.07 | 0.16 | 0.33 * | ||
| OC | −0.03 | −0.10 | 0.06 | 0.20 | −0.12 | 0.01 | −0.22 | |
| SY | 0.77 ** | 0.53 ** | 0.77 ** | 0.66 ** | 0.55 ** | 0.41 ** | −0.05 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandić, V.; Đorđević, S.; Đorđević, N.; Bijelić, Z.; Krnjaja, V.; Petričević, M.; Brankov, M. Genotype and Sowing Time Effects on Soybean Yield and Quality. Agriculture 2020, 10, 502. https://doi.org/10.3390/agriculture10110502
Mandić V, Đorđević S, Đorđević N, Bijelić Z, Krnjaja V, Petričević M, Brankov M. Genotype and Sowing Time Effects on Soybean Yield and Quality. Agriculture. 2020; 10(11):502. https://doi.org/10.3390/agriculture10110502
Chicago/Turabian StyleMandić, Violeta, Snežana Đorđević, Nikola Đorđević, Zorica Bijelić, Vesna Krnjaja, Maja Petričević, and Milan Brankov. 2020. "Genotype and Sowing Time Effects on Soybean Yield and Quality" Agriculture 10, no. 11: 502. https://doi.org/10.3390/agriculture10110502
APA StyleMandić, V., Đorđević, S., Đorđević, N., Bijelić, Z., Krnjaja, V., Petričević, M., & Brankov, M. (2020). Genotype and Sowing Time Effects on Soybean Yield and Quality. Agriculture, 10(11), 502. https://doi.org/10.3390/agriculture10110502





