A Two-Year Follow-Up Cohort Study—Improved Clinical Control over CVD Risk Factors through Weight Loss in Middle-Aged and Older Adults
Abstract
1. Introduction
2. Material and Methods
2.1. Data Verification
2.2. Anthropometric Measurements
2.3. Laboratory Measurements
2.4. Definitions of Outcome Variables
2.5. The Individual Health Status Questionnaire
2.6. Statistical Analyses
2.7. Sensitivity Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bessesen, D.H. Regulation of body weight: What is the regulated parameter? Physiol. Behav. 2011, 104, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Truesdale, K.P.; Bradshaw, P.T.; Cai, J.; Stevens, J. Three-year weight change and cardiometabolic risk factors in obese and normal weight adults who are metabolically healthy: The atherosclerosis risk in communities study. Int. J. Obes. 2015, 39, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Macek, P.; Biskup, M.; Terek-Derszniak, M.; Krol, H.; Smok-Kalwat, J.; Gozdz, S.; Zak, M. Optimal cut-off values for anthropometric measures of obesity in screening for cardiometabolic disorders in adults. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zomer, E.; Gurusamy, K.; Leach, R.; Trimmer, C.; Lobstein, T.; Morris, S.; James, W.; Finer, N. Interventions that cause weight loss and the impact on cardiovascular risk factors: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 1001–1011. [Google Scholar] [CrossRef]
- 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults|JACC: Journal of the American College of Cardiology. Available online: https://www.onlinejacc.org/content/63/25_Part_B/2985?_ga=2.134127054.1332637401.1560874631-256666033.1556036509 (accessed on 3 July 2020).
- Clifton, P.M.; Keogh, J.B. Effects of Different Weight Loss Approaches on CVD Risk. Curr. Atheroscler. Rep. 2018, 20, 27. [Google Scholar] [CrossRef]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle Weight-Loss Intervention Outcomes in Overweight and Obese Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef]
- Gummesson, A.; Nyman, E.; Knutsson, M.; Karpefors, M. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1295–1305. [Google Scholar] [CrossRef]
- Itoh, H.; Kaneko, H.; Kiriyama, H.; Nakanishi, K.; Mizuno, Y.; Daimon, M.; Morita, H.; Yamamichi, N.; Komuro, I. Effect of Body Weight Change on Blood Pressure in a Japanese General Population with a Body Mass Index ≥ 22 kg/m2. Int. Hear. J. 2019, 60, 1381–1386. [Google Scholar] [CrossRef]
- Williamson, D.F.; Thompson, T.J.; Thun, M.; Flanders, D.; Pamuk, E.; Byers, T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000, 23, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.; Montani, J.-P. Pathways from dieting to weight regain, to obesity and to the metabolic syndrome: An overview. Obes. Rev. 2015, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. 2015, 39, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Fayyad, R.; Laskey, R.; Demicco, D.A.; Messerli, F.H.; Waters, D.D. Body-Weight Fluctuations and Outcomes in Coronary Disease. N. Engl. J. Med. 2017, 376, 1332–1340. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.J.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Bell, J.A.; Hamer, M.; Sabia, S.; Singh-Manoux, A.; Batty, G.D.; Kivimäki, M. The Natural Course of Healthy Obesity Over 20 Years. J. Am. Coll. Cardiol. 2015, 65, 101–102. [Google Scholar] [CrossRef]
- Bradshaw, P.T.; Reynolds, K.R.; Wagenknecht, L.E.; Ndumele, C.E.; Stevens, J. Incidence of components of metabolic syndrome in the metabolically healthy obese over 9 years follow-up: The Atherosclerosis Risk In Communities study. Int. J. Obes. 2017, 42, 295–301. [Google Scholar] [CrossRef]
- Macek, P.; Biskup, M.; Terek-Derszniak, M.; Stachura, M.; Król, H.; Góźdź, S.; Zak, M. Optimal Body Fat Percentage Cut-Off Values in Predicting the Obesity-Related Cardiovascular Risk Factors: A Cross-Sectional Cohort Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1587–1597. [Google Scholar] [CrossRef]
- Macek, P.; Zak, M.; Terek-Derszniak, M.; Biskup, M.; Ciepiela, P.; Król, H.; Smok-Kalwat, J.; Góźdź, S. Age-Dependent Disparities in the Prevalence of Single and Clustering Cardiovascular Risk Factors: A Cross-Sectional Cohort Study in Middle-Aged and Older Adults. Clin. Interv. Aging 2020, 15, 161–169. [Google Scholar] [CrossRef]
- Biskup, M.; Macek, P.; Krol, H.; Terek-Derszniak, M.; Skowronek, T.; Sosnowska-Pasiarska, B.; Góźdź, S. The relationship between a sedentary lifestyle and human health in the light of the research of PONS-Healthy Kielce. Med. Stud. 2018, 34, 25–40. [Google Scholar] [CrossRef]
- Macek, P.; Terek-Derszniak, M.; Zak, M.; Biskup, M.; Ciepiela, P.; Krol, H.; Smok-Kalwat, J.; Gozdz, S. WHO recommendations on physical activity versus compliance rate within a specific urban population as assessed through IPAQ survey: A cross-sectional cohort study. BMJ Open 2019, 9, e028334. [Google Scholar] [CrossRef] [PubMed]
- Manczuk, M.; Boffetta, P.; Sartori, S.; Hashim, D.; Vatten, L.J.; Zatonski, W.A. Cohort Profile: The Polish-Norwegian Study (PONS) cohort. Int. J. Epidemiol. 2017, 46, e5. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals With Type 2 Diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Hill, J.O. Successful Weight Loss Maintenance. Annu. Rev. Nutr. 2001, 21, 323–341. [Google Scholar] [CrossRef]
- Stevens, J.; Truesdale, K.P.; McClain, J.E.; Cai, J. The definition of weight maintenance. Int. J. Obes. 2005, 30, 391–399. [Google Scholar] [CrossRef]
- Adults (US) NOEIEP on the I Evaluation, and Treatment of Obesity. In Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults; National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1998.
- Stern, J.S.; Hirsch, J.; Blair, S.N.; Foreyt, J.P.; Frank, A.; Kumanyika, S.K.; Madans, J.H.; Marlatt, G.A.; Jeor, S.T.S.; Stunkard, A.J. Weighing the options: Criteria for evaluating weight-management programs. The Committee to Develop Criteria for Evaluating the Outcomes of Approaches to Prevent and Treat Obesity. Obes. Res. 1995, 3, 591–604. [Google Scholar] [CrossRef]
- Soleymani, T.; Daniel, S.; Garvey, W.T. Weight maintenance: Challenges, tools and strategies for primary care physicians. Obes. Rev. 2015, 17, 81–93. [Google Scholar] [CrossRef]
- Krekora-Wollny, K.; Suliga, E. Changes in body mass during weight loss treatment – a two-year prospective study. Med. Stud. 2017, 33, 290–294. [Google Scholar] [CrossRef]
- Kearns, B.; Ara, R.; Young, T.; Relton, C. Association between body mass index and health-related quality of life, and the impact of self-reported long-term conditions—Cross-sectional study from the south Yorkshire cohort dataset. BMC Public Health 2013, 13, 1009. [Google Scholar] [CrossRef]
- Atkinson, R.L. Proposed Standards for Judging the Success of the Treatment of Obesity. Ann. Intern. Med. 1993, 119, 677. [Google Scholar] [CrossRef] [PubMed]
- Kahan, S. Overweight and obesity management strategies. Am. J. Manag. Care 2016, 22, s186–s196. [Google Scholar] [PubMed]
- Dong, S.-Y.; Yan, S.-T.; Wang, M.-L.; Li, Z.-B.; Fang, L.-Q.; Zeng, Q. Associations of body weight and weight change with cardiovascular events and mortality in patients with coronary heart disease. Atherosclerosis 2018, 274, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cash, R.E.; Bower, J.K.; Focht, B.C.; Paskett, E.D. Physical activity and risk of cardiovascular disease by weight status among U.S adults. PLoS ONE 2020, 15, e0232893. [Google Scholar] [CrossRef]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Baumann, M.; Tchicaya, A.; Lorentz, N.; Le Bihan, E. Life satisfaction and longitudinal changes in physical activity, diabetes and obesity among patients with cardiovascular diseases. BMC Public Health 2017, 17, 925. [Google Scholar] [CrossRef] [PubMed]
- Grazzi, G.; Mazzoni, G.; Myers, J.; Caruso, L.; Sassone, B.; Pasanisi, G.; Guerzoni, F.; Napoli, N.; Pizzolato, M.; Zerbini, V.; et al. Impact of Improvement in Walking Speed on Hospitalization and Mortality in Females with Cardiovascular Disease. J. Clin. Med. 2020, 9, 1755. [Google Scholar] [CrossRef]
- Dunn, C.; Haubenreiser, M.; Johnson, M.; Nordby, K.; Aggarwal, S.; Myer, S.; Thomas, C. Mindfulness Approaches and Weight Loss, Weight Maintenance, and Weight Regain. Curr. Obes. Rep. 2018, 7, 37–49. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures-2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2019, 16, 175–247. [Google Scholar] [CrossRef]
- Poobalan, A.S.; Aucott, L.; Smith, W.C.S.; Avenell, A.; Jung, R.; Broom, J.; Grant, A.M. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes—A systematic review. Obes. Rev. 2004, 5, 43–50. [Google Scholar] [CrossRef]
- Wadden, T.A.; Anderson, D.A.; Foster, G.D. Two-Year Changes in L ipids and Lipoproteins Associated with the Maintenance of a 5 % to 10% Reduction in Initial Weight: Some Findings and Some Questions. Obes. Res. 1999, 7, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Nakagami, T.; Oya, J.; Takahashi, K.; Isago, C.; Kurita, M.; Tanaka, Y.; Ito, A.; Kasahara, T.; Uchigata, Y. Body Weight Reduction of 5% Improved Blood Pressure and Lipid Profiles in Obese Men and Blood Glucose in Obese Women: A Four-Year Follow-up Observational Study. Metab. Syndr. Relat. Disord. 2019, 17, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, T.; Davey, R.; Gidlow, C.; Iqbal, Z.; Kumar, J.; Mawby, Y.; Chambers, R. Contribution of Individual Risk Factor Changes to Reductions in Population Absolute Cardiovascular Risk. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Lean, M.; Powrie, J.; Anderson, A.S.; Garthwaite, P. Obesity, Weight Loss and Prognosis in Type 2 Diabetes. Diabet. Med. 1990, 7, 228–233. [Google Scholar] [CrossRef] [PubMed]
- De Leiva, A. What are the benefits of moderate weight loss? Exp. Clin. Endocrinol. Diabetes 2009, 106, 10–13. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers. Clin. Diabetes 2018, 37, 11–34. [Google Scholar] [CrossRef]
- Page, R.; Harnden, K.; Cook, J.; Turner, R. Can Life-styles of Subjects With Impaired Glucose Tolerance Be Changed? A Feasibility Study. Diabet. Med. 1992, 9, 562–566. [Google Scholar] [CrossRef]
- Bourn, D.M.; Mann, J.I.; McSkimming, B.J.; Waldron, M.A.; Wishart, J.D. Impaired Glucose Tolerance and NIDDM: Does a Lifestyle Intervention Program Have an Effect? Diabetes Care 1994, 17, 1311–1319. [Google Scholar] [CrossRef]
- Eriksson, K.-F. Prevention of Type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise The 6-year Malmö feasibility study. Diabetologia 1991, 34, 891–898. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- The Diabetes Prevention Program Research Group; Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP). Diabetes Care 2002, 25, 2165–2171. [Google Scholar] [CrossRef]
- Rücker, V.; Keil, U.; Fitzgerald, A.P.; Malzahn, U.; Prugger, C.; Ertl, G.; Heuschmann, P.U.; Neuhauser, H. Predicting 10-Year Risk of Fatal Cardiovascular Disease in Germany: An Update Based on the SCORE-Deutschland Risk Charts. PLoS ONE 2016, 11, e0162188. [Google Scholar] [CrossRef] [PubMed]
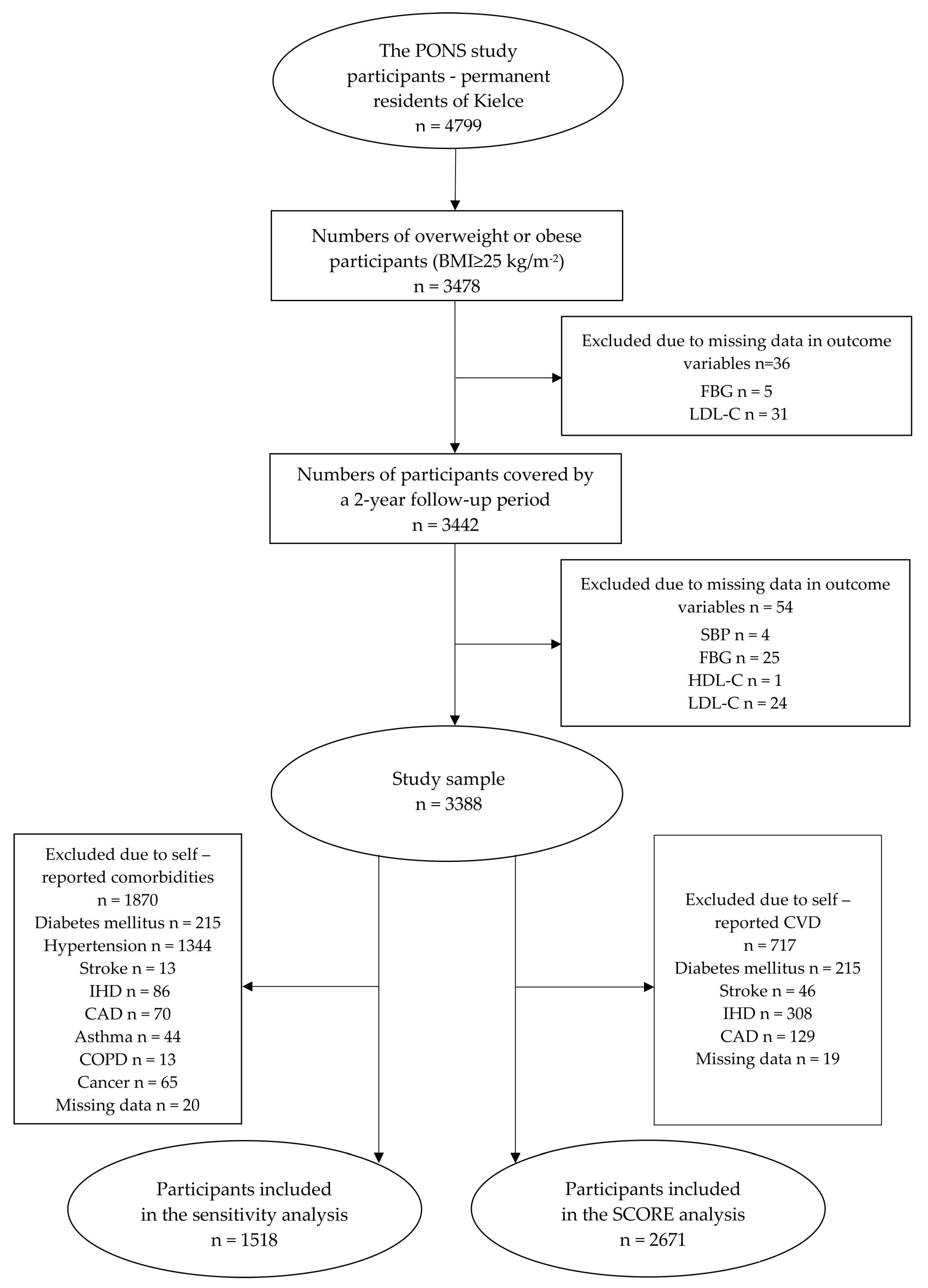
| Variables | Total | Stable | Gained | Lost | Lost | Lost | p |
|---|---|---|---|---|---|---|---|
| at Baseline | >3% and <3% | ≤3% | ≥3% and <5% | ≥5% and <10% | ≥10% | ||
| n (%) | 3388 (100) | 1975 (58.3) | 784 (23.1) | 310 (9.1) | 272 (8.0) | 47 (1.4) | <0.001 |
| Sex/men, n (%) | 1283 (37.9) | 800 (40.5) | 267 (34.1) | 109 (35.2) | 98 (36.0) | 9 (19.2) | <0.001 |
| Age (years) | 55.7 (43.0–64.0) | 55.8 (43.0–64.0) | 55.0 (43.0–64.0) | 56.6 (43.0–64.0) | 55.9 (43.0–64.0) | 55.6 (45.0–64.0) | <0.001 |
| Height (cm) | 164.8 (141.0–198.0) | 165.2 (141.0–198.0) | 164.6 (145.0–189.5) | 164.3 (145.0–190.0) | 164.3 (148.0–196.0) | 161.5 (148.0–184.0) | <0.01 |
| Weight (kg) | 80.6 (53.2–138.0) | 80.9 (53.2–138.0) | 79.7 (57.4–135.4) | 80.1 (57.8–123.8) | 81.3 (55.7–120.8) | 81.5 (61.5–120.5) | >0.05 |
| BF (%) | 34.6 (12.9–57.4) | 34.4 (12.9–57.4) | 34.6 (14.1–53.7) | 35.1 (18.9–53.7) | 35.1 (19.3–50.5) | 38.6 (20.4–47.8) | <0.001 |
| BMI (kg/m2) | 29.6 (25.0–52.3) | 29.6 (25.0–52.3) | 29.4 (25.0–47.2) | 29.6 (25.0–46.6) | 30.0 (25.0–47.8) | 31.2 (25.0–41.5) | <0.01 |
| WC (cm) | 95.2 (64.0–142) | 95.5 (68.0–135.0) | 94.2 (70.0–142) | 95.0 (71.0–140.0) | 95.7 (73.0–133.0) | 96.3 (64.0–118.0) | <0.05 |
| WHR | 0.9 (0.6–1.7) | 0.9 (0.6–1.7) | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) | 0.9 (0.7–1.0) | <0.05 |
| WHTR | 0.6 (0.4–0.9) | 0.6 (0.4–0.8) | 0.6 (0.5–0.8) | 0.6 (0.4–0.9) | 0.6 (0.5–0.8) | 0.6 (0.4–0.8) | <0.01 |
| SBP (mm/Hg) | 139.5 (92.5–230.0) | 140 (92.5–230.0) | 137.6 (93.5–207.0) | 138.6 (103.5–203.5) | 141.7 (98.0–214.0) | 144.3 (96.5–224.0) | <0.01 |
| DBP (mm/Hg) | 82.5 (50.0–136.5) | 82.9 (50.0–136.5) | 81.5 (57.5–123.0) | 81.8 (54.5–133.0) | 83.2 (56.5–114.5) | 82.3 (58.5–106.0) | <0.01 |
| FBG (mg/dL) | 99.5 (66.1–358.0) | 99.5 (70.0–358.0) | 98.1 (66.1–256.0) | 98.9 (72.0–219.0) | 102.5 (68.0–310.0) | 107.7 (75.0–238.0) | <0.05 |
| HDL-C (mg/dL) | 56.5 (20.0–134.0) | 56.1 (20.2–120.0) | 57.6 (27.0–134.0) | 55.7 (20.0–98.0) | 56.6 (27.2–104.0) | 60.8 (28.0–100.0) | <0.05 |
| LDL-C (mg/dL) | 127.1 (35.4–277.4) | 128.0 (41.2–277.4) | 124.2 (35.4–252.2) | 130.5 (52.6–211.6) | 125.6 (39.8–260.8) | 122.9 (64.7–179.2) | >0.05 |
| TC (mg/dL) | 208.1 (98.0–361.0) | 209.1 (100.0–360.0) | 204.8 (98.0–353.0) | 210.4 (103.0–316.0) | 206.6 (110.0–361.0) | 209.9 (142.9–290.0) | >0.05 |
| TG (mg/dL) | 122.2 (28.0–397.0) | 125.2 (28.0–389.0) | 114.5 (36.0–353.0) | 121.3 (37.0–397.0) | 122.1 (34.0–371.0) | 130.6 (52.0–338.0) | <0.001 |
| BMI ≥ 30, n (%) | 1248 (36.8) | 732 (37.1) | 270 (34.4) | 113 (36.5) | 109 (40.1) | 24 (51.1) | >0.5 |
| Smoker, n (%) | 483 (14.3) | 267 (13.5) | 137 (17.5) | 44 (14.2) | 29 (10.7) | 6 (12.8) | <0.05 |
| Drinker, n (%) | 2912 (86.0) | 1714 (86.8) | 667 (85.1) | 266 (85.8) | 227 (83.5) | 38 (80.9) | >0.05 |
| MVPA, n (%) | 1062 (31.4) | 653 (33.1) | 234 (29.9) | 94 (30.3) | 73 (26.8) | 8 (17.0) | <0.05 |
| Variables | Total at Baseline | Stable | Gained | Lost | Lost | Lost |
|---|---|---|---|---|---|---|
| >−3% and <3% | ≥3% | ≥3% and <5% | ≥5% and <10% | ≥10% | ||
| n (%) | 3388 (100) | 1975 (58.3) | 784 (23.1) | 310 (9.1) | 272 (8.0) | 47 (1.4) |
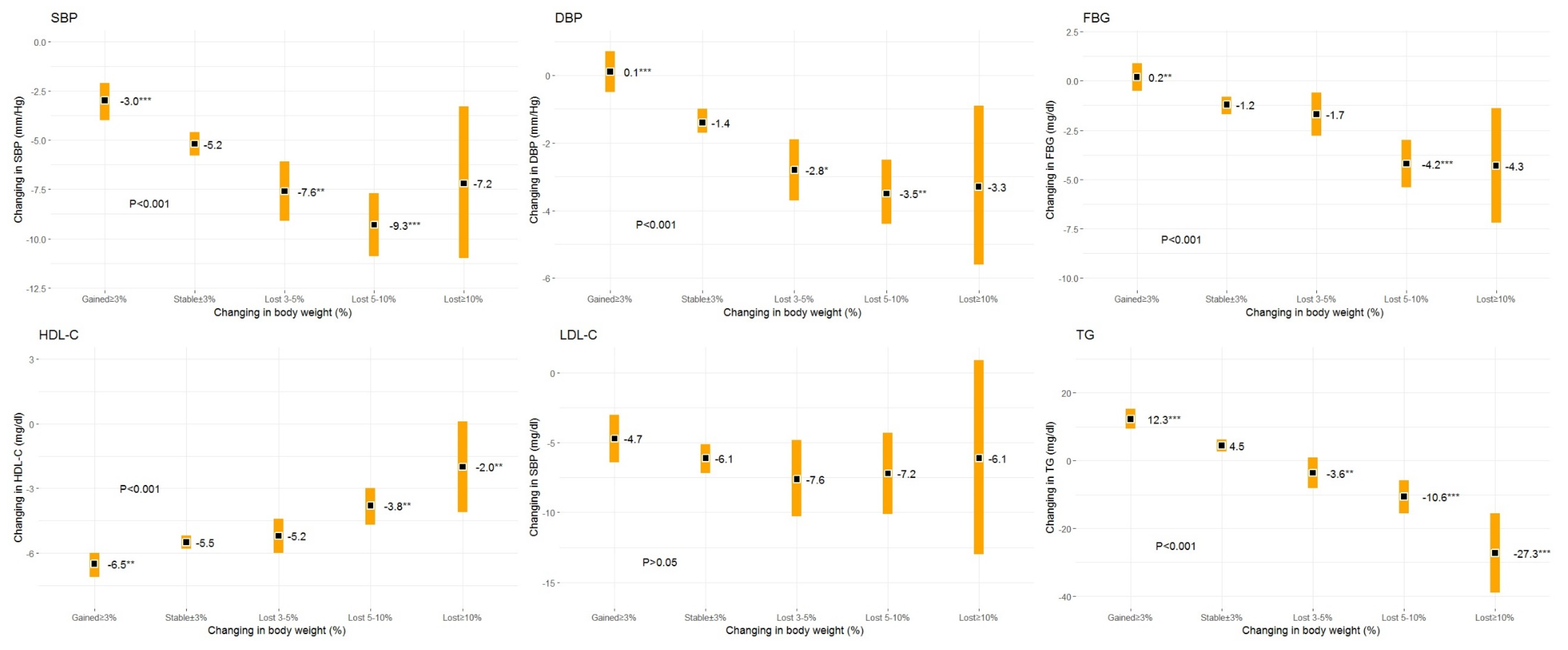
| SBP (mm/Hg) | 139.5 ± 18.9 | −4.9 ± 16.0 | −2.1 ± 16.2 *** | −6.9 ± 16.0 | −9.7 ± 16.3 *** | −9.5 ± 17.3 |
| DBP (mm/Hg) | 82.5 ± 10.2 | 1.5 ± 9.1 | 0.4 ± 9.4 *** | −2.5 ± 10.3 | −3.8 ± 9.4 *** | −3.1 ± 10.0 |
| FBG (mg/dL) | 99.5 ± 19.4 | −0.4 ± 16.3 | 1.5 ± 14.0 ** | −1.1 ± 16.8 | −4.4 ± 18.7 *** | −9.4 ± 25.1 ** |
| HDL-C (mg/dL) | 56.5 ± 13.7 | −4.7 ± 8.8 | −6.1 ± 9.5 ** | −4.2 ± 8.0 | −2.8 ± 10.4 ** | −2.3 ± 11.4 |
| LDL-C (mg/dL) | 127.1 ± 33.6 | −6.7 ± 31.7 | −3.4 ± 30.5 ** | −8.6 ± 29.0 | −6.3 ± 31.0 | −2.5 ± 29.3 |
| TG (mg/dL) | 122.2 ± 58.1 | 7.9 ± 54.4 | 21.5 ± 52.0 *** | −1.4 ± 47.1 *** | −5.3 ± 55.5 *** | −30.9 ± 58.8 *** |
| Variables | Gained ≥3% | Stable >−3% and <3% | Lost ≥3 to <5% | Lost ≥5 to <10% | Lost ≥10% |
|---|---|---|---|---|---|
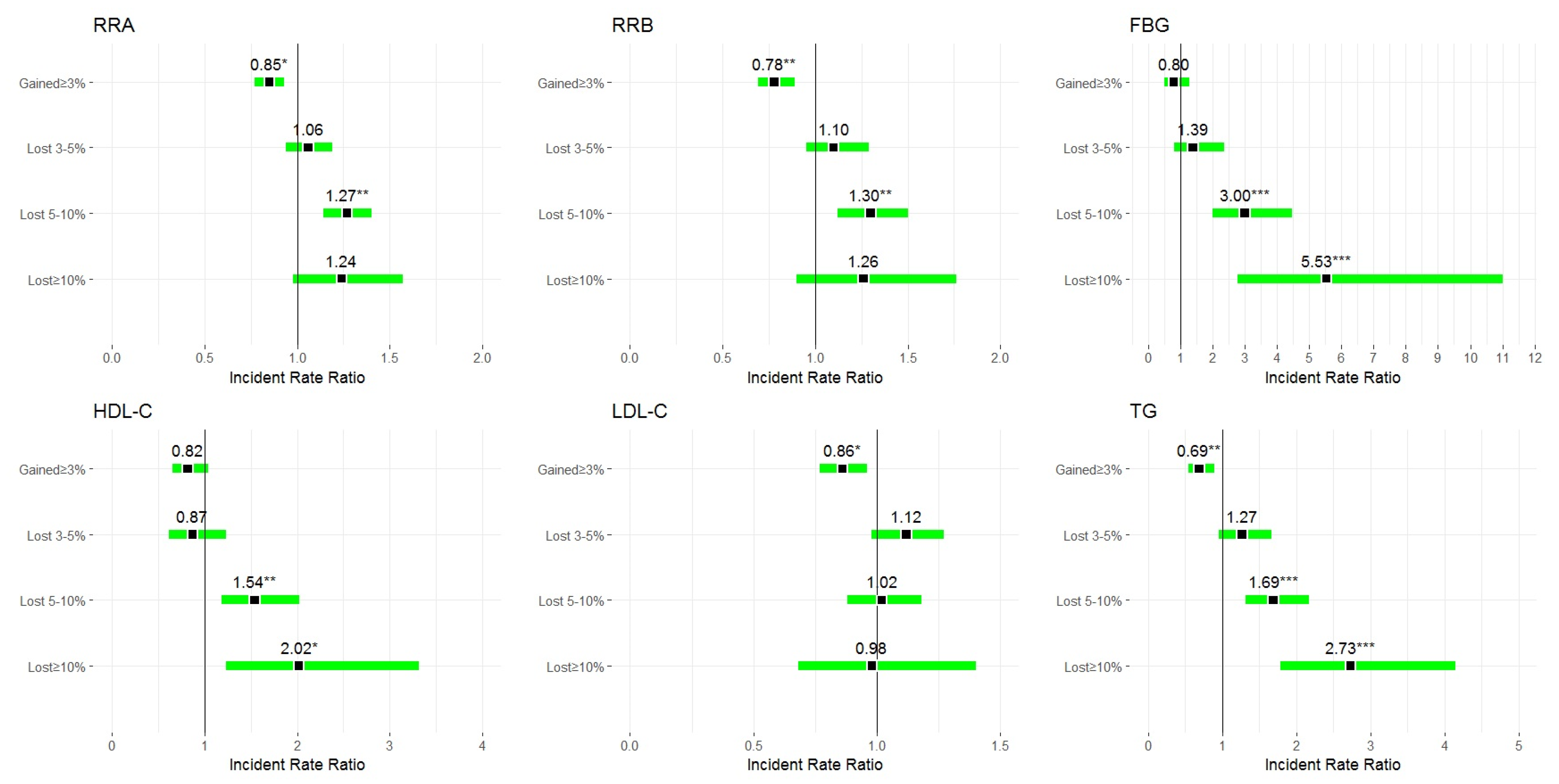
| SBP 5 mm/Hg decrease | 0.81 (0.70, 0.93) * | 1 (ref) | 0.99 (0.83, 1.18) | 1.40 (1.22, 1.60) ** | 1.15 (0.74, 1.77) |
| DBP 5 mm/Hg decrease | 0.74 (0.60, 0.90) * | 1 (ref) | 1.21 (0.97, 1.51) | 1.45 (1.18, 1.78) * | 1.27 (0.72, 2.24) |
| FBG 20 mg/dL decrease | 1.60 (0.82, 3.10) | 1 (ref) | 2.19 (0.99, 4.83) | 3.34 (1.64, 6.79) ** | 2.58 (0.34, 19.71) |
| HDL-C 5 mg/dL increase | 0.80 (0.56, 1.15) | 1 (ref) | 1.11 (0.70, 1.78) | 1.99 (1.37, 2.91) ** | 2.11 (0.89, 5.01) |
| LDL-C 10 mg/dL decrease | 0.80 (0.67, 0.95) * | 1 (ref) | 1.07 (0.88, 1.31) | 1.04 (0.83, 1.29) | 1.29 (0.80, 2.10) |
| TGC 40 mg/dL decrease | 0.73 (0.50, 1.06) | 1 (ref) | 1.56 (1.08, 2.25) * | 1.80 (1.23, 2.64) ** | 2.11 (0.85, 5.25) |
| Absolute | Total | Stable | Gained | Lost | Lost | Lost | p |
|---|---|---|---|---|---|---|---|
| Risk | >−3% and <3% | ≥3% | ≥3% and <5% | ≥5% and <10% | ≥10% | ||
| Baseline | <0.001 | ||||||
| Low | 223 (8.3) | 127 (8.2) | 66 (10.4) | 14 (5.8) | 14 (6.6) | 2 (5.9) | |
| Moderate | 1859 (69.6) | 1035 (66.9) | 454 (71.4) | 176 (72.4) | 165 (78.2) | 29 (85.3) | |
| High to very high | 589 (22.1) | 385 (24.9) | 116 (18.2) | 53 (21.8) | 32 (15.2) | 3 (8.8) | |
| Follow-up | <0.001 | ||||||
| Low | 226 (8.5) | 127 (8.2) | 71 (11.2) | 13 (5.4) | 13 (6.2) | 2 (5.9) | |
| Moderate | 1949 (73.0) ** | 1101 (71.2) ** | 458 (72.0) | 186 (76.5) | 174 (82.5) | 30 (88.2) | |
| High to very high | 496 (18.6) ** | 319 (20.6) ** | 107 (16.8) * | 44 (18.1) | 24 (11.4) | 2 (5.9) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macek, P.; Terek-Derszniak, M.; Biskup, M.; Krol, H.; Smok-Kalwat, J.; Gozdz, S.; Zak, M. A Two-Year Follow-Up Cohort Study—Improved Clinical Control over CVD Risk Factors through Weight Loss in Middle-Aged and Older Adults. J. Clin. Med. 2020, 9, 2904. https://doi.org/10.3390/jcm9092904
Macek P, Terek-Derszniak M, Biskup M, Krol H, Smok-Kalwat J, Gozdz S, Zak M. A Two-Year Follow-Up Cohort Study—Improved Clinical Control over CVD Risk Factors through Weight Loss in Middle-Aged and Older Adults. Journal of Clinical Medicine. 2020; 9(9):2904. https://doi.org/10.3390/jcm9092904
Chicago/Turabian StyleMacek, Pawel, Malgorzata Terek-Derszniak, Malgorzata Biskup, Halina Krol, Jolanta Smok-Kalwat, Stanislaw Gozdz, and Marek Zak. 2020. "A Two-Year Follow-Up Cohort Study—Improved Clinical Control over CVD Risk Factors through Weight Loss in Middle-Aged and Older Adults" Journal of Clinical Medicine 9, no. 9: 2904. https://doi.org/10.3390/jcm9092904
APA StyleMacek, P., Terek-Derszniak, M., Biskup, M., Krol, H., Smok-Kalwat, J., Gozdz, S., & Zak, M. (2020). A Two-Year Follow-Up Cohort Study—Improved Clinical Control over CVD Risk Factors through Weight Loss in Middle-Aged and Older Adults. Journal of Clinical Medicine, 9(9), 2904. https://doi.org/10.3390/jcm9092904






