Time Trends of Crohn’s Disease in Catalonia from 2011 to 2017. Increasing Use of Biologics Correlates with a Reduced Need for Surgery
Abstract
1. Introduction
2. Material and Methods
2.1. Statistical Methods
2.2. Ethical Issues
3. Results
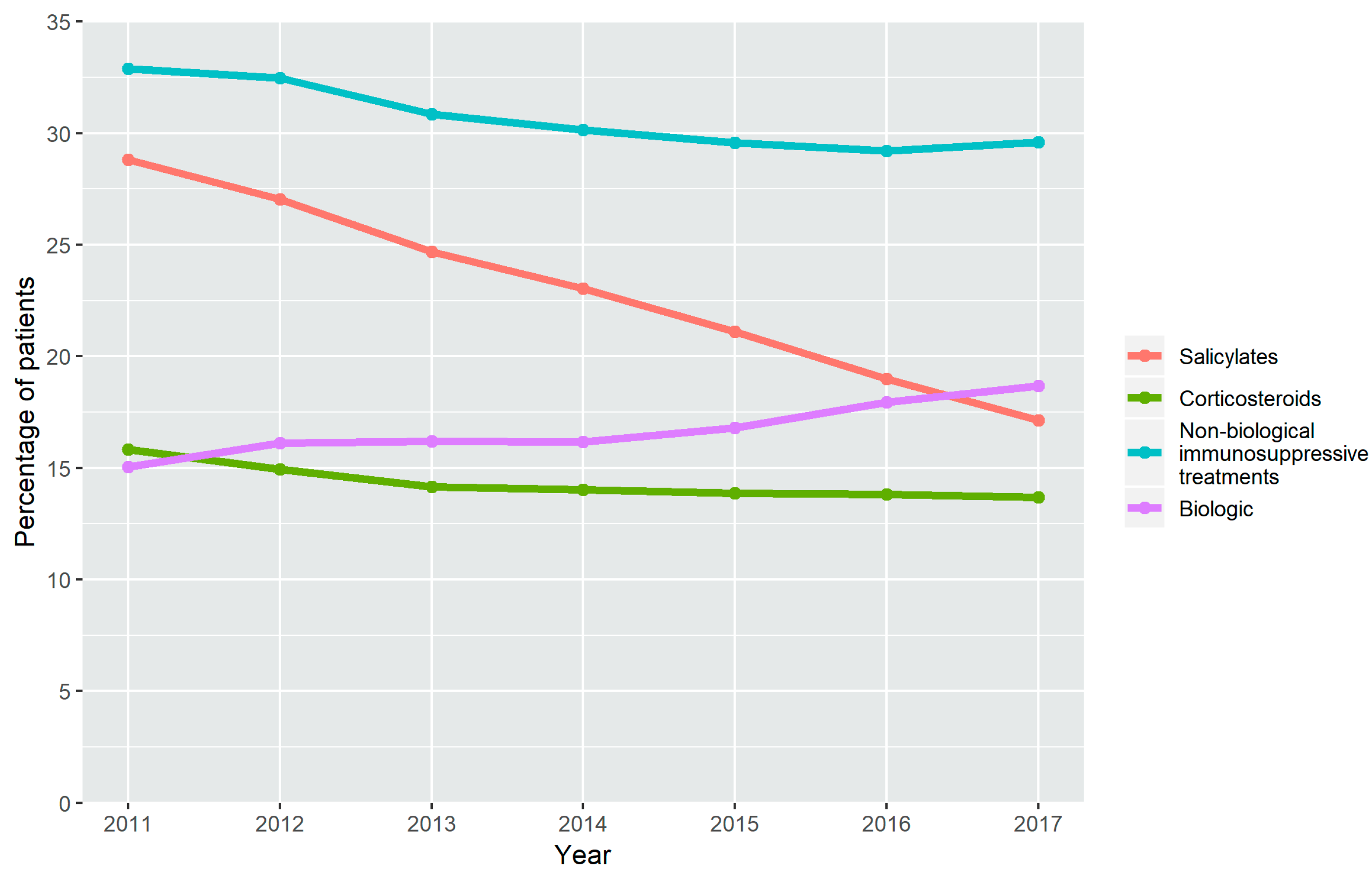
3.1. Treatment Time Trends in the Use of Drugs
3.2. Surgical Procedures
3.3. Hospital Admissions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Kiudelis, G.; Kupcinskas, L.; Kievit, H.A.L.; Andersen, K.W.; Andersen, V.; Salupere, R.; Pedersen, N.; Kjeldsen, J.; D’Incà, R.; et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: An Epi-IBD study. Gut 2019, 68, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Pajares, J.M.; Gisbert, J.P.; Pajares, J.M.; Gisbert, J.P. Epidemiology of inflammatory bowel disease in Spain. A systematic review. Rev. Esp. Enferm. Dig. 2001, 93, 9–20. [Google Scholar] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Golovics, P.A.; Mandel, M.D.; Lovasz, B.D.; Lakatos, P.L. Inflammatory bowel disease course in Crohn’s disease: Is the natural history changing? World J. Gastroenterol. 2014, 20, 3198–3207. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Van Assche, G.; Sandborn, W.J.; Douglas, W.C.; Geboes, K.; Colombel, J.F.; Reinisch, W.; Kumar, A.; Lazar, A.; Camez, A.; et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: Data from the EXTEND trial. Gastroenterology 2012, 142, 1102–1111. [Google Scholar] [CrossRef]
- Colombel, J.F.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rutgeerts, P.; Tang, K.L.; Oortwijn, A.; Bevelander, G.S.; Cornillie, F.J.; Sandborn, W.J. Randomised clinical trial: Deep remission in biologic and immunomodulator naïve patients with Crohn’s disease—A SONIC post hoc analysis. Aliment. Pharmacol. Ther. 2015, 41, 734–746. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, A.; et al. Manteniance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Colombel, J.; Sandborn, W.J.; Rutgeerts, P.; Enns, R.; Hanauer, S.B.; Panaccione, R.; Schreiber, S.; Byczkowski, D.; Li, J.; Kent, J.D.; et al. Adalimumab for Maintenance of Clinical Response and Remission in Patients with Crohn’s Disease: The CHARM Trial. Gastroenterology 2006, 132, 52–65. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Sands, B.E.; Gasink, C.; Yeager, B.; Jacobstein, D.; Gao, L.L.; Daly, K.; Ghosh, S.; Rutgeerts, P.; Hanauer, S.B.; et al. Sa1743 Reduced Rates of Crohn’s- Related Surgeries; Hospitalizations and Alternate Biologic Initiation with Ustekinumab in the Im-Uniti Study Through 2 Years. Gastroenterology 2018, 154, S-377–S-378. [Google Scholar] [CrossRef]
- Vester-Andersen, M.K.; Prosberg, M.V.; Jess, T.; Andersson, M.; Bengtsson, B.G.; Blixt, T.; Munkholm, P.; Bendtsen, F.; Vind, I. Disease course and surgery rates in inflammatory bowel disease: A population-based, 7-year follow-up study in the era of immunomodulating therapy. Am. J. Gastroenterol. 2014, 109, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Rungoe, C.; Langholz, E.; Andersson, M.; Basit, S.; Nielsen, N.M.; Wohlfahrt, J.; Jess, T. Changes in medical treatment and surgery rates in inflammatory bowel disease: A nationwide cohort study 1979–2011. Gut 2014, 63, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Holko, P.; Kawalec, P.; Pilc, A. Impact of biologic treatment of Crohn’s disease on the rate of surgeries and other healthcare resources: An analysis of a nationwide database from Poland. Front. Pharmacol. 2018, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Verdon, C. Surgery and hospitalisations rates in inflammatory bowel disease patients in the Québec provincial database from 1996. In Proceedings of the 14th Congress of ECCO, Copenhagen, Denmark, 6–9 March 2019. [Google Scholar]
- Sonnenberg, A. Hospitalization for inflammatory bowel disease in the United States between 1970 and 2004. J. Clin. Gastroenterol. 2009, 43, 297–300. [Google Scholar] [CrossRef]
- Agència de Qualitat i Avaluació Sanitàries de Catalunya; Departament de Salut; Generalitat de Catalunya; Observatori del Sistema de Salut de Catalunya. Desigualtats Socioeconòmiques en la Salut i la Utilització dels Serveis Sanitaris públics en la Població de Catalunya. Observatori sobre els efectes de la crisi en la salut de la població. Barcelona. 2017. Available online: http//observatorisalut.gencat.cat/web/.content/minisite/observatorisalut/ossc_crisi_salut/Fitxers_crisi/Salut_crisi_informe_2016.pdf; http://observatorisalut.gencat.cat (accessed on 27 October 2017).
- Brunet, E.; Roig-Ramos, C.; Vela, E.; Clèries, M.; Melcarne, L.; Villòria, A.; Pontes, C.; Calvet, X. Prevalence, incidence and mortality of inflammatory bowel disease in Catalonia. A population-based analysis. Ann. Med. 2018, 50, 613–619. [Google Scholar] [CrossRef]
- The PLOS Medicine Editors. Observational studies: Getting clear about transparency. PLoS Med. 2014, 11, e1001711. [Google Scholar]
- Rahman, A.; Jairath, V.; Feagan, B.G.; Khanna, R.; Shariff, S.Z.; Allen, B.N.; Jenkyn, K.B.; Vinden, C.; Jeyarajah, J.; Mosli, M.; et al. Declining hospitalisation and surgical intervention rates in patients with Crohn’s disease: A population-based cohort. Aliment. Pharmacol. Ther. 2019, 50, 1086–1093. [Google Scholar] [CrossRef]
- Yu, H.; MacIsaac, D.; Wong, J.J.; Sellers, Z.M.; Wren, A.A.; Bensen, R.; Kin, C.; Park, K.T. Market Share and Costs of Biologic Therapies for Inflammatory Bowel Disease in the United States. Aliment. Pharmacol. Ther. 2018, 47, 364–370. [Google Scholar] [CrossRef]
- Lirhus, S.S.; Høivik, M.L.; Moum, B.; Melberg, H.O. Regional differences in anti-TNF-α therapy and surgery in the treatment of inflammatory bowel disease patients: A Norwegian nationwide cohort study. Scand. J. Gastroenterol. 2018, 53, 952–957. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Lemaitre, M.; Rudnichi, A.; Racine, A.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: Analysis of the French administrative health databases 2009–2014. Aliment. Pharmacol. Ther. 2017, 45, 37–49. [Google Scholar] [CrossRef]
- Kurti, Z.; Vegh, Z.; Golovics, P.A.; Fadgyas-Freyler, P.; Gecse, K.B.; Gonczi, L.; Gimesi-Orszagh, J.; Lovasz, B.D.; Lakatos, P.L. Nationwide prevalence and drug treatment practices of inflammatory bowel diseases in Hungary: A population-based study based on the National Health Insurance Fund database. Dig. Liver Dis. 2016, 48, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Vegh, Z.; Burisch, J.; Pedersen, N.; Kaimakliotis, I.; Duricova, D.; Bortlik, M.; Vinding, K.K.; Avnstrøm, S.; Olsen, J.; Nielsen, K.R.; et al. Treatment Steps, Surgery, and Hospitalization Rates During the First Year of Follow-up in Patients with Inflammatory Bowel Diseases from the 2011 ECCO-Epicom Inception Cohort. J. Crohn’s Colitis 2015, 9, 747–753. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Annese, V.; Duricova, D.; Gower-Rousseau, C.; Jess, T.; Langholz, E. Impact of New Treatments on Hospitalisation, Surgery, Infection, and Mortality in IBD: A Focus Paper by the Epidemiology Committee of ECCO. J. Crohn’s Colitis 2016, 10, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Chachu, K.A.; Vajravelu, R.K.; Vaughn, B.P.; Ni, J.; Osterman, M.T.; Cheifetz, A.S. Improved Long-term Outcomes of Patients with Inflammatory Bowel Disease Receiving Proactive Compared with Reactive Monitoring of Serum Concentrations of Infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, 1580–1588.e3. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Loftus, E.V.; Ng, S.C.; Lakatos, P.L.; Moum, B. Hospitalisations and surgery in Crohn’s disease. Gut 2012, 61, 622–629. [Google Scholar] [CrossRef]
- Mao, E.J.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 3–13. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Leombruno, J.P.; Nguyen, G.C.; Grootendorst, P.; Juurlink, D.; Einarson, T. Hospitalization and surgical rates in patients with Crohn’s disease treated with infliximab: A matched analysis. Pharmacoepidemiol. Drug Saf. 2011, 20, 838–848. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Cosnes, J. Complications and surgery in the inflammatory bowel diseases biological era. Curr. Opin. Gastroenterol. 2014, 30, 378–384. [Google Scholar] [CrossRef]
- Lazarev, M.; Saul, M.; Kip, K.E.; Regueiro, M.; Schraut, W.H.; Ullman, T. Small bowel resection rates in Crohnʼs disease and the indication for surgery over time: Experience from a large tertiary care center. Inflamm. Bowel Dis. 2009, 16, 830–835. [Google Scholar] [CrossRef]
- Malarcher, C.A.; Wheaton, A.G.; Liu, Y.; Greenlund, S.F.; Greenlund, S.J.; Lu, H.; Croft, J.B. Hospitalizations for Crohn’s Disease—United States, 2003–2013. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 377–381. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Underwood, F.E.; Panaccione, N.; Quan, J.; Windsor, W.; Kotze, P.G.; Ng, S.C.; Ghosh, S.; Lakatos, P.L.; Jess, T.; et al. Trends in hospitalisation rates for inflammatory bowel disease in western versus newly industrialised countries: A population-based study of countries in the Organisation for Economic Co-operation and Development. Lancet Gastroenterol. Hepatol. 2019, 4, 287–295. [Google Scholar] [CrossRef]
- Burisch, J.; Pedersen, N.; Cukovic-Cavka, S.; Turk, N.; Kaimakliotis, I.; Duricova, D.; Shonová, O.; Vind, I.; Avnstrøm, S.; Thorsgaard, N.; et al. Initial disease course and treatment in an inflammatory bowel disease inception cohort in Europe: The ECCO-EpiCom cohort. Inflamm. Bowel Dis. 2014, 20, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K.; Begum, J.; Benchimol, E.I.; Bernstein, C.N.; Kaplan, G.G.; McCurdy, J.D.; Singh, H.; Targownik, L.; Taljaard, M. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut 2020, 69, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Golovics, P.A.; Lakatos, L.; Mandel, M.D.; Lovasz, B.D.; Vegh, Z.; Kurti, Z.; Szita, I.; Kiss, L.S.; Pandur, T.; Lakatos, P.L. Prevalence and predictors of hospitalization in Crohn’s disease in a prospective population-based inception cohort from 2000–2012. World J. Gastroenterol. 2017, 21, 7272–7280. [Google Scholar] [CrossRef]
- Lakatos, P.L.; David, G.; Pandur, T.; Erdelyi, Z.; Mester, G.; Balogh, M.; Szipocs, I.; Molnar, C.; Komaromi, E.; Kiss, L.S.; et al. IBD in the elderly population: Results from a population-based study in Western Hungary, 1977–2008. J. Crohn’s Colitis 2011, 5, 5–13. [Google Scholar] [CrossRef]
- Shen, H.; Lipka, S.; Katz, S. Increased hospitalizations in elderly with inflammatory bowel disease on anti-tumor necrosis factor therapy but not increased infections: A community practice experience. J. Crohn’s Colitis 2014, 8, 898–899. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunet, E.; Vela, E.; Melcarne, L.; Clèries, M.; Pontes, C.; Llovet, L.P.; García-Iglesias, P.; Gallach, M.; Villòria, A.; Vergara, M.; et al. Time Trends of Crohn’s Disease in Catalonia from 2011 to 2017. Increasing Use of Biologics Correlates with a Reduced Need for Surgery. J. Clin. Med. 2020, 9, 2896. https://doi.org/10.3390/jcm9092896
Brunet E, Vela E, Melcarne L, Clèries M, Pontes C, Llovet LP, García-Iglesias P, Gallach M, Villòria A, Vergara M, et al. Time Trends of Crohn’s Disease in Catalonia from 2011 to 2017. Increasing Use of Biologics Correlates with a Reduced Need for Surgery. Journal of Clinical Medicine. 2020; 9(9):2896. https://doi.org/10.3390/jcm9092896
Chicago/Turabian StyleBrunet, Eduard, Emili Vela, Luigi Melcarne, Montserrat Clèries, Caridad Pontes, Laura Patricia Llovet, Pilar García-Iglesias, Marta Gallach, Albert Villòria, Mercedes Vergara, and et al. 2020. "Time Trends of Crohn’s Disease in Catalonia from 2011 to 2017. Increasing Use of Biologics Correlates with a Reduced Need for Surgery" Journal of Clinical Medicine 9, no. 9: 2896. https://doi.org/10.3390/jcm9092896
APA StyleBrunet, E., Vela, E., Melcarne, L., Clèries, M., Pontes, C., Llovet, L. P., García-Iglesias, P., Gallach, M., Villòria, A., Vergara, M., & Calvet, X. (2020). Time Trends of Crohn’s Disease in Catalonia from 2011 to 2017. Increasing Use of Biologics Correlates with a Reduced Need for Surgery. Journal of Clinical Medicine, 9(9), 2896. https://doi.org/10.3390/jcm9092896




