Restless Legs Syndrome During Pregnancy and 12 Weeks Postpartum and Its Links to Cardiovascular Diseases, Stressful Life Events, and Psychiatric History
Abstract
1. Introduction
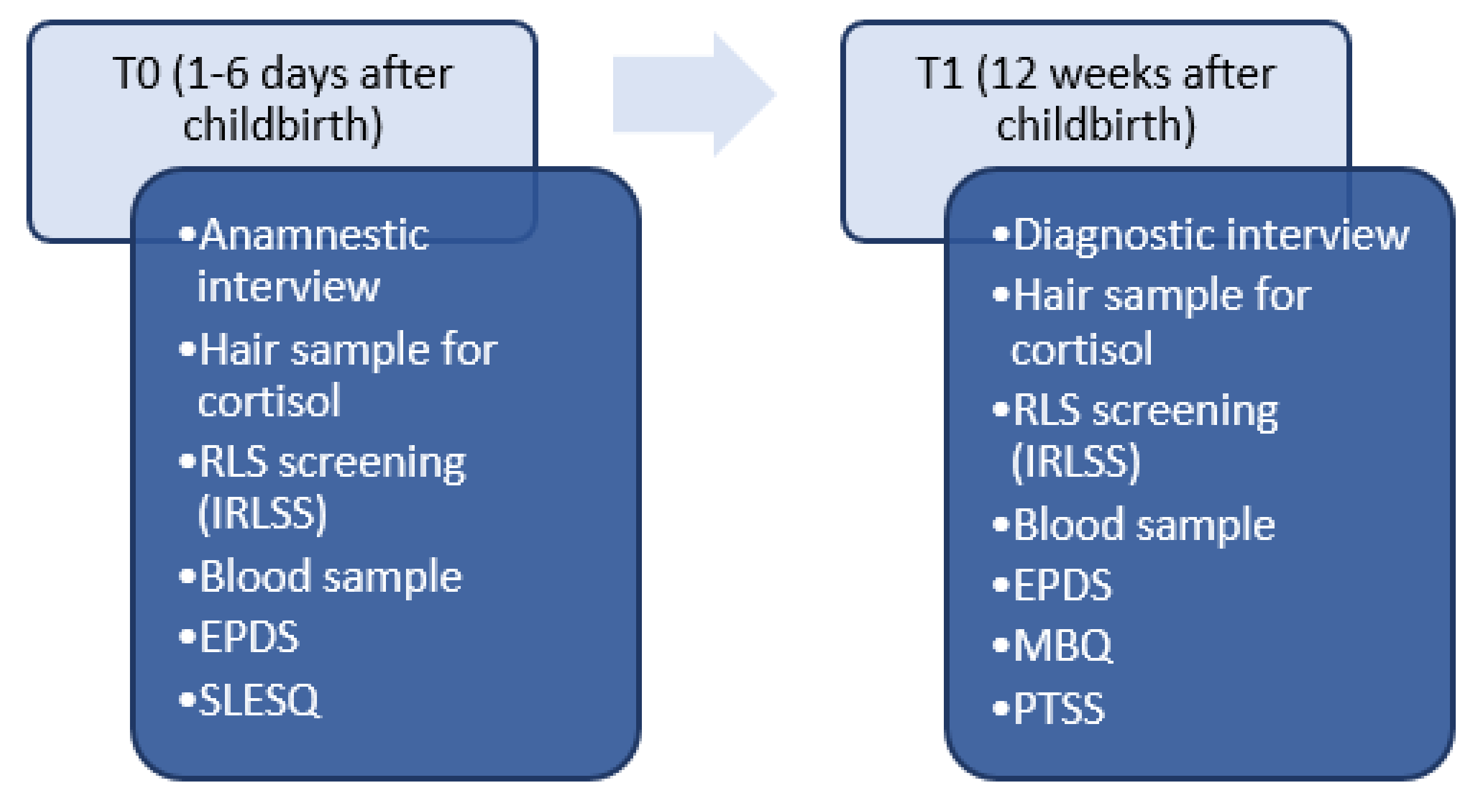
2. Methods
2.1. Hair Sample Collection and Preparation
2.2. Analysis
3. Results
3.1. Course of RLS in Pregnancy in the Sample
3.2. Differences between the RLS and non-RLS Groups
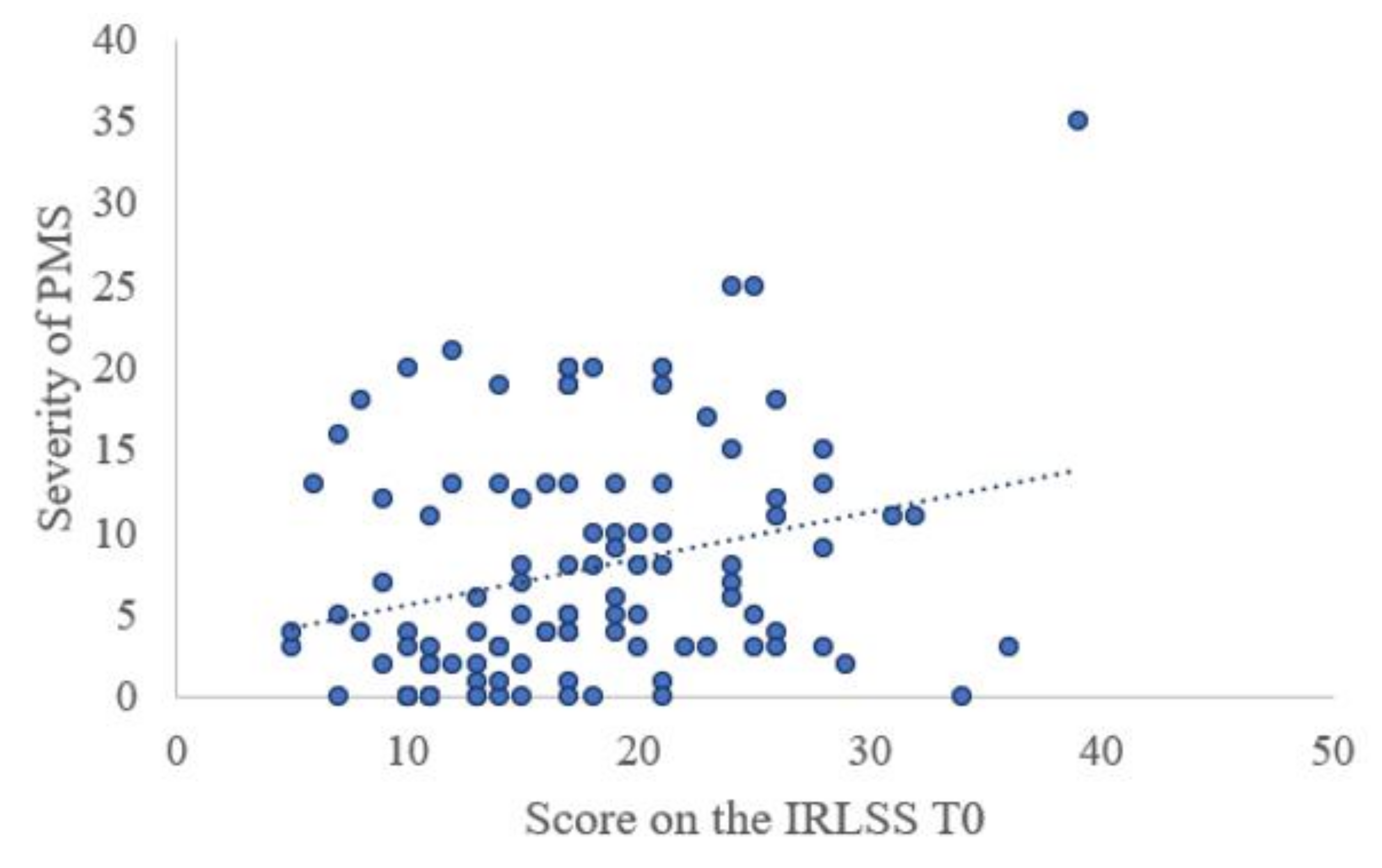
3.3. Differences between Pregnancy-Related RLS and Persistent RLS
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Variable | n (%) | M (SD) | Measured Study Range |
|---|---|---|---|
| Age | 561 (100) | 32.13 (4.79) | 18–45 |
| Married | 413 (73.9) | ||
| Unmarried | 146 (26.1) | ||
| Single Parent | 16 (2.9) | ||
| Number of Children | 1–5 | ||
| 1 | 296 (53.0) | ||
| 2 | 187 (33.5) | ||
| 3 | 59 (10.6) | ||
| 4 | 13 (2.3) | ||
| 5 | 3 (0.5) | ||
| Highest Education | |||
| No school graduation | 4 (0.7) | ||
| Secondary school degree | 23 (4.2) | ||
| Junior high school degree | 79 (14.5) | ||
| University entrance | 202 (37.1) | ||
| College degree | 195 (35.8) | ||
| Doctoral degree | 40 (7.4) |
| Pregnancy-Associated Characteristics | n (%) | M (SD) | Measured Study Range | Scale/Method Range |
|---|---|---|---|---|
| Birth Mode | ||||
| Spontaneous | 307 (55.9) | |||
| Ventouse | 36 (6.6) | |||
| Planned C-section | 134 (24.4) | |||
| Emergency C-section | 72 (13.1) | |||
| Twin birth | 18 (3.2) | |||
| Pregnancy-Related Complications | ||||
| Pre-eclampsia | 11 (2.0) | |||
| Eclampsia | 2 (0.4) | |||
| HELLP (hemolysis, elevated liver enzymes, and a low platelet count) | 1 (0.2) | |||
| Gestational Diabetes Mellitus | 78 (13.9) | |||
| Hypothyroidism | 129 (23.0) | |||
| Hypertension | 25 (4.5) | |||
| Premature birth | 49 (8.7) | |||
| Postpartum anemia | 179 (31.9) | |||
| Birth weight of child | 3284.14 (567.94) | 840–5350 | ||
| Days of gestation | 272.88 (13.43) | 192-294 | ||
| Breastfeeding T0 | 499 (89.0) | |||
| Breastfeeding T1 | 432 (78.0) | |||
| RLS Characteristics | ||||
| Restless Legs Syndrome (RLS) T0 | 119 (21.3) | |||
| Severity of RLS symptoms in pregnancy (IRLSS score T0) | 17.54 (6.98) | 5–39 | 0–40 | |
| Restless Legs Syndrome (RLS) T1 | 23 (4.1) | |||
| Severity of RLS symptoms 12 weeks postpartum (IRLSS score T1) | 16.04 (7.77) | 0–32 | 0–40 | |
| RLS in previous pregnancy | 34 (6.1) | |||
| Psychiatric Characteristics | ||||
| Depression Score T0 (EPDS) | 5.49 (4.19) | 0–22 | 0–30 | |
| Depression Score T1 (EPDS) | 3.93 (4.16) | 0–22 | 0–30 | |
| Stressful Life Events (SLESQ) | 284 (50.7) | |||
| Psychiatric history | 111 (20.1) | |||
| Depression | 83 (15.0) | |||
| Eating disorder | 6 (1.1) | |||
| Anxiety disorder | 14 (2.5) | |||
| Other | 8 (1.4) | |||
| Laboratory Tests | ||||
| HCC T0 (pg/mg) | 9.84 (12.10) | 0.58–91.55 | 2.2–72.2 1 | |
| HCC T1 (pg/mg) | 11.21 (79.58) | 0.33–1468.22 | 2.2–72.2 1 | |
| Hemoglobin (g/dL) T0 | 10.78 (1.48) | 7.7–14.6 | 6.2–15.1 | |
| TSH T0 | 2.98 (1.56) | 32–15.96 | ||
| TSH T1 | 1.44 (2.50) | 01–33.87 |
Appendix B
| Sociodemographic Characteristics | RLS (n = 119) | No RLS (n = 442) | p |
|---|---|---|---|
| M (SD) | M (SD) | ||
| Age | 32.97 (4.75) | 31.91 (4.79) | 0.05 |
| Family Status | n (%) | n (%) | 0.05 |
| Married | 84 (70.6) | 329 (74.8) | |
| Unmarried | 35 (29.4) | 111 (25.2) | |
| Number of Children | 0.05 | ||
| 1 | 58 (48.7) | 238 (54.2) | |
| 2 | 41 (34.5) | 146 (33.3) | |
| 3 | 13 (10.9) | 46 (10.5) | |
| 4 | 6 (5.0) | 7 (1.6) | |
| 5 | 1 (0.8) | 2 (0.5) | |
| Highest Degree of Education | 0.05 | ||
| No school graduation | 1 (0.8) | 3 (0.7) | |
| Secondary school degree | 8 (6.8) | 16 (3.8) | |
| Junior high school degree | 14 (11.9) | 65 (15.3) | |
| University entrance | 39 (33.1) | 163 (38.3) | |
| College degree | 45 (38.1) | 150 (35.2) | |
| Doctoral degree | 11 (9.3) | 29 (6.8) | |
| Pregnancy-Associated Characteristics | |||
| Birth Mode | |||
| Spontaneous | 68 (59.1) | 239 (55.1) | |
| Ventouse | 6 (5.2) | 30 (6.9) | |
| C-section | 25 (21.7) | 109 (25.1) | |
| Emergency C-section | 16 (13.9) | 56 (12.9) | |
| Child Relocation after Birth | 36 (30.3) | 119 (26.9) | 0.05 |
| Pregnancy-Related Complications | |||
| Pre-eclampsia | 1 (0.8) | 10 (2.3) | 0.05 |
| Eclampsia | 0 (0.0) | 2 (0.5) | 0.05 |
| HELLP (hemolysis, elevated liver enzymes, and a low platelet count) | 0 (0.0) | 1 (0.2) | 0.05 |
| Gestational Diabetes Mellitus (GDM) | 23 (19.3) | 55 (12.4) | 0.054 |
| Insulin-dependent GDM | 14 (11.8) | 25 (5.7) | |
| Dietary-set GDM | 9 (7.6) | 28 (6.3) | |
| GDM not specified | 0 (0.0) | 2 (0.5) | |
| Hypothyroidism | 34 (28.6) | 95 (21.5) | 0.05 |
| Hypertension | 9 (7.6) | 16 (3.6) | 0.064 |
| Postpartum anemia | 34 (28.6) | 145 (32.8) | 0.05 |
| M (SD) | M (SD) | ||
| Length of pregnancy in days | 272.46 (11.81) | 272.99 (13.74) | 0.05 |
| Child’s birth weight (in gram) | 3246.87 (534.43) | 3294.17 (576.46) | 0.05 |
| n (%) | n (%) | ||
| Breastfeeding at T0 | 109 (91.6) | 390 (88.2) | |
| Breastfeeding at T1 | 97 (82.9) | 335 (76.7) | |
| RLS Characteristics | |||
| Iron supplements | 72 (60.5) | - | |
| RLS in previous pregnancy | 43 (6.1) | - | |
| Most severe RLS symptoms (n = 110) | |||
| First trimester | 2 (1.8) | - | |
| Second trimester | 8 (7.3) | - | |
| Third trimester | 85 (77.3) | - | |
| Equally high over all trimesters | 15 (13.6) | - | |
| Severity of RLS Symptoms T0 (IRLSS) | |||
| Mild | 19 (16.0) | ||
| Moderate | 63 (52.9) | ||
| Severe | 31 (26.1) | ||
| Very severe | 6 (5.0) | ||
| Psychiatric Characteristics | |||
| Depression score EPDS T0 | 6.27 (4.61) | 5.29 (4.06) | 0.023 |
| Depression score EPDS T1 | 3.85 (3.81) | 3.95 (4.25) | 0.05 |
| Stressful life events (SLESQ) | 76 (63.9) | 208 (47.2) | 0.001 |
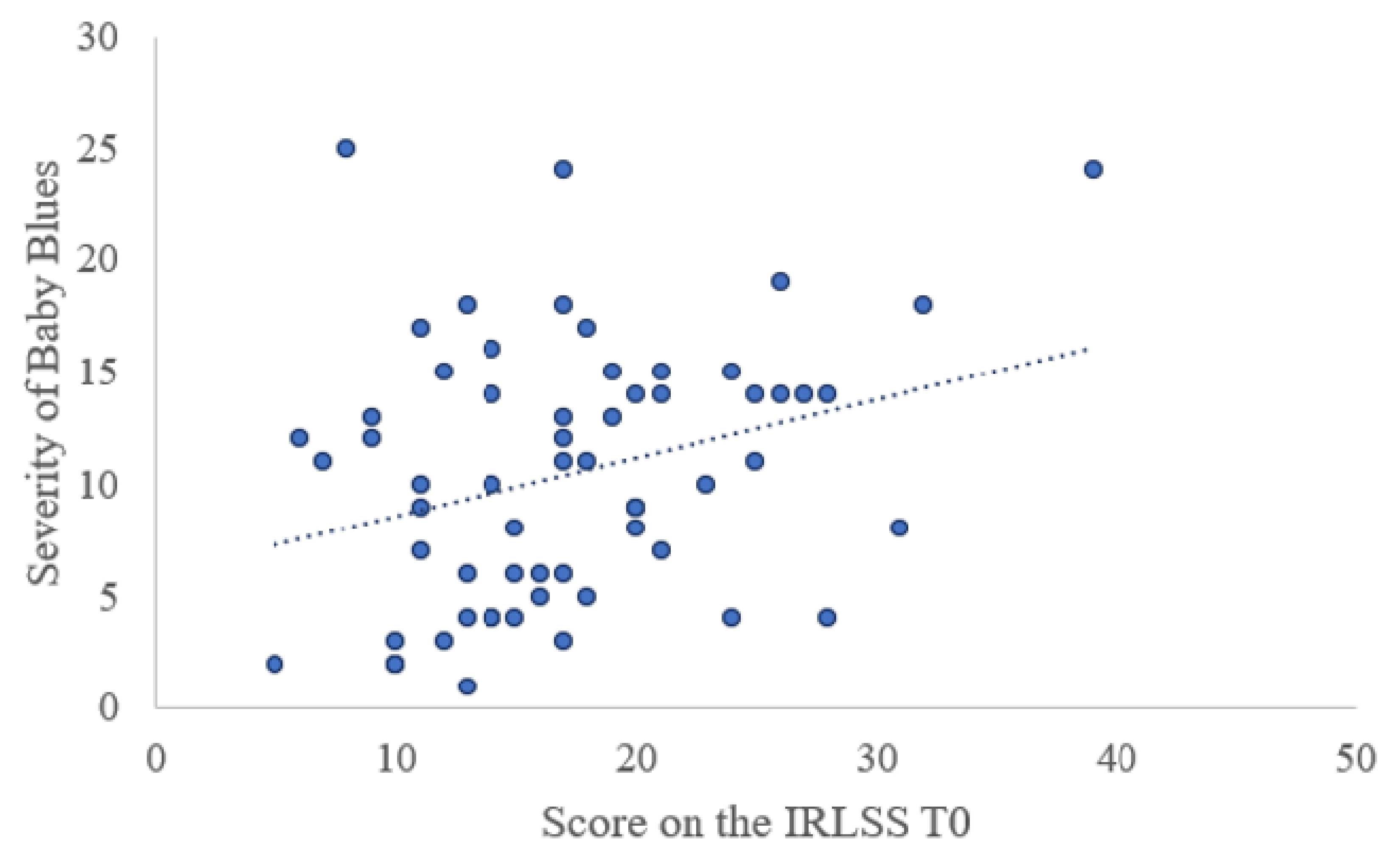
| Baby blues | 64 (53.8) | 191 (43.5) | 0.046 |
| Severity of baby blues | 10.52 (5.84) | 9.29 (5.25) | 0.05 |
| Psychiatric history | 32 (27.1) | 79 (18.2) | 0.032 |
| Depression | 25 (21.2) | 58 (13.4) | |
| Eating disorder | 2 (1.7) | 4 (0.9) | |
| Anxiety disorder | 4 (3.4) | 11 (2.5) | |
| Other | 1 (0.8) | 6 (0.8) | |
| Laboratory Tests | n (%) | n (%) | |
| HCC T0 (pg/mg) | 10.89 (14.14) | 9.56 (11.52) | 0.05 |
| HCC T1 (pg/mg) | 6.03 (5.81) | 12.55 (11.52) | 0.05 |
| Hemoglobin (g/dL) T0 | 12.06 (1.12) | 11.93 (1.18) | 0.05 |
| TSH T0 | 2.88 (2.04) | 3.01 (1.37) | 0.05 |
| TSH T1 | 1.16 (0.69) | 1.54 (2.87) | 0.05 |
Appendix C
| Variable | Pregnancy-Associated RLS (n = 96) | Persistent RLS (n = 23) | p |
|---|---|---|---|
| M (SD) | M (SD) | ||
| Sociodemographic Characteristics | |||
| Age | 33.07 (4.83) | 32.52 (4.46) | 0.05 |
| Family Status | 0.05 | ||
| Married | 66 (68.8) | 18 (78.3) | |
| Unmarried | 30 (31.3) | 5 (21.7) | |
| Number of Children | 0.05 | ||
| 1 | 45 (46.9) | 13 (56.5) | |
| 2 | 34 (35.4) | 7 (30.4) | |
| 3 | 11 (11.5) | 2 (8.7) | |
| 4 | 5 (5.2) | 1 (4.3) | |
| 5 | 1 (1.0) | 0 (0.0) | |
| Highest Degree of Education | 0.05 | ||
| No school graduation | 1 (1.1) | 0 (0.0) | |
| Secondary school degree | 8 (8.4) | 0 (0.0) | |
| Junior high school degree | 13 (13.7) | 1 (4.3) | |
| University entrance | 27 (28.4) | 12 (52.2) | |
| College degree | 35 (36.8) | 10 (43.5) | |
| Doctoral degree | 11 (11.6) | 0 (0.0) | |
| Pregnancy-Associated Characteristics | |||
| Birth mode | 0.05 | ||
| Spontaneous | 55 | 13 | |
| Ventouse | 4 | 2 | |
| C-section | 22 | 3 | |
| Emergency C-section | 11 | 5 | |
| Child Relocation after Birth | 32 (33.3) | 4 (17.4) | 0.05 |
| Pregnancy-related complications | 0.05 | ||
| Pre-eclampsia | 1 (1.0) | 0 (0.0) | |
| Gestational Diabetes Mellitus (GDM) | |||
| Insulin-dependent GDM | 10 (10.4) | 4 (17.4) | |
| Dietary-set GDM | 9 (9.4) | 0 (0.0) | |
| Hypothyroidism | 26 (27.1) | 8 (34.8) | |
| Hypertension | 6 (6.3) | 3 (13.0) | |
| Postpartum anemia | 29 (30.2) | 5 (21.7) | |
| M (SD) | M (SD) | ||
| Length of pregnancy in days | 272.48 (11.95) | 272.35 (11.48) | 0.05 |
| Child’s birth weight (in gram) | 3282.32 (548.51) | 3098.91 (452.82) | 0.05 |
| n (%) | n (%) | ||
| Breastfeeding at T0 | 89 (92.7) | 20 (87.0) | 0.05 |
| Breastfeeding at T1 | 78 (83.0) | 19 (82.6) | 0.05 |
| RLS Characteristics | |||
| Iron supplements | 59 (61.5) | 13 (56.5) | 0.05 |
| IRLSS score T0 | 17.27 (6.96) | 18.65 (7.09) | 0.05 |
| IRLSS score T1 | - | 16.04 (7.77) | - |
| RLS in previous pregnancy | 27 (28.1) | 7 (30.4) | 0.05 |
| Most severe RLS symptoms (n = 110) | 0.021 | ||
| First trimester | 2 (2.2) | 0 (0.0) | |
| Second trimester | 7 (7.8) | 1 (5.0) | |
| Third trimester | 73 (81.1) | 12 (60.0) | |
| Equally high over all trimesters | 8 (8.9) | 7 (35.0) | |
| Severity of RLS Symptoms T0 (IRLSS) | 0.05 | ||
| Mild | 2 (8.7) | 17 (17.7) | |
| Moderate | 12 (52.2) | 51 (53.1) | |
| Severe | 8 (34.8) | 23 (24.0) | |
| Very severe | 1 (4.3) | 5 (5.2) | |
| Psychiatric Characteristics | M (SD) | M (SD) | |
| Depression score EPDS T0 | 6.01 (4.48) | 7.35 (5.06) | 0.05 |
| Depression score EPDS T1 | 3.78 (3.86) | 4.13 (3.67) | 0.05 |
| n (%) | n (%) | ||
| Stressful life events (SLESQ) | 60 (62.5) | 16 (69.6) | 0.05 |
| Baby blues | 55 (57.3) | 9 (39.1) | 0.05 |
| Severity of baby blues | 10.67 (5.76) | 9.67 (6.56) | 0.05 |
| Psychiatric History | 0.05 | ||
| Depression | 19 (20.0) | 6 (26.1) | |
| Eating disorder | 1 (1.1) | 1 (4.3) | |
| Anxiety disorder and depression | 3 (3.2) | 1 (4.3) | |
| Other | 1 (1.1) | 0 (0.0) | |
| Laboratory Tests | M (SD) | M (SD) | |
| HCC T0 (pg/mg) | 11.06 (15.48) | 10.31 (8.42) | 0.05 |
| HCC T1 (pg/mg) | 6.13 (5.88) | 5.74 (5.77) | 0.05 |
| Hemoglobin (g/dL) T0 | 10.74 (1.49) | 11.07 (1.73) | 0.05 |
| TSH T0 | 2.74 (1.12) | 3.70 (4.65) | 0.05 |
| TSH T1 | 1.17 (0.69) | 1.11 (0.77) | 0.05 |
Appendix D
| Question | All Women with RLS (n = 119) | Pregnancy-Associated RLS (n = 96) | Persistent RLS (n = 23) | Statistical Comparison between Pregnancy-Associated and Persistent RLS |
|---|---|---|---|---|
| M (SD) | ||||
| IRLSS score T0 (Severity of RLS symptoms in pregnancy) | 17.54 (6.98) | 17.27 (6.96) | 18.61 (7.06) | t (117) = 0.825, p 0.05 |
| IRLSS score T1 (Severity of RLS symptoms 12 weeks postpartum) | 3.39 (7.45) | - | 16.04 (7.77) | |
| RLS in previous pregnancy | n = 34 (28.6%) | n = 27 (28.1%) | n = 7 (30.4%) | |
| Q1. How would you rate the RLS-related unpleasant sensations in your legs or arms? | 2.04 (0.89) | 2.01 (0.89) | 2.17 (0.94) | t (117) = 0.784, p 0.05 |
| Q2. How would you rate your urge to move because of the unpleasant sensations? | 2.50 (0.99) | 2.43 (0.98) | 2.83 (0.98) | t (117) = 1.75, p 0.05 |
| Q3. To what extent were the unpleasant sensations in your legs or arms alleviated by exercise? | 1.83 (0.93) | 1.82 (0.92) | 1.87 (1.01) | t (117) = 0.215, p 0.05 |
| Q4. How badly was your sleep affected by the unpleasant sensations? | 1.80 (1.29) | 1.77 (1.27) | 1.91 (1.44) | t (117) = −2.13, p 0.05 |
| Q5. How tired or sleepy were you during the day because of the unpleasant sensations? | 1.32 (1.24) | 1.34 (1.26) | 1.22 (1.20) | t (117) = 0.470, p 0.05 |
| Q6. Overall, how strong were the unpleasant sensations? | 2.00 (0.86) | 1.96 (0.86) | 2.17 (0.89) | t (117) = −0.437, p 0.05 |
| Q7. How often did the unpleasant sensations occur? | 2.41 (0.96) | 2.33 (0.98) | 2.74 (0.81) | t (117) = 1.08, p 0.05 |
| Q8. If you had unpleasant sensations, how severe were they on average? | 1.95 (0.77) | 1.88 (0.73) | 2.26 (0.86) | t (117) = 2.20, p = 0.30 |
| Q9. To what extent did your unpleasant sensations affect your ability to carry out daily activities? | 0.65 (0.95) | 0.70 (0.96) | 0.43 (0.90) | t (117) = −1.19, p 0.05 |
| Q10. How badly was your mood affected by the unpleasant sensations (making you, for example, angry, depressed, sad, anxious, or irritable)? | 1.03 (1.13) | 1.03 (1.11) | 1.00 (1.24) | t (117) = −0.119, p 0.05 |
References
- Ohayon, M.M.; O’Hara, R.; Vitiello, M.V. Epidemiology of restless legs syndrome: A synthesis of the literature. Sleep. Med. Rev. 2012, 16, 283–295. [Google Scholar] [CrossRef]
- Berger, K.; Luedemann, J.; Trenkwalder, C.; John, U.; Kessler, C. Sex and the risk of restless legs syndrome in the general population. Arch. Intern. Med. 2004, 164, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Ismailogullari, S.; Ozturk, A.; Mazicioglu, M.M.; Serin, S.; Gultekin, M.; Aksu, M. Restless legs syndrome and pregnancy in Kayseri, Turkey: A hospital based survey. Sleep. Biol. Rhyth. 2010, 8, 137–143. [Google Scholar] [CrossRef]
- Neau, J.-P.; Porcheron, A.; Mathis, S.; Julian, A.; Meurice, J.-C.; Paquereau, J.; Godeneche, G.; Ciron, J.; Bouche, G. Restless legs syndrome and pregnancy: A questionnaire study in the Poitiers District, France. Eur. Neurol. 2010, 64, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Neau, J.P.; Marion, P.; Mathis, S.; Julian, A.; Godeneche, G.; Larrieu, D.; Meurice, J.-C.; Paquereau, J.; Ingrand, P. Restless legs syndrome and pregnancy: Follow-up of pregnant women before and after delivery. Eur. Neurol. 2010, 64, 361–366. [Google Scholar] [CrossRef]
- Pantaleo, N.P.; Hening, W.A.; Allen, R.P.; Earley, C.J. Pregnancy accounts for most of the gender difference in prevalence of familial RLS. Sleep Med. 2010, 11, 310–313. [Google Scholar] [CrossRef]
- Manconi, M.; Ulfberg, J.; Berger, K.; Ghorayeb, I.; Wesström, J.; Fulda, S.; Allen, R.P.; Pollmächer, T. When gender matters: Restless legs syndrome. Report of the “RLS and woman” workshop endorsed by the European RLS Study Group. Sleep Med. Rev. 2012, 16, 297–307. [Google Scholar] [CrossRef]
- Gupta, R.; Dhyani, M.; Kendzerska, T.; Pandi-Perumal, S.R.; BaHammam, A.S.; Srivanitchapoom, P.; Pandey, S.; Hallett, M. Restless legs syndrome and pregnancy: Prevalence, possible pathophysiological mechanisms and treatment. Acta Neurol. Scand. 2016, 133, 320–329. [Google Scholar] [CrossRef]
- Srivanitchapoom, P.; Pandey, S.; Hallett, M. Restless legs syndrome and pregnancy: A review. Parkinsonism Relat. Disord. 2014, 20, 716–722. [Google Scholar] [CrossRef]
- Darvishi, N.; Daneshkhah, A.; Khaledi-Paveh, B.; Vaisi-Raygani, A.; Mohammadi, M.; Salari, N.; Darvishi, F.; Abdi, A.; Jalali, R. The prevalence of Restless Legs Syndrome/Willis-ekbom disease (RLS/WED) in the third trimester of pregnancy: A systematic review. BMC Neurol. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Manconi, M.; Govoni, V.; De Vito, A.; Economou, N.T.; Cesnik, E.; Casetta, I.; Mollica, G.; Ferini-Strambi, L.; Granieri, E. Restless legs syndrome and pregnancy. Neurology 2004, 63, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Cesnik, E.; Casetta, I.; Turri, M.; Govoni, V.; Granieri, E.; Strambi, L.F.; Manconi, M. Transient RLS during pregnancy is a risk factor for the chronic idiopathic form. Neurology 2010, 75, 2117–2120. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Guterman, P.; Leuner, B.; Galea, L.A.M. The long and short term effects of motherhood on the brain. Front. Neuroendocrinol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sonagra, A.D.; Biradar, S.M.; Dattatreya, K.; DS, J.M. Normal pregnancy-a state of insulin resistance. J. Clin. Diagn. Res. JCDR 2014, 8, CC01. [Google Scholar] [CrossRef]
- Gao, X.; Schwarzschild, M.A.; Wang, H.; Ascherio, A. Obesity and restless legs syndrome in men and women. Neurology 2009, 72, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Haider, B.A.; Olofin, I.; Wang, M.; Spiegelman, D.; Ezzati, M.; Fawzi, W.W.; Group, N.I.M.S. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2013, 346, f3443. [Google Scholar] [CrossRef]
- Sikandar, R.; Khealani, B.A.; Wasay, M. Predictors of restless legs syndrome in pregnancy: A hospital based cross sectional survey from Pakistan. Sleep Med. 2009, 10, 676–678. [Google Scholar] [CrossRef]
- Balendran, J.; Champion, D.; Jaaniste, T.; Welsh, A. A common sleep disorder in pregnancy: Restless legs syndrome and its predictors. Aust. New Zeal. J. Obstet. Gynaecol. 2011, 51, 262–264. [Google Scholar] [CrossRef]
- Chen, P.-H.; Liou, K.-C.; Chen, C.-P.; Cheng, S.-J. Risk factors and prevalence rate of restless legs syndrome among pregnant women in Taiwan. Sleep Med. 2012, 13, 1153–1157. [Google Scholar] [CrossRef]
- Hübner, A.; Krafft, A.; Gadient, S.; Werth, E.; Zimmermann, R.; Bassetti, C.L. Characteristics and determinants of restless legs syndrome in pregnancy: A prospective study. Neurology 2013, 80, 738–742. [Google Scholar] [CrossRef]
- Vahdat, M.; Sariri, E.; Miri, S.; Rohani, M.; Kashanian, M.; Sabet, A.; Zamani, B. Prevalence and associated features of restless legs syndrome in a population of Iranian women during pregnancy. Int. J. Gynecol. Obstet. 2013, 123, 46–49. [Google Scholar] [CrossRef]
- Ma, S.; Shang, X.; Guo, Y.; Liu, G.; Yang, J.; Xue, R. Restless legs syndrome and hypertension in Chinese pregnant women. Neurol. Sci. 2015, 36, 877–881. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Winkelman, J.W.; Walters, A.S.; Han, J.; Hu, F.B.; Gao, X. Prospective study of restless legs syndrome and total and cardiovascular mortality among women. Neurology 2018, 90, e135–e141. [Google Scholar] [CrossRef]
- Ramirez, J.O.; Cabrera, S.A.S.; Hidalgo, H.; Cabrera, S.G.; Linnebank, M.; Bassetti, C.L.; Kallweit, U. Is preeclampsia associated with restless legs syndrome? Sleep Med. 2013, 14, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Innes, K.E.; Kandati, S.; Flack, K.L.; Agarwal, P.; Selfe, T.K. The association of restless legs syndrome to history of gestational diabetes in an Appalachian primary care population. J. Clin. Sleep Med. 2015, 11, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B. Restless legs syndrome/Willis–Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.A.; Corcoran, C.; Turner, K.; Yuan, N.; Green, B.L. Assessing traumatic event exposure: General issues and preliminary findings for the Stressful Life Events Screening Questionnaire. J. Trauma. Stress 1998, 11, 521–542. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Bergant, A.M.; Nguyen, T.; Heim, K.; Ulmer, H.; Dapunt, O. German language version and validation of the Edinburgh postnatal depression scale. Dtsch. Med. Wochenschr. 1998, 123, 35–40. [Google Scholar] [CrossRef]
- Kennerley, H.; Gath, D. Maternity blues: I. Detection and measurement by questionnaire. Br. J. Psychiatry 1989, 155, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Haskett, R.F.; Carroll, B.J. Premenstrual tension syndrome: The development of research diagnostic criteria and new rating scales. Acta Psychiatr. Scand. 1980, 62, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Kirschbaum, C. Analysis of cortisol in hair–state of the art and future directions. Brain. Behav. Immun. 2012, 26, 1019–1029. [Google Scholar] [CrossRef]
- Liu, B.-P.; Wang, X.-T.; Liu, Z.-Z.; Wang, Z.-Y.; Liu, X.; Jia, C.-X. Stressful life events, insomnia and suicidality in a large sample of Chinese adolescents. J. Affect. Disord. 2019, 249, 404–409. [Google Scholar] [CrossRef]
- Kessler, R.C. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997, 48, 191–214. [Google Scholar] [CrossRef]
- Carballo, J.J.; Llorente, C.; Kehrmann, L.; Flamarique, I.; Zuddas, A.; Purper-Ouakil, D.; Hoekstra, P.J.; Coghill, D.; Schulze, U.M.E.; Dittmann, R.W. Psychosocial risk factors for suicidality in children and adolescents. Eur. Child Adolesc. Psychiatry 2019, 1–18. [Google Scholar] [CrossRef]
- Zhuang, S.; Na, M.; Winkelman, J.W.; Ba, D.; Liu, C.-F.; Liu, G.; Gao, X. Association of restless legs syndrome with risk of suicide and self-harm. JAMA Netw. Open 2019, 2, e199966. [Google Scholar] [CrossRef]
- Chandan, J.S.; Thomas, T.; Raza, K.; Bandyopadhyay, S.; Nirantharakumar, K.; Taylor, J. Association between child maltreatment and central sensitivity syndromes: A systematic review protocol. BMJ Open 2019, 9, e025436. [Google Scholar] [CrossRef]
- Winkelmann, J.; Prager, M.; Lieb, R.; Pfister, H.; Spiegel, B.; Wittchen, H.U.; Holsboer, F.; Trenkwalder, C.; Ströhle, A. “Anxietas tibiarum”. Depression and anxiety disorders in patients with restless legs syndrome. J. Neurol. 2005, 252, 67–71. [Google Scholar] [CrossRef]
- Li, Y.; Mirzaei, F.; O’reilly, E.J.; Winkelman, J.; Malhotra, A.; Okereke, O.I.; Ascherio, A.; Gao, X. Prospective study of restless legs syndrome and risk of depression in women. Am. J. Epidemiol. 2012, 176, 279–288. [Google Scholar] [CrossRef]
- Koo, B.B.; Blackwell, T.; Lee, H.B.; Stone, K.L.; Louis, E.D.; Redline, S.; Osteoporotic Fractures in Men (MrOS) Study Group. Restless legs syndrome and depression: Effect mediation by disturbed sleep and periodic limb movements. Am. J. Geriatr. Psychiatry 2016, 24, 1105–1116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Auvinen, P.; Mäntyselkä, P.; Koponen, H.; Kautiainen, H.; Korniloff, K.; Ahonen, T.; Vanhala, M. Prevalence of restless legs symptoms according to depressive symptoms and depression type: A cross-sectional study. Nord. J. Psychiatry 2018, 72, 51–56. [Google Scholar] [CrossRef]
- Cho, C.H.; Kim, L.; Lee, H.J. Individuals with restless legs syndrome tend to have severe depressive symptoms: Findings from a community-based cohort study. Psychiatry Investig. 2017, 14, 887. [Google Scholar] [CrossRef] [PubMed]
- Wesström, J.; Nilsson, S.; Sundström-Poromaa, I.; Ulfberg, J. Restless legs syndrome among women: Prevalence, co-morbidity and possible relationship to menopause. Climacteric 2008, 11, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Pengo, M.F.; Won, C.H.; Bourjeily, G. Sleep in women across the life span. Chest 2018, 154, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Dzaja, A.; Wehrle, R.; Lancel, M.; Pollmächer, T. Elevated estradiol plasma levels in women with restless legs during pregnancy. Sleep 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Castillo, P.R.; Mera, R.M.; Fredrickson, P.A.; Zambrano, M.; Del Brutto, V.J.; Del Brutto, O.H. Psychological distress in patients with restless legs syndrome (Willis-Ekbom disease): A population-based door-to-door survey in rural Ecuador. BMC Res. Notes 2014, 7, 911. [Google Scholar] [CrossRef]
- Lu, Q.; Mouri, A.; Yang, Y.; Kunisawa, K.; Teshigawara, T.; Hirakawa, M.; Mori, Y.; Yamamoto, Y.; Libo, Z.; Nabeshima, T. Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav. Brain Res. 2019, 372, 112053. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Somers, V.K.; Punjabi, N.M.; Winkelman, J.W. Restless legs syndrome and cardiovascular disease: A research roadmap. Sleep Med. 2017, 31, 10–17. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Blackwell, T.; Stone, K.; Ancoli-Israel, S.; Redline, S. Associations of incident cardiovascular events with restless legs syndrome and periodic leg movements of sleep in older men, for the outcomes of sleep disorders in older men study (MrOS Sleep Study). Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Trenkwalder, C.; Allen, R.; Högl, B.; Paulus, W.; Winkelmann, J. Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology 2016, 86, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Panvatvanich, S.; Lolekha, P. Restless legs syndrome in pregnant Thai women: Prevalence, predictive factors, and natural course. J. Clin. Neurol. 2019, 15, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Yang, J.; Guo, Y.; Ma, S.; Jia, Z.; Xue, R. Restless legs syndrome among pregnant women in China: Prevalence and risk factors. Sleep Breath. 2015, 19, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Earley, C.J.; Connor, J.; Garcia-Borreguero, D.; Jenner, P.; Winkelman, J.; Zee, P.C.; Allen, R. Altered brain iron homeostasis and dopaminergic function in restless legs syndrome (Willis–Ekbom disease). Sleep Med. 2014, 15, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Allen, R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS). Sleep Med. 2004, 5, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, J.; Hornyak, M.; Voderholzer, U.; Kryger, M.; Skomrow, R.; Lipinski, J.F.; Masood, A.; Phillips, B.; Oertel, W.H.; Stiasny, K.; et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003, 4, 121–132. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goecke, T.W.; Schnakenberg, P.; Frensch, M.; Chechko, N. Restless Legs Syndrome During Pregnancy and 12 Weeks Postpartum and Its Links to Cardiovascular Diseases, Stressful Life Events, and Psychiatric History. J. Clin. Med. 2020, 9, 3046. https://doi.org/10.3390/jcm9093046
Goecke TW, Schnakenberg P, Frensch M, Chechko N. Restless Legs Syndrome During Pregnancy and 12 Weeks Postpartum and Its Links to Cardiovascular Diseases, Stressful Life Events, and Psychiatric History. Journal of Clinical Medicine. 2020; 9(9):3046. https://doi.org/10.3390/jcm9093046
Chicago/Turabian StyleGoecke, Tamme W., Patricia Schnakenberg, Markus Frensch, and Natalia Chechko. 2020. "Restless Legs Syndrome During Pregnancy and 12 Weeks Postpartum and Its Links to Cardiovascular Diseases, Stressful Life Events, and Psychiatric History" Journal of Clinical Medicine 9, no. 9: 3046. https://doi.org/10.3390/jcm9093046
APA StyleGoecke, T. W., Schnakenberg, P., Frensch, M., & Chechko, N. (2020). Restless Legs Syndrome During Pregnancy and 12 Weeks Postpartum and Its Links to Cardiovascular Diseases, Stressful Life Events, and Psychiatric History. Journal of Clinical Medicine, 9(9), 3046. https://doi.org/10.3390/jcm9093046





