Longitudinal Evaluation of Working Memory in Duchenne Muscular Dystrophy
Abstract
:1. Introduction
2. Experimental Section
2.1. Methods
2.1.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.1.2. Study Design and Subjects
2.1.3. Study Measures
2.1.4. Study Participant Grouping Based on Location of nmDMD Mutation
2.2. Statistical Analyses
3. Results
3.1. Subject Demographics
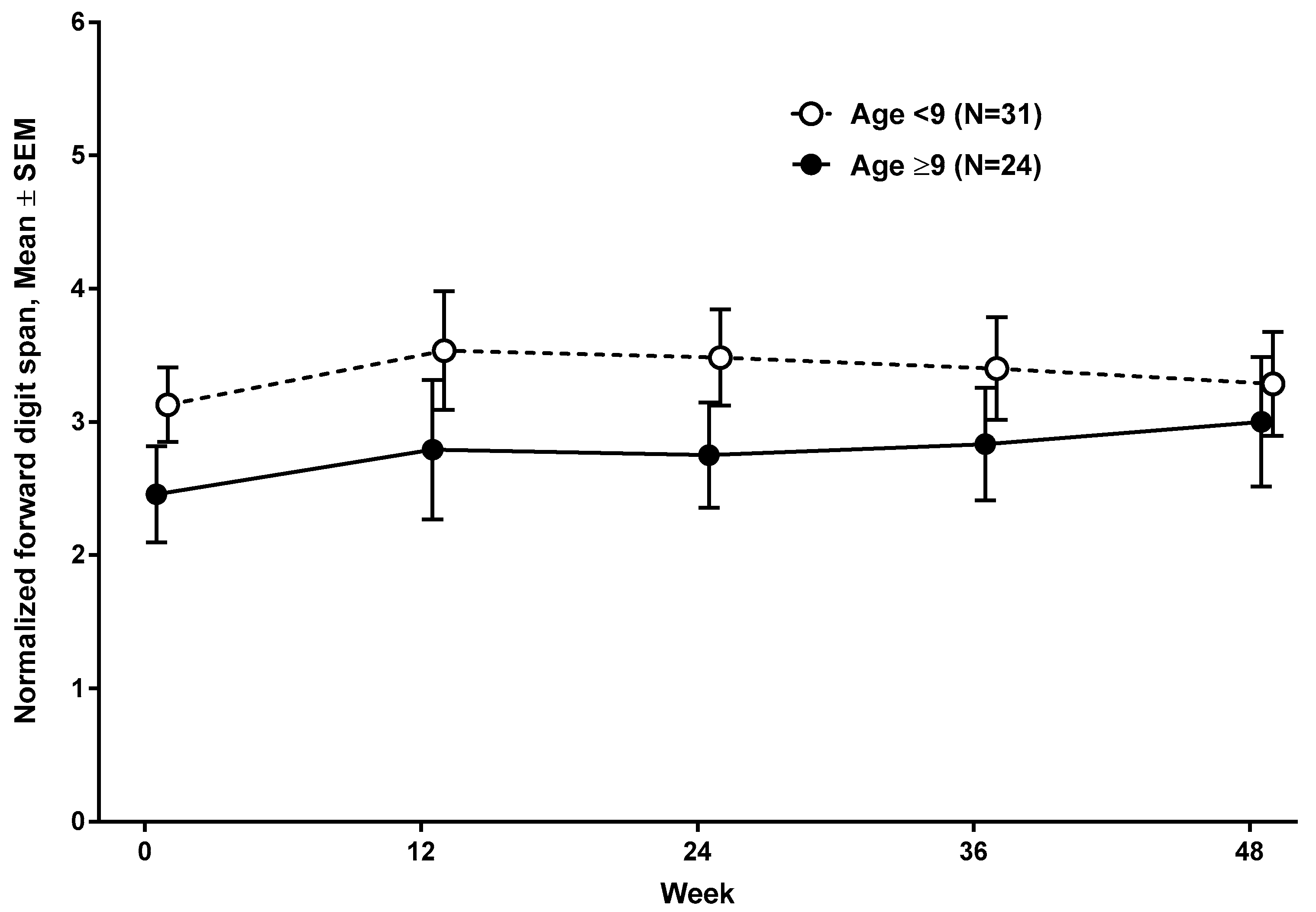
3.2. Developmental Change in Working Memory as a Function of Age, Oral Corticosteroid Treatment, and nmDMD Mutation Location
3.3. Developmental Growth of Working Memory Based on nmDMD Mutation Location
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Darras, B.T.; Urion, D.K.; Ghosh, P.S. Dystrophinopathies. In GeneReviews; Adam, M.P., Ardinger, H.H., Eds.; University of Washington: Seattle, WA, USA, 2000. [Google Scholar]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Colvin, M.K.; Cripe, L.; Herron, A.R.; Kennedy, A.; Kinnett, K.; et al. DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018, 17, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, E.P.; Brown, R.H., Jr.; Kunkel, L.M. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Muntoni, F.; Torelli, S.; Ferlini, A. Dystrophin and mutations: One gene, several proteins, multiple phenotypes. Lancet Neurol. 2003, 2, 731–740. [Google Scholar] [CrossRef]
- Lidov, H.G.; Selig, S.; Kunkel, L.M. Dp140: A novel 140 kDa CNS transcript from the dystrophin locus. Hum. Mol. Genet. 1995, 4, 329–335. [Google Scholar] [CrossRef]
- Feener, C.A.; Koenig, M.; Kunkel, L.M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature 1989, 338, 509–511. [Google Scholar] [CrossRef]
- Blake, D.J.; Hawkes, R.; Benson, M.A.; Beesley, P.W. Different dystrophin-like complexes are expressed in neurons and glia. J. Cell Biol. 1999, 147, 645–658. [Google Scholar] [CrossRef] [Green Version]
- Aranmolate, A.; Tse, N.; Colognato, H. Myelination is delayed during postnatal brain development in the mdx mouse model of Duchenne muscular dystrophy. BMC Neurosci. 2017, 18, 63. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Wu, K.; Black, I.B. Deficiency of brain synaptic dystrophin in human Duchenne muscular dystrophy. Ann. Neurol. 1995, 38, 446–449. [Google Scholar] [CrossRef]
- Eyermann, C.; Czaplinski, K.; Colognato, H. Dystroglycan promotes filopodial formation and process branching in differentiating oligodendroglia. J. Neurochem. 2012, 120, 928–947. [Google Scholar] [CrossRef]
- Galvin, J.; Eyermann, C.; Colognato, H. Dystroglycan modulates the ability of insulin-like growth factor-1 to promote oligodendrocyte differentiation. J. Neurosci. Res. 2010, 88, 3295–3307. [Google Scholar] [CrossRef] [Green Version]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar] [CrossRef]
- Ervasti, J.M.; Campbell, K.P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 1993, 122, 809–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybakova, I.N.; Patel, J.R.; Ervasti, J.M. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J. Cell Biol. 2000, 150, 1209–1214. [Google Scholar] [CrossRef]
- Ervasti, J.M.; Campbell, K.P. Dystrophin and the membrane skeleton. Curr. Opin. Cell Biol. 1993, 5, 82–87. [Google Scholar] [CrossRef]
- Connolly, A.M.; Florence, J.M.; Cradock, M.M.; Malkus, E.C.; Schierbecker, J.R.; Siener, C.A.; Wulf, C.O.; Anand, P.; Golumbek, P.T.; Zaidman, C.M.; et al. MDA DMD Clinical Research Network. Motor and cognitive assessment of infants and young boys with Duchenne Muscular Dystrophy: Results from the Muscular Dystrophy Association DMD Clinical Research Network. Neuromuscul. Disord. 2013, 23, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Nichols, B.; Takeda, S.; Yokota, T. Nonmechanical Roles of Dystrophin and Associated Proteins in Exercise, Neuromuscular Junctions, and Brains. Brain Sci. 2015, 5, 275–298. [Google Scholar] [CrossRef] [Green Version]
- Chieffo, D.; Brogna, C.; Berardinelli, A.; D’Angelo, G.; Mallardi, M.; D’Amico, A.; Alfieri, P.; Mercuri, E.; Pane, M. Early Neurodevelopmental Findings Predict School Age Cognitive Abilities in Duchenne Muscular Dystrophy: A Longitudinal Study. PLoS ONE 2015, 10, e0133214. [Google Scholar] [CrossRef] [Green Version]
- Ricotti, V.; Mandy, W.P.; Scoto, M.; Pane, M.; Deconinck, N.; Messina, S.; Mercuri, E.; Skuse, D.H.; Muntoni, F. Neurodevelopmental, emotional, and behavioral problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child Neurol. 2016, 58, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Kuban, K.C.; Allred, E.; Shapiro, F.; Darras, B.T. Association of Duchenne muscular dystrophy with autism spectrum disorder. J. Child Neurol. 2005, 20, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, J.G.; Vles, J.S. Neuropsychiatric disorders in males with duchenne muscular dystrophy: Frequency rate of attention-deficient hyperactivity disorder (ADHD), autism spectrum disorder, and obsessive-compulsive disorder. J. Child Neurol. 2008, 23, 477–481. [Google Scholar] [CrossRef]
- Cotton, N.M.; Voudouris, N.J.; Greenwood, K.M. Association between intellectual functioning and age in children and young adults with Duchenne muscular dystrophy: Further results from a meta-analysis. Dev. Med. Child Neurol. 2005, 47, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogasawara, A. Downward shift in IQ in persons with Duchenne muscular dystrophy compared to those with spinal muscular atrophy. Am. J. Ment. Retard. 1989, 93, 544–547. [Google Scholar] [PubMed]
- Billard, C.; Gillet, P.; Signoret, J.L.; Uicaut, E.; Bertrand, P.; Fardeau, M.; Barthez-Carpentier, M.A.; Santini, J.J. Cognitive functions in Duchenne muscular dystrophy: A reappraisal and comparison with spinal muscular atrophy. Neuromuscul. Disord. 1992, 2, 371–378. [Google Scholar] [CrossRef]
- Doorenweerd, N.; Straathof, C.S.; Dumas, E.M.; Spitali, P.; Ginjaar, I.B.; Wokke, B.H.; Schrans, D.G.; van den Bergen, J.C.; van Zwet, E.W.; Webb, A.; et al. Reduced cerebral grey matter and altered white matter in boys with Duchenne muscular dystrophy. Ann. Neurol. 2014, 76, 403–411. [Google Scholar] [CrossRef]
- Felisari, G.; Martinelli Boneschi, F.; Bardoni, A.; Sironi, M.; Comi, G.P.; Robotti, M.; Turconi, A.C.; Lai, M.; Corrao, G.; Bresolin, N. Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology 2000, 55, 559–564. [Google Scholar] [CrossRef]
- Dorman, C.; Hurley, A.D.; D’Avignon, J. Language and learning disorders of older boys with Duchenne muscular dystrophy. Dev. Med. Child Neurol. 1988, 30, 316–327. [Google Scholar] [CrossRef]
- Wingeier, K.; Giger, E.; Strozzi, S.; Kreis, R.; Joncourt, F.; Conrad, B.; Gallati, S.; Steinlin, M. Neuropsychological impairments and the impact of dystrophin mutations on general cognitive functioning of patients with Duchenne muscular dystrophy. J. Clin. Neurosci. 2011, 18, 90–95. [Google Scholar] [CrossRef]
- Cyrulnik, S.E.; Fee, R.J.; Batchelder, A.; Kiefel, J.; Goldstein, E.; Hinton, V.J. Cognitive and adaptive deficits in young children with Duchenne muscular dystrophy (DMD). J. Int. Neuropsychol. Soc. 2008, 14, 853–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, V.J.; De Vivo, D.C.; Nereo, N.E.; Goldstein, E.; Stern, Y. Selective deficits in verbal working memory associated with a known genetic etiology: The neuropsychological profile of Duchenne muscular dystrophy. J. Int. Neuropsychol. Soc. 2001, 7, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Cyrulnik, S.E.; Fee, R.J.; De Vivo, D.C.; Goldstein, E.; Hinton, V.J. Delayed developmental language milestones in children with Duchenne’s muscular dystrophy. J. Pediatr. 2007, 150, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Hinton, V.J.; De Vivo, D.C.; Nereo, N.E.; Goldstein, E.; Stern, Y. Poor verbal working memory across intellectual level in boys with Duchenne dystrophy. Neurology 2000, 54, 2127–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaffer, E.B.; Fee, R.J.; Hinton, V.J. Digit Span Performance in Children with Dystrophinopathy: A Verbal Span or Working Memory Contribution? J. Int. Neuropsychol. Soc. 2016, 22, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Thangarajh, M.; Elfring, G.L.; Trifillis, P.; McIntosh, J.; Peltz, S.W.; Ataluren Phase 2b Study Group. The relationship between deficit in digit span and genotype in nonsense mutation Duchenne muscular dystrophy. Neurology 2018, 91, e1215–e1219. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.K.; Willard, V.W.; Allen, T.M.; Bonner, M.J. Working memory training in survivors of pediatric cancer: A randomized pilot study. Psychooncology 2013, 22, 1856–1865. [Google Scholar] [CrossRef] [Green Version]
- Conklin, H.M.; Ogg, R.J.; Ashford, J.M.; Scoggins, M.A.; Zou, P.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Jeha, S.; et al. Computerized Cognitive Training for Amelioration of Cognitive Late Effects among Childhood Cancer Survivors: A Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 3894–3902. [Google Scholar] [CrossRef] [Green Version]
- Kerr, E.N.; Blackwell, M.C. Near-transfer effects following working memory intervention (Cogmed) in children with symptomatic epilepsy: An open randomized clinical trial. Epilepsia 2015, 56, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Bushby, K.; Finkel, R.; Wong, B.; Barohn, R.; Campbell, C.; Comi, G.P.; Connolly, A.M.; Day, J.W.; Flanigan, K.M.; Goemans, N.; et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve 2014, 50, 477–487. [Google Scholar] [CrossRef]
- Weschler, D. Weschler Intelligence Scale for Children, 4th ed.; The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Van Deutekom, J.C.; Fokkema, I.F.; Van Ommen, G.J.; Den Dunnen, J.T. Entries in the Leiden Duchenne muscular dystrophy mutation database: An overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 2006, 34, 135–144. [Google Scholar] [CrossRef]
- Juan-Mateu, J.; González-Quereda, L.; Rodríguez, M.J.; Verdura, E.; Lázaro, K.; Jou, C.; Nascimento, A.; Jiménez-Mallebrera, C.; Colomer, J.; Monges, S.; et al. Interplay between DMD point mutations and splicing signals in Dystrophinpathy phenotypes. PLoS ONE 2013, 8, e59916. [Google Scholar] [CrossRef] [Green Version]
- Gardner, R.A. Digits forward and digits backward as two separate tests: Normative data on 1567 school children. J. Clin. Child Psychol. 1981, 10, 131–135. [Google Scholar] [CrossRef]
- Gómez, C.M.; Barriga-Paulino, C.I.; Rodríguez-Martínez, E.I.; Rojas-Benjumea, M.A.; Arjona, A.; Gómez-González, J. The neurophysiology of working memory development: From childhood to adolescence and young adulthood. Rev. Neurosci. 2018, 29, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Crone, E.A.; Wendelken, C.; Donohue, S.; van Leijenhorst, L.; Bunge, S.A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. USA 2006, 103, 9315–9320. [Google Scholar] [CrossRef] [Green Version]
- Kolskår, K.K.; Alnæs, D.; Kaufmann, T.; Richard, G.; Sanders, A.M.; Ulrichsen, K.M.; Moberget, T.; Andreassen, O.A.; Nordvik, J.E.; Westlye, L.T. Key Brain Network Nodes Show Differential Cognitive Relevance and Developmental Trajectories during Childhood and Adolescence. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Durston, S.; Davidson, M.C.; Tottenham, N.; Galvan, A.; Spicer, J.; Fossella, J.A.; Casey, B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006, 9, 1–8. [Google Scholar] [CrossRef]
- Benedict, R.H.; Zgaljardic, D.J. Practice effects during repeated administrations of memory tests with and without alternate forms. J. Clin. Exp. Neuropsychol. 1998, 20, 339–352. [Google Scholar] [CrossRef]
- Wilson, B.A.; Watson, P.C.; Baddeley, A.D.; Emslie, H.; Evans, J.J. Improvement or simply practice? The effects of twenty repeated assessments on people with and without brain injury. J. Int. Neuropsychol. Soc. 2000, 6, 469–479. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W. Neuropsychological Assessment, 3rd ed.; Oxford University Press: New York, NY, USA, 1995; ISBN 978-0195090314. [Google Scholar]
- Slade, P.D.; Townes, B.D.; Rosenbaum, G.; Martins, I.P.; Luis, H.; Bernardo, M.; Martin, M.D.; Derouen, T.A. The serial use of child neurocognitive tests: Development versus practice effects. Psychol. Assess. 2008, 20, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.J.; Rourke, B.P.; Cicchetti, D.V. Reliability of tests and measures used in the neuropsychological assessment of children. Clin. Neuropsychol. 1989, 3, 353–368. [Google Scholar] [CrossRef]
- Dikmen, S.S.; Heaton, R.K.; Grant, I.; Temkin, N.R. Test-retest reliability and practice effects of expanded Halstead-Reitan Neuropsychological Test Battery. J. Int. Neuropsychol. Soc. 1999, 5, 346–356. [Google Scholar] [CrossRef]
- Hellebrekers, D.M.J.; Doorenweerd, N.; Sweere, D.J.J.; van Kuijk, S.M.J.; Aartsma-Rus, A.M.; Klinkenberg, S.; Vles, J.S.H.; Hendriksen, J.G.M. Longitudinal follow-up of verbal span and processing speed in Duchenne muscular dystrophy. Eur. J. Paediatr. Neurol. 2020, 25, 120–126. [Google Scholar] [CrossRef]
- Hardy, S.J.; Hardy, K.K.; Schatz, J.C.; Thompson, A.L.; Meier, E.R. Feasibility of Home-Based Computerized Working Memory Training with Children and Adolescents with Sickle Cell Disease. Pediatr. Blood Cancer 2016, 63, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Conklin, H.M.; Ashford, J.M.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Ogg, R.J.; Jeha, S.; Huang, L.; Zhang, H. Long-Term Efficacy of Computerized Cognitive Training among Survivors of Childhood Cancer: A Single-Blind Randomized Control Trial. J. Pediatr. Psychol. 2017, 42, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, A.; Kerr, E.N. Maintenance effects of working memory intervention (Cogmed) in children with symptomatic epilepsy. Epilepsy Behav. 2017, 67, 51–59. [Google Scholar] [CrossRef]
- Bigorra, A.; Garolera, M.; Guijarro, S.; Hervás, A. Long-term far-transfer effects of working memory training in children with ADHD: A randomized controlled trial. Eur. Child Adolesc. Psychiatry 2016, 25, 853–867. [Google Scholar] [CrossRef]
- Gathercole, S.E.; Pickering, S.J.; Knight, C.; Stegmann, Z. Working memory skills and educational attainment: Evidence from national curriculum assessments at 7 and 14 years of age. Appl. Cogn. Psychol. 2003, 18, 1–16. [Google Scholar] [CrossRef]
- Alloway, T.P.; Alloway, R.G. Investigating the predictive roles of working memory and IQ in academic attainment. J. Exp. Child Psychol. 2010, 106, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Sedek, G.; Krejtz, I.; Rydzewska, K.; Kaczan, R.; Rycielski, P. Three functional aspects of working memory as strong predictors of early school achievements: The review and illustrative evidence. Pol. Psychol. Bull. 2016, 47, 103–111. [Google Scholar] [CrossRef]
- Hinton, V.J.; De Vivo, D.C.; Fee, R.; Goldstein, E.; Stern, Y. Investigation of Poor Academic Achievement in Children with Duchenne Muscular Dystrophy. Learn. Disabil. Res. Pract. 2004, 19, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Allan, J.L.; McMinn, D.; Daly, M. A Bidirectional Relationship between Executive Function and Health Behavior: Evidence, Implications, and Future Directions. Front. Neurosci. 2016, 10, 386. [Google Scholar] [CrossRef]
- Hinson, J.M.; Jameson, T.L.; Whitney, P. Impulsive decision making and working memory. J. Exp. Psychol. Learn. Mem. Cogn. 2003, 29, 298–306. [Google Scholar] [CrossRef]
- Bickel, W.K.; Yi, R.; Landes, R.D.; Hill, P.F.; Baxter, C. Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biol. Psychiatry 2011, 69, 260–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Kawai, M.; Kimura, E.; Ogata, K.; Takahashi, T.; Kobayashi, M.; Takada, H.; Kuru, S.; Mikata, T.; Matsumura, T.; et al. Study of Duchenne muscular dystrophy long-term survivors aged 40 years and older living in specialized institutions in Japan. Neuromuscul. Disord. 2017, 27, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kieny, P.; Chollet, S.; Delalande, P.; Le Fort, M.; Magot, A.; Pereon, Y.; Perrouin Verbe, B. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann. Phys. Rehabil. Med. 2013, 56, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Griggs, R.; Bryne, B.; Connolly, A.M.; Finkel, R.; Grajkowska, L.; Haidet-Phillips, A.; Hagerty, L.; Ostrander, R.; Orlando, L.; et al. Maximizing the Benefit of Life-Saving Treatments for Pompe Disease, Spinal Muscular Atrophy, and Duchenne Muscular Dystrophy Through Newborn Screening: Essential Steps. JAMA Neurol. 2019. [Google Scholar] [CrossRef]
- Thangarajh, M.; Kaat, A.J.; Bibat, G.; Mansour, J.; Summerton, K.; Gioia, A.; Berger, C.; Hardy, K.K.; Wagner, K.R. The NIH Toolbox for cognitive surveillance in Duchenne muscular dystrophy. Ann. Clin. Transl. Neurol. 2019, 6, 1696–1706. [Google Scholar] [CrossRef] [Green Version]





| Time of Evaluation | Number of Subjects | Mean, Median (SD) | Range |
|---|---|---|---|
| Week 0 | 55 | 2.84, 3.00 (1.68) | 1–7 |
| Week 12 | 52 | 3.19, 2.50 (2.47) | 1–12 |
| Week 24 | 55 | 3.16, 3.00 (2.00) | 1–8 |
| Week 36 | 54 | 3.15, 2.50 (2.10) | 1–10 |
| Week 48 | 52 | 3.15, 3.00 (2.21) | 1–10 |
| Time of Evaluation | Number of Subjects | Mean, Median (SD) | Range |
|---|---|---|---|
| Week 0 | 54 | 1.17, 1.00 (0.54) | 1–4 |
| Week 12 | 52 | 1.23, 1.00 (0.73) | 1–5 |
| Week 24 | 54 | 1.22, 1.00 (0.77) | 1–5 |
| Week 36 | 53 | 1.13, 1.00 (0.52) | 1–4 |
| Week 48 | 52 | 1.19, 1.00 (0.66) | 1–4 |
| Location of nmDMD Mutation by DMD Exon |
|---|
| 3 |
| 4 |
| 6 |
| 7 |
| 7 |
| 7 |
| 11 |
| 12 |
| 12 |
| 14 |
| 14 |
| 15 |
| 16 |
| 18 |
| 19 |
| 20 |
| 21 |
| 21 |
| 22 |
| 23 |
| 23 |
| 24 |
| 24 |
| 25 |
| 29 |
| 29 |
| 33 |
| 33 |
| 33 |
| 35 |
| 35 |
| 38 |
| 39 |
| 40 |
| 40 |
| 44 |
| 44 |
| 44 |
| 52 |
| 55 |
| 55 |
| 55 |
| 59 |
| 60 |
| 61 |
| 61 |
| 65 |
| 66 |
| 66 |
| 68 |
| 68 |
| 70 |
| 70 |
| 70 |
| 70 |
| 70 |
| 70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangarajh, M.; Elfring, G.L.; Trifillis, P., on behalf of the Ataluren Phase b Study Group. Longitudinal Evaluation of Working Memory in Duchenne Muscular Dystrophy. J. Clin. Med. 2020, 9, 2940. https://doi.org/10.3390/jcm9092940
Thangarajh M, Elfring GL, Trifillis P on behalf of the Ataluren Phase b Study Group. Longitudinal Evaluation of Working Memory in Duchenne Muscular Dystrophy. Journal of Clinical Medicine. 2020; 9(9):2940. https://doi.org/10.3390/jcm9092940
Chicago/Turabian StyleThangarajh, Mathula, Gary L. Elfring, and Panayiota Trifillis on behalf of the Ataluren Phase b Study Group. 2020. "Longitudinal Evaluation of Working Memory in Duchenne Muscular Dystrophy" Journal of Clinical Medicine 9, no. 9: 2940. https://doi.org/10.3390/jcm9092940
APA StyleThangarajh, M., Elfring, G. L., & Trifillis, P., on behalf of the Ataluren Phase b Study Group. (2020). Longitudinal Evaluation of Working Memory in Duchenne Muscular Dystrophy. Journal of Clinical Medicine, 9(9), 2940. https://doi.org/10.3390/jcm9092940




