Increased Antioxidant Capacity and Pro-Homeostatic Lipid Mediators in Ocular Hypertension—A Human Experimental Model
Abstract
:1. Introduction
2. Experimental Section
2.1. Power Calculations
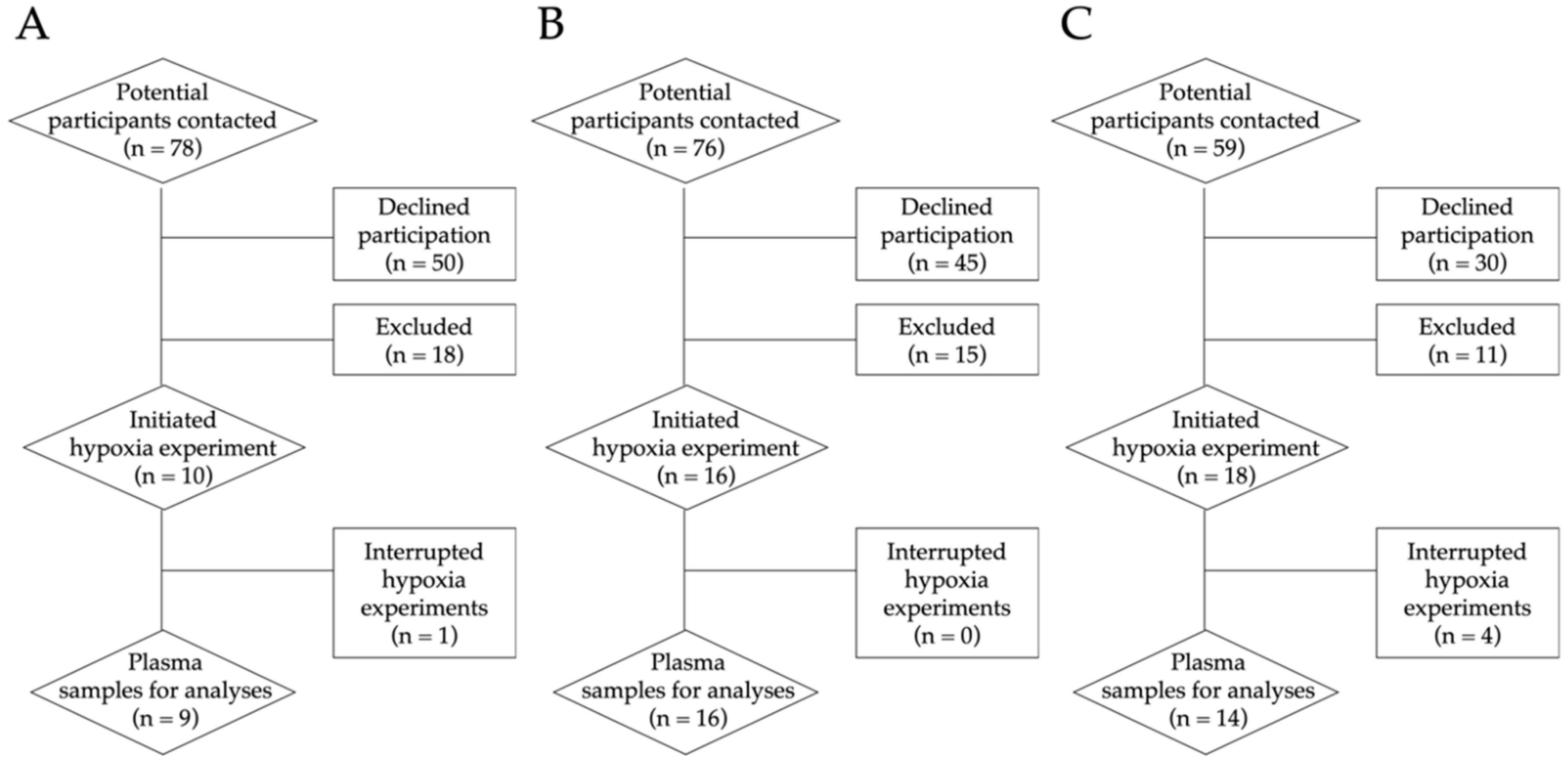
2.2. Recruitment Process
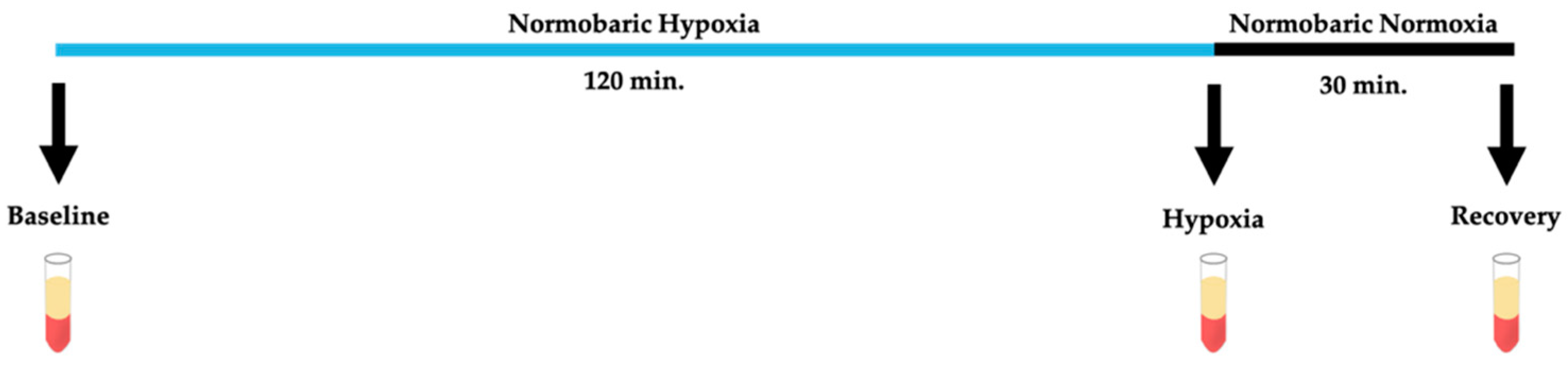
2.3. Hypoxia Model
2.4. Antioxidant–Oxidant Status
2.4.1. Total Antioxidant Capacity Analysis
2.4.2. Superoxide Dismutase 3 Analysis
2.4.3. Malondialdehyde Analysis
2.4.4. Free Radical Analysis
2.5. Lipidomics
2.5.1. Lipid Extraction
2.5.2. LC-MS/MS
2.6. Statistical Analysis
3. Results
3.1. Demographics and Ophthalmological Characteristics
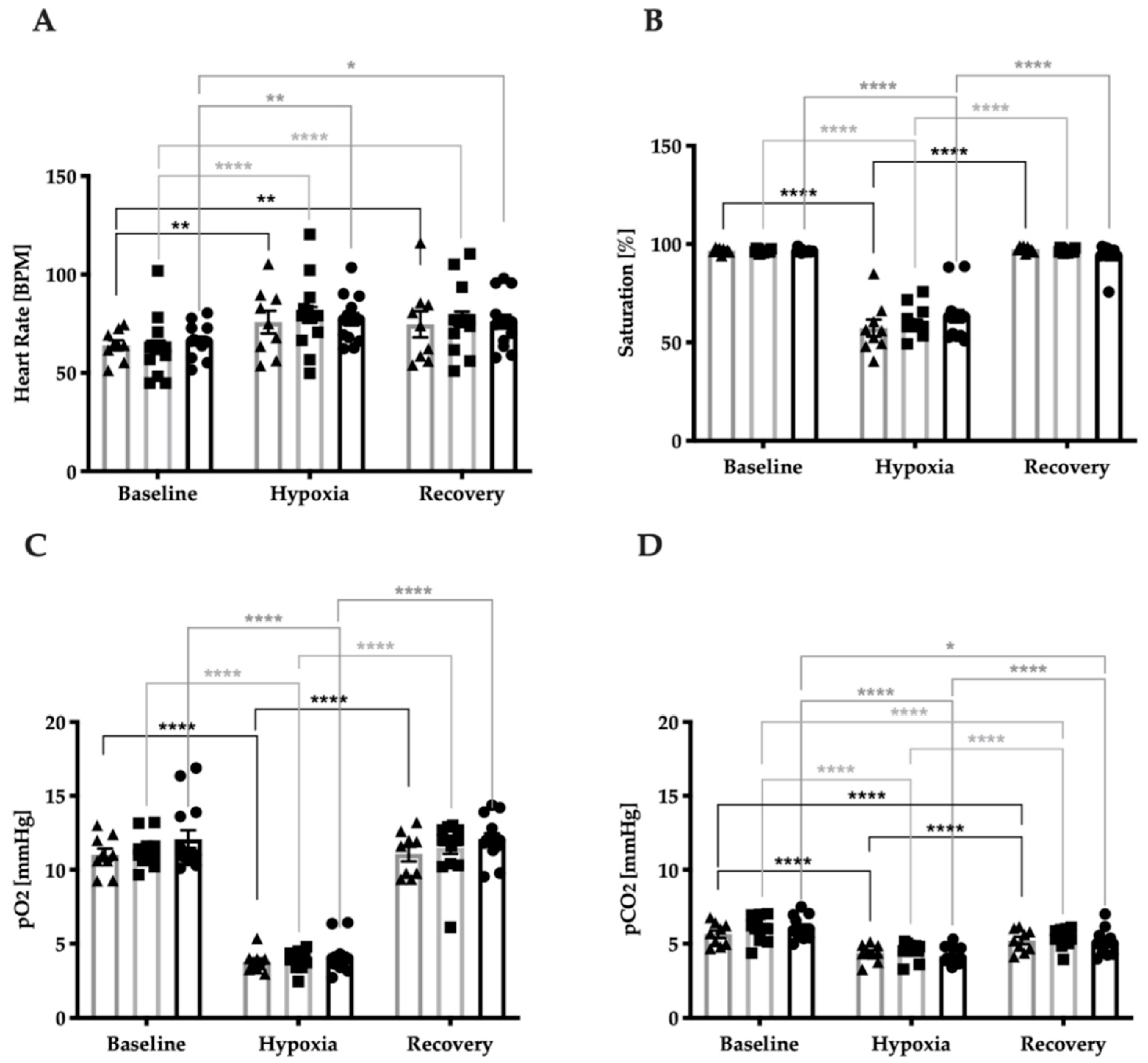
3.2. Stress Response Regarding Systemic Vital Parameters
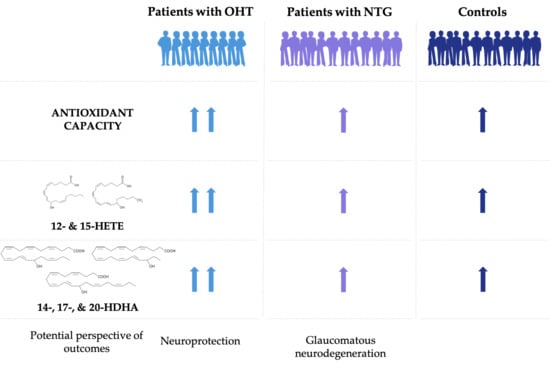
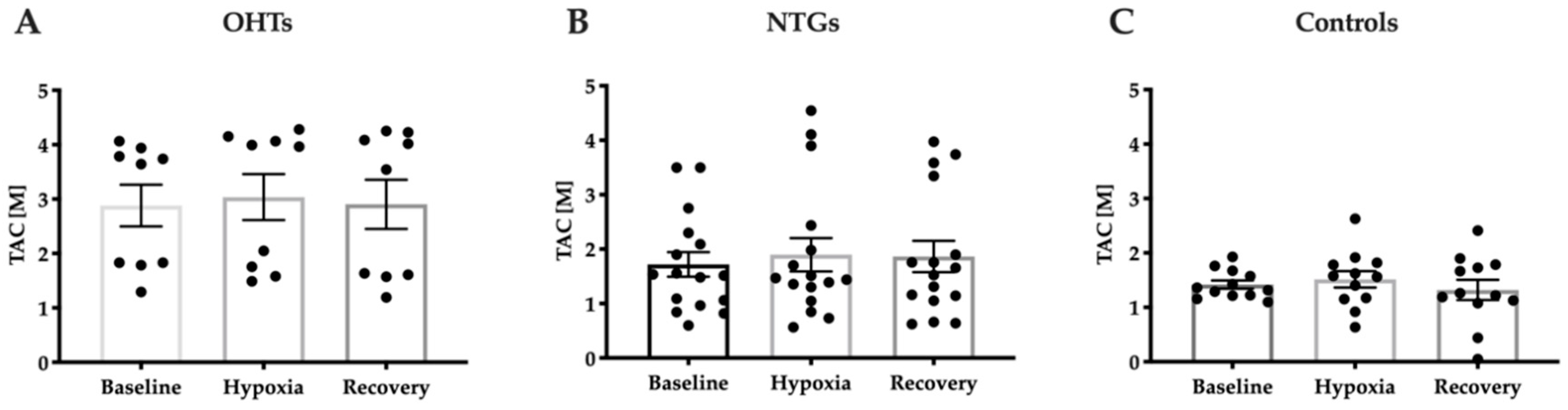
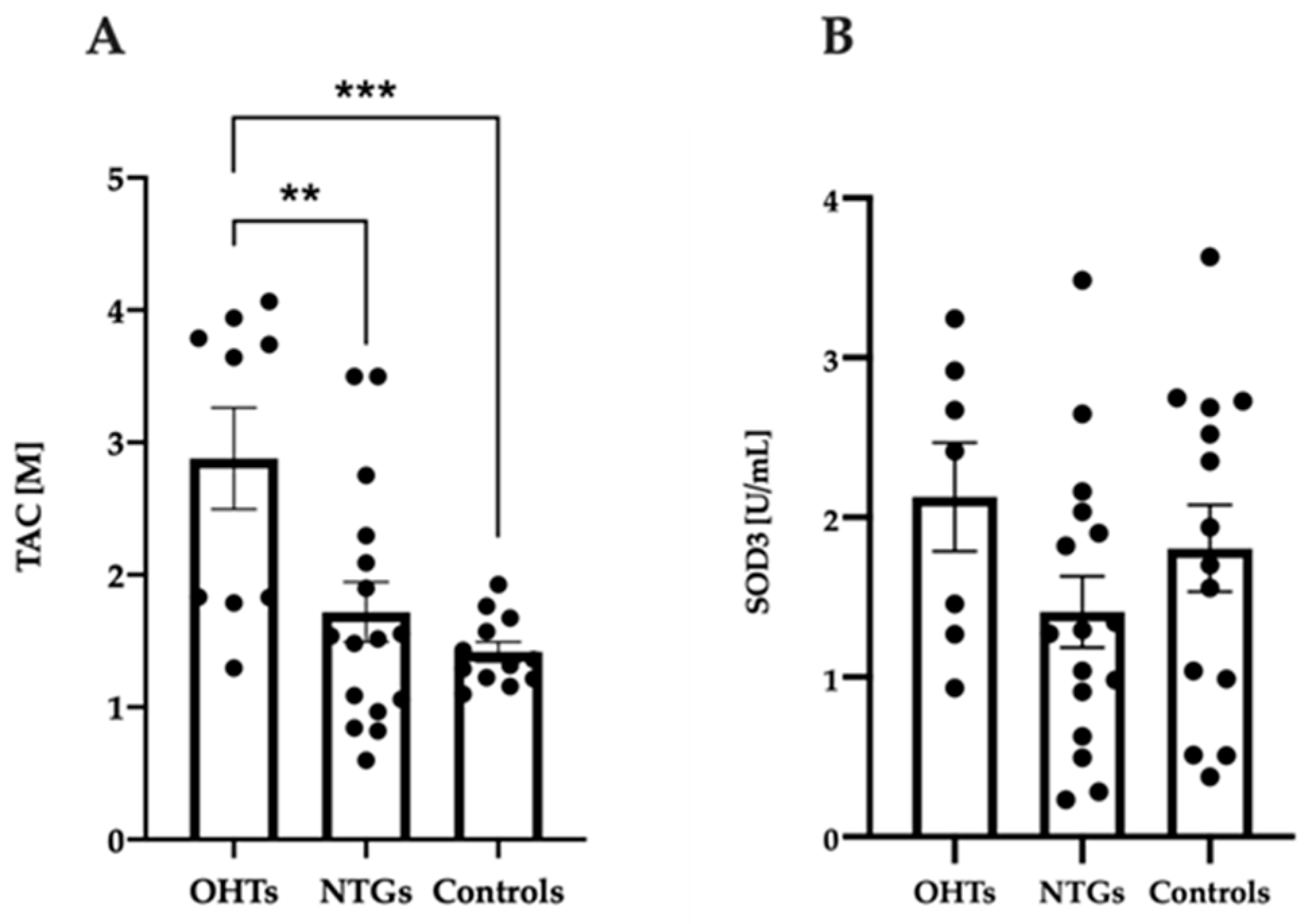
3.3. Increased Antioxidant Capacity in Patients with OHT Compared to NTG and Controls
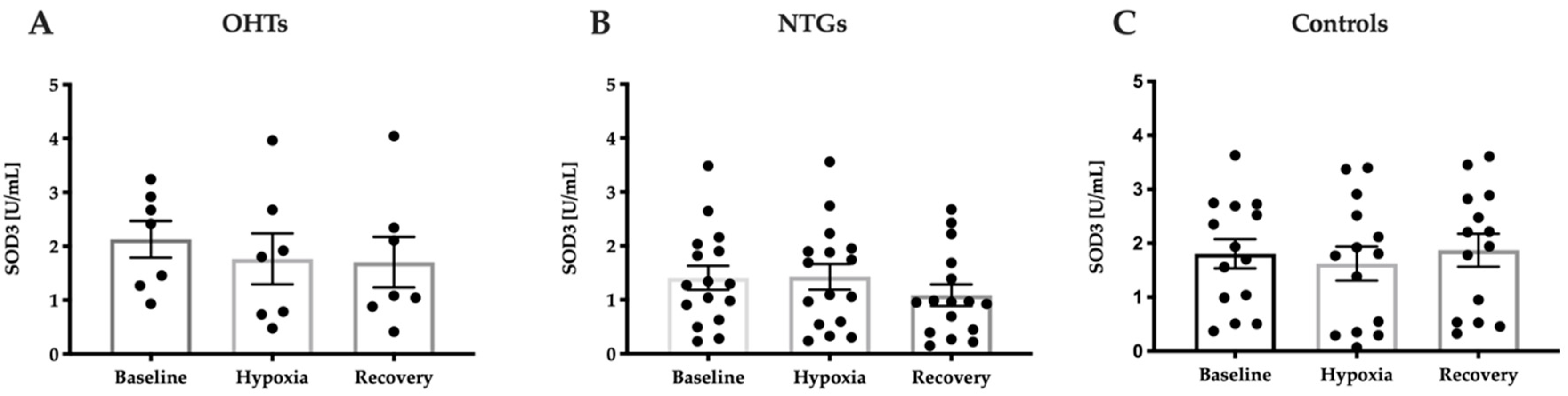
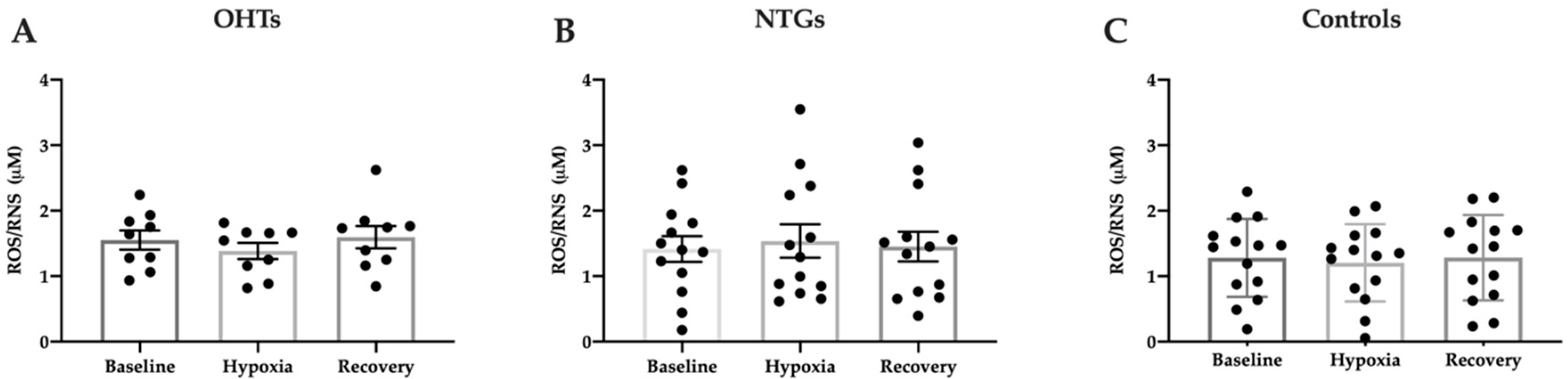
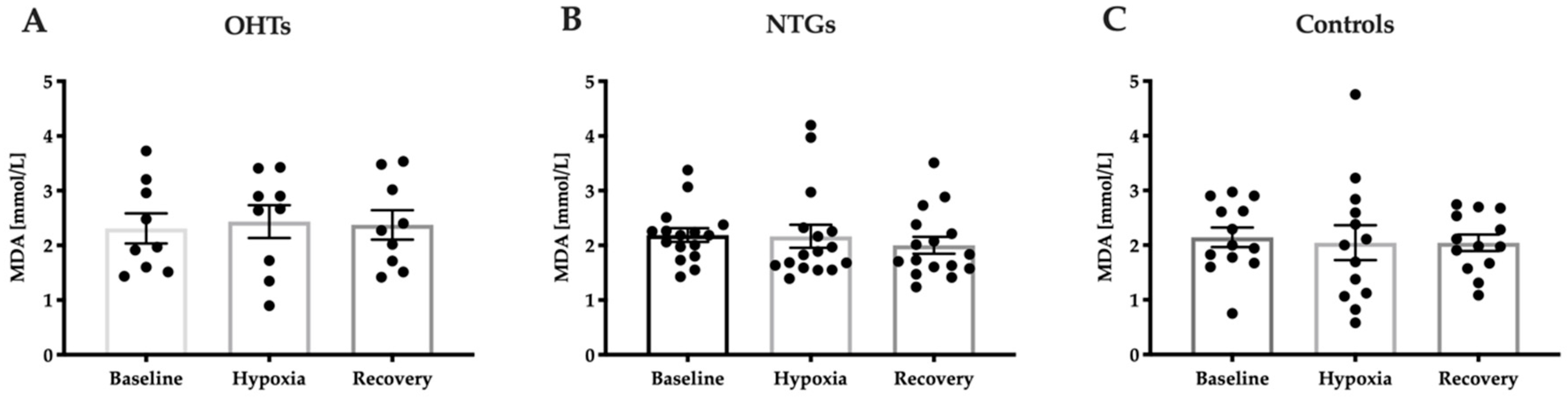
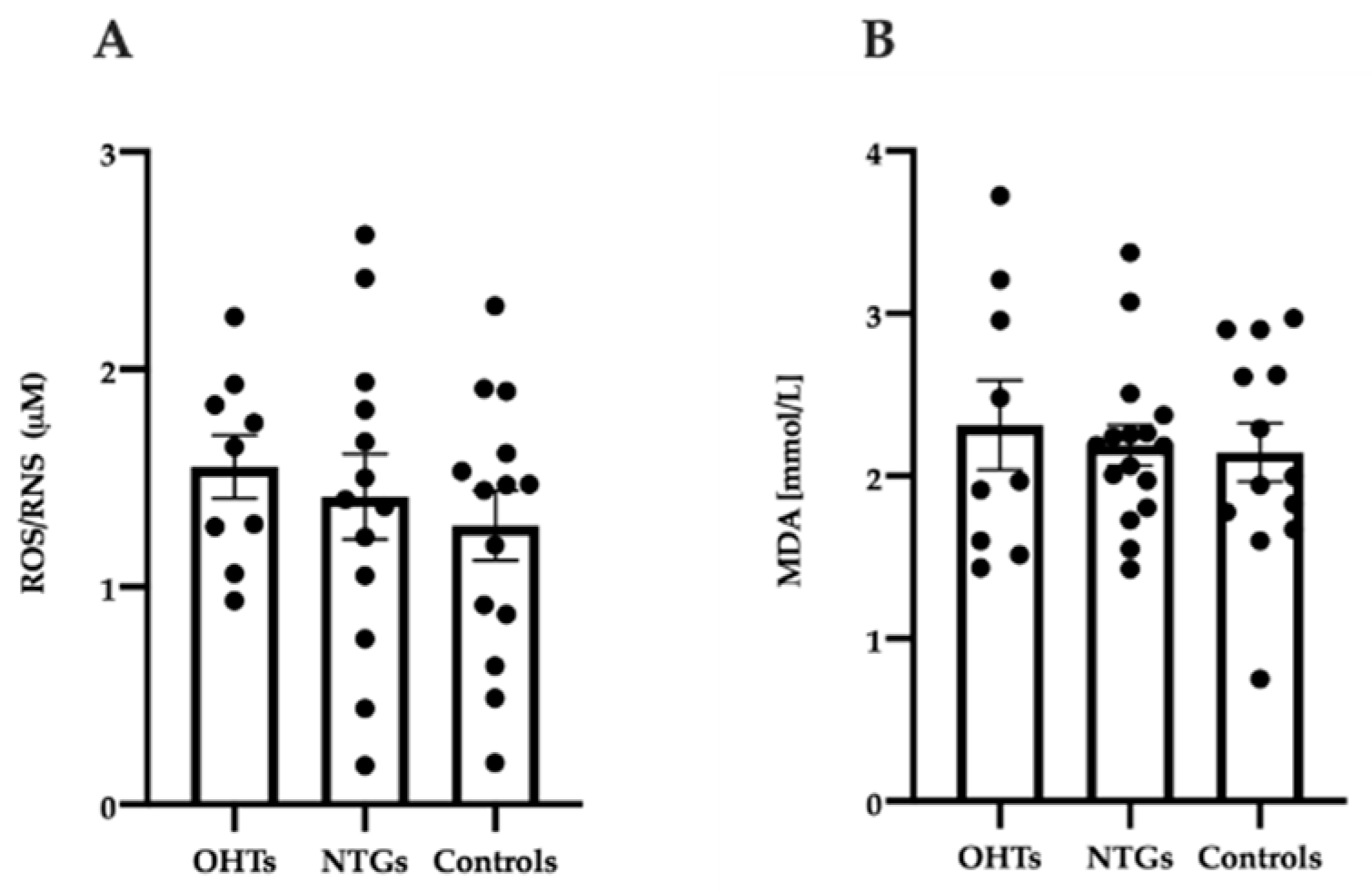
3.4. Oxidative Stress in Study Groups
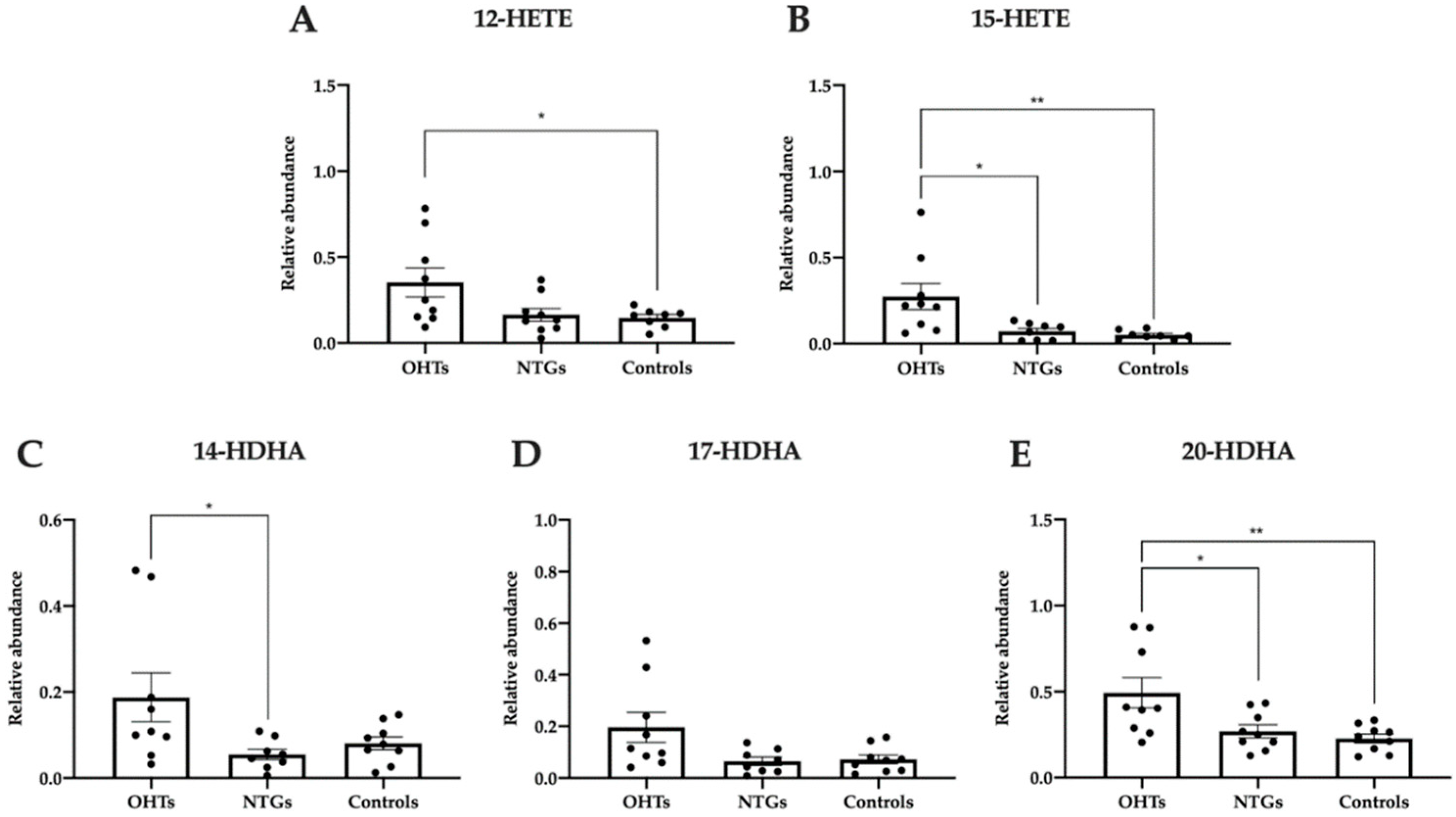
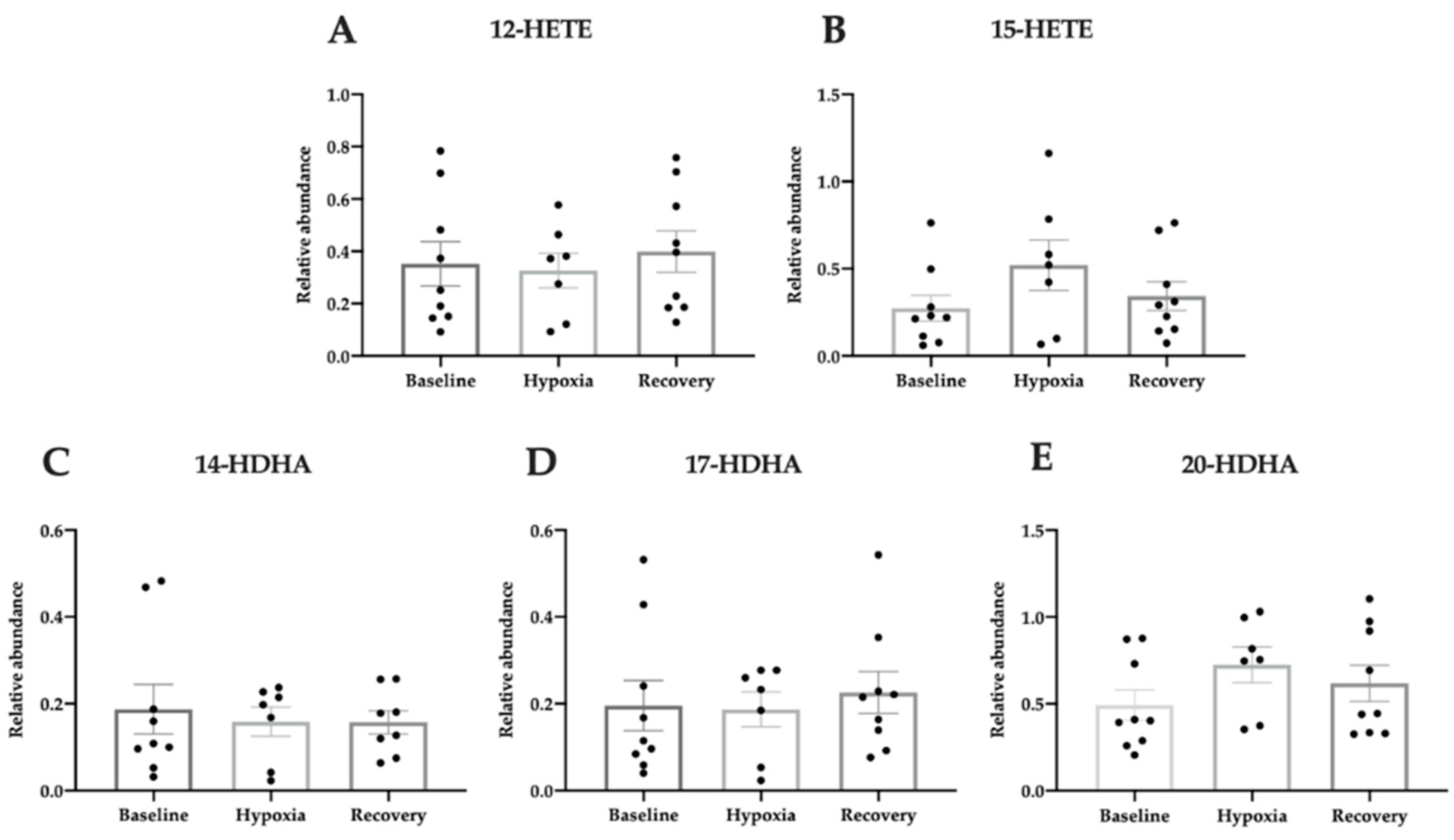
3.5. Pro-Homeostatic Lipid Mediators Correlate with Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| DHA | docosahexaenoic acid |
| HDHA | hydroxydocosahexaenoic acids |
| HETE | hydroxyeicosatetraenoic acids |
| HR | heart rate |
| HTG | high-tension glaucoma |
| IOP | intraocular pressure |
| LC-MS/MS | liquid chromatography with tandem mass spectrometry |
| M | molar |
| MD | mean defect |
| MDA | malondialdehyde |
| NTG | normal-tension glaucoma |
| OCT | ocular coherence tomography |
| OD | oculus dexter |
| OHT | ocular hypertension |
| OS | oculus sinister |
| pCO2 | partial pressure of carbon dioxide |
| pO2 | partial pressure of oxygen |
| POAG | primary open-angle glaucoma |
| PUFAs | polyunsaturated fatty acids |
| RBCs | red blood cells |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SAT | saturation |
| SOD3 | superoxide dismutase 3 |
| TAC | total antioxidant capacity |
Appendix A
Appendix B
References
- Lascaratos, G.; Chau, K.-Y.; Zhu, H.; Gkotsi, D.; King, R.; Gout, I.; Kamal, D.; Luthert, P.J.; Schapira, A.H.V.; Garway-Heath, D. Resistance to the most common optic neuropathy is associated with systemic mitochondrial efficiency. Neurobiol. Dis. 2015, 82, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kolko, M.; Horwitz, A.; Thygesen, J.; Jeppesen, J.; Torp-Pedersen, C. The Prevalence and Incidence of Glaucoma in Denmark in a Fifteen Year Period: A Nationwide Study. PLoS ONE 2015, 10, e0132048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokicki, W.; Zalejska-Fiolka, J.; Pojda-Wilczek, D.; Kabiesz, A.; Majewski, W. Oxidative stress in the red blood cells of patients with primary open-angle glaucoma. Clin. Hemorheol. Microcirc. 2016, 62, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Bengtsson, B.; Oskarsdottir, S.E. Prevalence and Severity of Undetected Manifest Glaucoma. Ophthalmology 2013, 120, 1541–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolko, M. Detection and prevention of blindness in patients with glaucoma is a socio-economical challenge. Ugeskr. Laeger 2017, 179, 179. [Google Scholar]
- Topouzis, F.; Anastasopoulos, E. Glaucoma—The Importance of Early Detection and Early Treatment. Eur. Ophthalmic Rev. 2007, 13. [Google Scholar] [CrossRef]
- Noecker, R.J. The management of glaucoma and intraocular hypertension: Current approaches and recent advances. Ther. Clin. Risk Manag. 2006, 2, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.R. Normal-tension glaucoma (Low-tension glaucoma). Indian J. Ophthalmol. 2011, 59, S97–S101. [Google Scholar] [CrossRef]
- Shields, M.B. Normal-tension glaucoma: Is it different from primary open-angle glaucoma? Curr. Opin. Ophthalmol. 2008, 19, 85–88. [Google Scholar] [CrossRef]
- López-Riquelme, N.; Villalba, C.; Tormo, C.; Belmonte, A.; Fernandez, C.; Torralba, G.; Hernández, F. Endothelin-1 levels and biomarkers of oxidative stress in glaucoma patients. Int. Ophthalmol. 2014, 35, 527–532. [Google Scholar] [CrossRef]
- Gugleta, K.; Kochkorov, A.; Waldmann, N.; Polunina, A.; Katamay, R.; Flammer, J.; Orgül, S. Dynamics of retinal vessel response to flicker light in glaucoma patients and ocular hypertensives. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 250, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherecheanu, A.P.; Garhofer, G.; Schmidl, D.; Werkmeister, R.; Schmetterer, L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcmonnies, C. Reactive oxygen species, oxidative stress, glaucoma and hyperbaric oxygen therapy. J. Optom. 2018, 11, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Erdurmuş, M.; Yagci, R.; Atis, O.; Karadag, R.; Akbaş, A.; Hepsen, I.F. Antioxidant Status and Oxidative Stress in Primary Open Angle Glaucoma and Pseudoexfoliative Glaucoma. Curr. Eye Res. 2011, 36, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Yue, B.Y. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: The trabecular meshwork. J. Cell. Physiol. 1999, 180, 182–189. [Google Scholar] [CrossRef]
- Majsterek, I.; Malinowska, K.; Stanczyk, M.; Kowalski, M.; Blaszczyk, J.; Kurowska, A.K.; Kamińska, A.; Szaflik, J.; Szaflik, J.P. Evaluation of oxidative stress markers in pathogenesis of primary open-angle glaucoma. Exp. Mol. Pathol. 2011, 90, 231–237. [Google Scholar] [CrossRef]
- Awai-Kasaoka, N.; Inoue, T.; Kameda, T.; Fujimoto, T.; Inoue-Mochita, M.; Tanihara, H. Oxidative stress response signaling pathways in trabecular meshwork cells and their effects on cell viability. Mol. Vis. 2013, 19, 1332–1340. [Google Scholar]
- He, Y.; Leung, K.W.; Zhang, Y.; Duan, S.; Zhong, X.; Jiang, R.-Z.; Peng, Z.; Tombran-Tink, J.; Ge, J. Mitochondrial Complex I Defect Induces ROS Release and Degeneration in Trabecular Meshwork Cells of POAG Patients: Protection by Antioxidants. Investig. Opthalmology Vis. Sci. 2008, 49, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Maccarrone, M.; Chiurchiù, V. Proresolving Lipid Mediators: Endogenous Modulators of Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 8107265. [Google Scholar] [CrossRef]
- Saccà, S.C.; Cutolo, C.A.; Ferrari, D.; Corazza, P.; Traverso, C.E. The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients 2018, 10, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqui, A.A. Lipid Mediators in the Neural Cell Nucleus: Their Metabolism, Signaling, and Association with Neurological Disorders. Neuroscience 2009, 15, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Gronert, K. Resolution, the grail for healthy ocular inflammation. Exp. Eye Res. 2010, 91, 478–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaou, A.; Mauro, C.; Urquhart, P.; Marelli-Berg, F.M. Polyunsaturated Fatty Acid-Derived Lipid Mediators and T Cell Function. Front. Immunol. 2014, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Antony, R.; Lukiw, W.J.; Bazan, N.G. Neuroprotectin D1 Induces Dephosphorylation of Bcl-xLin a PP2A-dependent Manner during Oxidative Stress and Promotes Retinal Pigment Epithelial Cell Survival. J. Boil. Chem. 2010, 285, 18301–18308. [Google Scholar] [CrossRef] [Green Version]
- Faghiri, Z.; Bazan, N.G. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp. Eye Res. 2010, 90, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. From The Cover: Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, N.; Hopkins, S.R.; Elliott, A.R.; Aaron, E.; Weinger, M.B.; Powell, F.L. Ventilatory response to 2-h sustained hypoxia in humans. Respir. Physiol. 2000, 124, 11–22. [Google Scholar] [CrossRef]
- Johansson, P.I.; Bergstrã¶m, A.; Aachmann-Andersen, N.J.; Meyer, M.A.S.; Ostrowski, S.R.; Nordsborg, N.B.; Olsen, N.V.; Bergström, A. Effect of acute hypobaric hypoxia on the endothelial glycocalyx and digital reactive hyperemia in humans. Front. Physiol. 2014, 5, 459. [Google Scholar] [CrossRef] [Green Version]
- Easton, P.A.; Slykerman, L.J.; Anthonisen, N.R. Recovery of the ventilatory response to hypoxia in normal adults. J. Appl. Physiol. 1988, 64, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Dalgaard, L.M.; Vibæk, J.; Langbøl, M.A.; Bergersen, L.H.; Olsen, N.V.; Hassel, B.; Chaudhry, F.A.; Kolko, M.; Vibaek, J. Potential metabolic markers in glaucoma and their regulation in response to hypoxia. Acta Ophthalmol. 2019, 97, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y. Retinal Vessel Diameters and Their Associations with Age and Blood Pressure. Investig. Opthalmol. Vis. Sci. 2003, 44, 4644–4650. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 7. [Google Scholar] [CrossRef] [Green Version]
- Abu-Amero, K.K.; Morales, J.; Bosley, T.M. Mitochondrial Abnormalities in Patients with Primary Open-Angle Glaucoma. Investig. Opthalmol. Vis. Sci. 2006, 47, 2533–2541. [Google Scholar] [CrossRef]
- D’Azy, C.B.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166915. [Google Scholar] [CrossRef]
- Bulboaca, A.E.; Nicula, C.; Bulboaca, A.; Blidaru, M.; Boarescu, P.M.; Festila, D.; Dogaru, G.; Stanescu, I. Oxidative stress/antioxidant balance implication in reducing of intra-ocular pressure in patients with stroke, nicergoline therapy and open-angle glaucoma. Balneo Res. J. 2019, 10, 259–264. [Google Scholar] [CrossRef]
- Chang, D.; Sha, Q.; Zhang, X.; Liu, P.; Rong, S.; Han, T.; Liu, P.; Pan, H. The Evaluation of the Oxidative Stress Parameters in Patients with Primary Angle-Closure Glaucoma. PLoS ONE 2011, 6, e27218. [Google Scholar] [CrossRef]
- Engin, K.N.; Yemişci, B.; Yiğit, U.; Ağaçhan, A.; Coşkun, C. Variability of serum oxidative stress biomarkers relative to biochemical data and clinical parameters of glaucoma patients. Mol. Vis. 2010, 16, 1260–1271. [Google Scholar]
- Hondur, G.; Göktaş, E.; Yang, X.; Al-Aswad, L.; Auran, J.D.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M.; Suh, L.H.; Trief, D.; et al. Oxidative Stress–Related Molecular Biomarker Candidates for Glaucoma. Investig. Opthalmol. Vis. Sci. 2017, 58, 4078–4088. [Google Scholar] [CrossRef] [Green Version]
- Nucci, C.; Di Pierro, N.; Varesi, C.; Ciuffoletti, E.; Russo, R.; Gentile, R.; Cedrone, C.; Duran, M.D.P.; Coletta, M.; Mancino, R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol. Vis. 2013, 19, 1841–1846. [Google Scholar] [PubMed]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Javadzadeh, A.; Rashtchizadeh, N.; Moharrery, M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol. Vis. 2011, 17, 41–46. [Google Scholar] [PubMed]
- Yıldırım, Ö.; Ateş, N.; Ercan, B.; Muşlu, N.; Ünlü, A.; Tamer, L.; Atik, U.; Kanık, A.; Ate, N. Role of oxidative stress enzymes in open-angle glaucoma. Eye 2004, 19, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Murat, D.; Kimura, I.; Tsubota, K. Increased serum total antioxidant status and decreased urinary 8-hydroxy-2′-deoxyguanosine levels in patients with normal-tension glaucoma. Acta Ophthalmol. 2010, 88, e259–e264. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Lerner, S.F.; Brunzini, R.; Evelson, P.; Llesuy, S. Oxidative stress markers in aqueous humor of glaucoma patients. Am. J. Ophthalmol. 2004, 137, 62–69. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.; Mousa, A.; Osman, E.A.; Al-Obeidan, S.A. Decreased Total Antioxidants in Patients with Primary Open Angle Glaucoma 1. Curr. Eye Res. 2013, 38, 959–964. [Google Scholar] [CrossRef]
- Mousa, A.; Kondkar, A.A.; Al-Obeidan, S.A.; Azad, T.A.; Sultan, T.; Osman, E.; Abu-Amero, K.K. Association of total antioxidants level with glaucoma type and severity. Saudi Med. J. 2015, 36, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zanon-Moreno, V.C.; Marco-Ventura, P.; Lleó-Pérez, A.; Pons-Vázquez, S.; García-Medina, J.J.; Vinuesa-Silva, I.; Moreno-Nadal, M.A.; Pinazo-Durán, M.D. Oxidative Stress in Primary Open-angle Glaucoma. J. Glaucoma 2008, 17, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Durán, M.D.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanon-Moreno, V.C.; Nucci, C.; Dolz-Marco, R.; Martinez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J. Signal Transduct. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.A.; Arafa, L.F.; El-Baz, A. Oxidative Stress Markers in Patients with Primary Open-Angle Glaucoma. Curr. Eye Res. 2010, 35, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of Oxidative Stress Markers in Aqueous Humor of Primary Open Angle Glaucoma and Primary Angle Closure Glaucoma Patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; Ferreira, G.C.; Trevenzoli, I.H.; Oliveira, K.D.J.; Reis, R.A.D.M. Fatty Acids, Antioxidants and Physical Activity in Brain Aging. Nutrients 2017, 9, 1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorlaksdottir, A.Y.; Skuladottir, G.V.; Petursdottir, A.L.; Tryggvadóttir, L.; Ogmundsdottir, H.M.; Eyfjord, J.E.; Jonsson, J.J.; Hardardottir, I. Positive association between plasma antioxidant capacity and n−3 PUFA in red blood cells from women. Lipids 2006, 41, 119–125. [Google Scholar] [CrossRef]
- Nakamura, N.; Kumasaka, R.; Osawa, H.; Yamabe, H.; Shirato, K.-I.; Fujita, T.; Murakami, R.-I.; Shimada, M.; Nakamura, M.; Okumura, K.; et al. Effects of eicosapentaenoic acids on oxidative stress and plasma fatty acid composition in patients with lupus nephritis. In Vivo 2005, 19, 879–882. [Google Scholar]
- Barbosa, D.S.; Cecchini, R.; El Kadri, M.Z.; Rodríguez, M.A.M.; Burini, R.C.; Dichi, J.B. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil ω-3 fatty acids. Nutrients 2003, 19, 837–842. [Google Scholar] [CrossRef]
- Cittadini, A.; Calviello, G.; Su, H.-M.; Weylandt, K. ω-3 PUFAs in the Prevention and Cure of Inflammatory, Degenerative, and Neoplastic Diseases. BioMed Res. Int. 2013, 2013, 1–2. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.-W.; Han, J.; Liang, H.; Wang, N.; Cheng, Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARγ: A possible neuroprotective effect in ischemic brain. J. Lipid Res. 2015, 56, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Nazarewicz, R.R.; Zenebe, W.J.; Parihar, A.; Parihar, M.S.; Vaccaro, M.; Rink, C.; Sen, C.K.; Ghafourifar, P. 12(S)-Hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch. Biochem. Biophys. 2007, 468, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Mao, M.; Qiu, Y.; Liu, G.; Sheng, T.; Yu, X.; Wang, S.; Zhu, D. Key Role of ROS in the Process of 15-Lipoxygenase/15-Hydroxyeicosatetraenoiccid-Induced Pulmonary Vascular Remodeling in Hypoxia Pulmonary Hypertension. PLoS ONE 2016, 11, e0149164. [Google Scholar] [CrossRef] [PubMed]
- Debevec, T.; Millet, G.P.; Pialoux, V. Hypoxia-Induced Oxidative Stress Modulation with Physical Activity. Front. Physiol. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.M.; Taudorf, S.; Berg, R.M.G.; Lundby, C.; McEneny, J.; Young, I.S.; Evans, K.A.; James, P.E.; Shore, A.; Hullin, D.A.; et al. Increased cerebral output of free radicals during hypoxia: Implications for acute mountain sickness? Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R1283–R1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhaes, J.; Ascensão, A.; Viscor, G.; Soares, J.; Oliveira, J.; Marques, F.; Duarte, J. Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat. Space Environ. Med. 2004, 75, 16–22. [Google Scholar]
- Strapazzon, G.; Malacrida, S.; Vezzoli, A.; Cappello, T.D.; Falla, M.; Lochner, P.; Moretti, S.; Procter, E.; Brugger, H.; Mrakic-Sposta, S. Oxidative stress response to acute hypobaric hypoxia and its association with indirect measurement of increased intracranial pressure: A field study. Sci. Rep. 2016, 6, 32426. [Google Scholar] [CrossRef]
- Møller, P.; Loft, S.; Lundby, C.; Olsen, N.V. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001, 15, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Subudhi, A.W.; Jacobs, K.A.; Hagobian, T.; Fattor, J.; Fulco, C.S.; Muza, S.R.; Rock, P.B.; Hoffman, A.R.; Cymerman, A.; Friedlander, A.L. Antioxidant supplementation does not attenuate oxidative stress at high altitude. Aviat. Space Environ. Med. 2004, 75, 881–888. [Google Scholar]
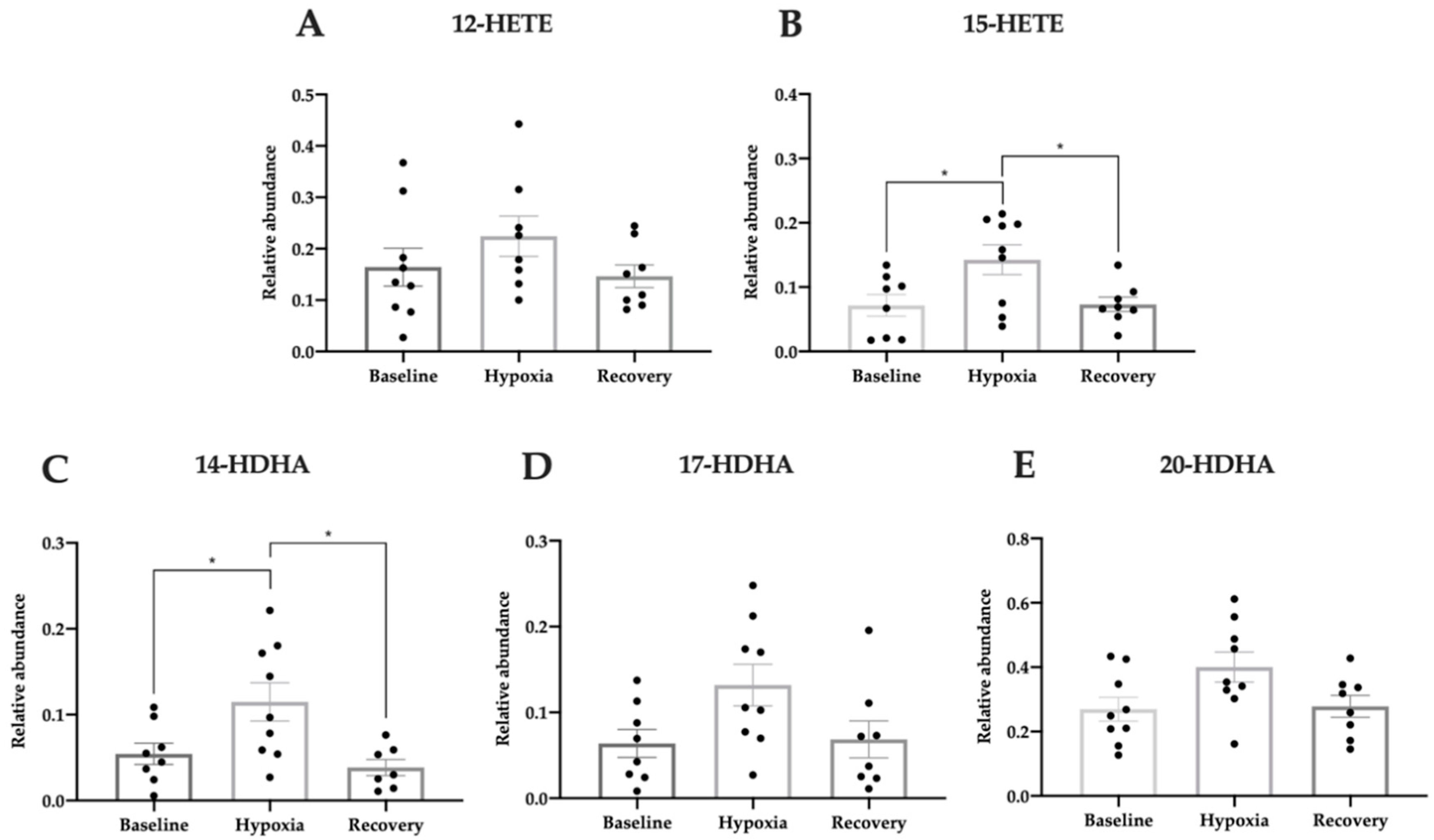
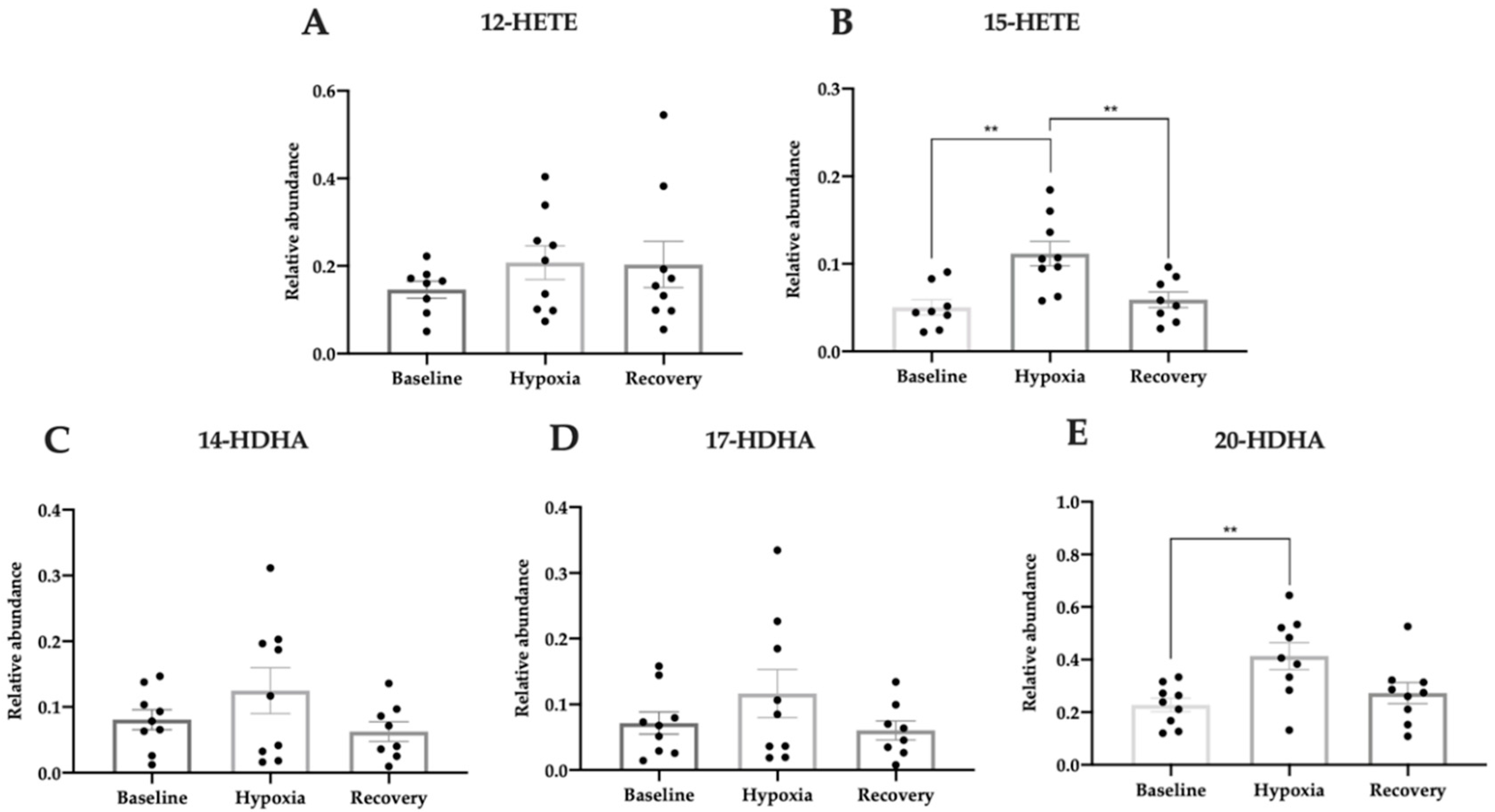
| OHTs | NTGs | Controls | |
|---|---|---|---|
| Age (years) | 72 ± 4 | 70 ± 6 | 66 ± 7 |
| Body Weight (kg) | 74.2 ± 10.2 | 73.9 ± 14.9 | 74.9 ± 7.4 |
| Height (cm) | 172 ± 8 | 174 ± 10 | 173 ± 9 |
| BMI 1 | 25 ± 2 | 24 ± 3 | 25 ± 3 |
| Gender (F/M) | 4/5 | 8/8 | 6/8 |
| OHTs | NTGs | Controls | |
|---|---|---|---|
| IOP OD (mmHg) | 28 ± 3 | 12 ± 1 **** | 13 ± 2 **** |
| IOP OS (mmHg) | 30 ± 4 | 12 ± 1 **** | 13 ± 2 **** |
| MD OD (dB) | 0.84 ± 2.73 # | 5.68 ± 6.63 | 0.96 ± 0.075 ## |
| MD OS (dB) | 0.88 ± 4.97 ## | 8.82 ± 7.87 | 0.68 ± 0.32 ### |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langbøl, M.; Saruhanian, S.; Baskaran, T.; Tiedemann, D.; Mouhammad, Z.A.; Toft-Kehler, A.K.; Jun, B.; Vohra, R.; Bazan, N.G.; Kolko, M. Increased Antioxidant Capacity and Pro-Homeostatic Lipid Mediators in Ocular Hypertension—A Human Experimental Model. J. Clin. Med. 2020, 9, 2979. https://doi.org/10.3390/jcm9092979
Langbøl M, Saruhanian S, Baskaran T, Tiedemann D, Mouhammad ZA, Toft-Kehler AK, Jun B, Vohra R, Bazan NG, Kolko M. Increased Antioxidant Capacity and Pro-Homeostatic Lipid Mediators in Ocular Hypertension—A Human Experimental Model. Journal of Clinical Medicine. 2020; 9(9):2979. https://doi.org/10.3390/jcm9092979
Chicago/Turabian StyleLangbøl, Mia, Sarkis Saruhanian, Thisayini Baskaran, Daniel Tiedemann, Zaynab A. Mouhammad, Anne Katrine Toft-Kehler, Bokkyoo Jun, Rupali Vohra, Nicolas G. Bazan, and Miriam Kolko. 2020. "Increased Antioxidant Capacity and Pro-Homeostatic Lipid Mediators in Ocular Hypertension—A Human Experimental Model" Journal of Clinical Medicine 9, no. 9: 2979. https://doi.org/10.3390/jcm9092979
APA StyleLangbøl, M., Saruhanian, S., Baskaran, T., Tiedemann, D., Mouhammad, Z. A., Toft-Kehler, A. K., Jun, B., Vohra, R., Bazan, N. G., & Kolko, M. (2020). Increased Antioxidant Capacity and Pro-Homeostatic Lipid Mediators in Ocular Hypertension—A Human Experimental Model. Journal of Clinical Medicine, 9(9), 2979. https://doi.org/10.3390/jcm9092979