Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient’s Selection
2.2. Procedures and Instruments
2.3. Outcome Measures and Analyzed Confounders
2.4. Statistical Analysis
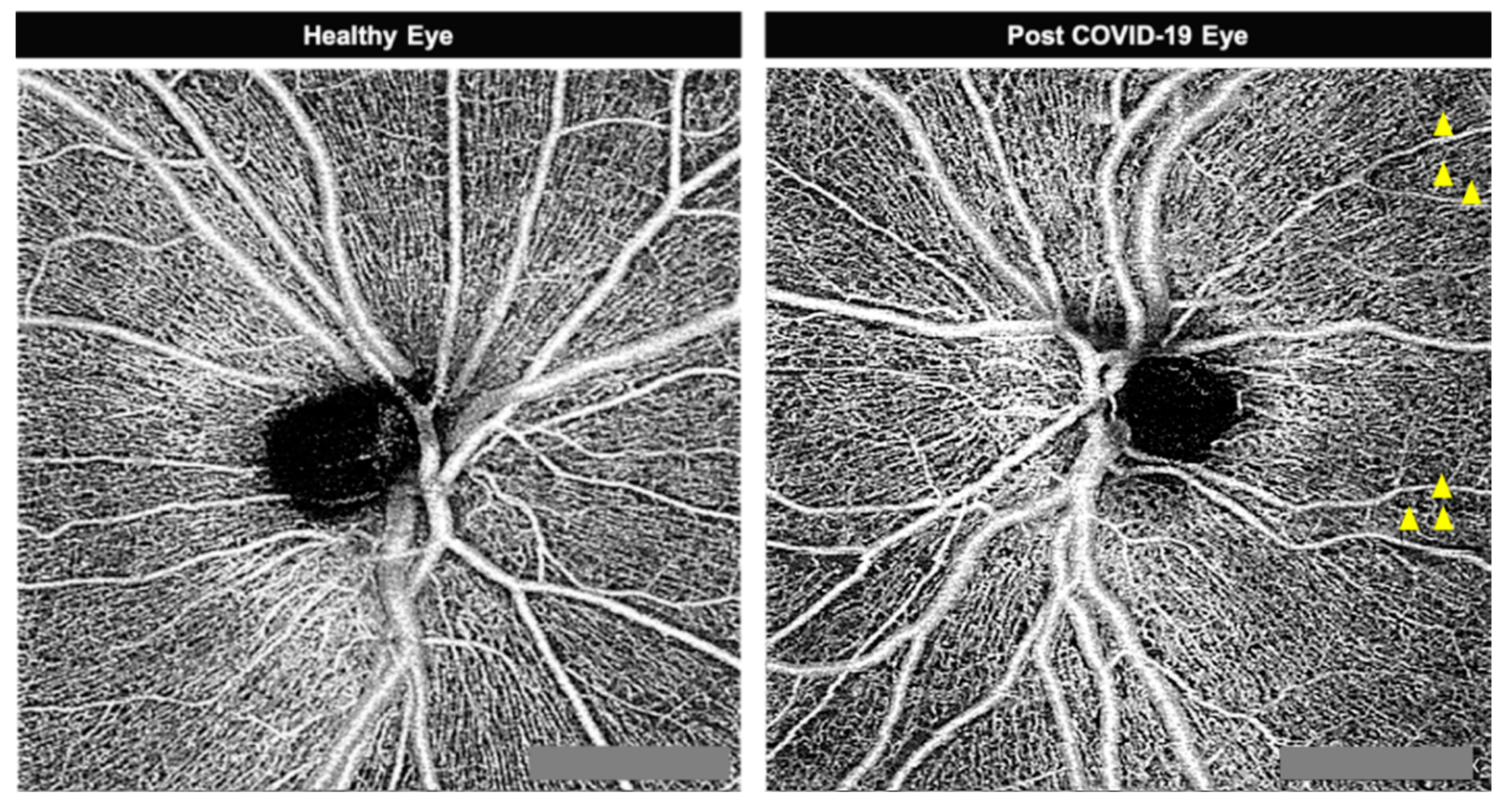
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Appl. Microbiol. 2018, 100, 163–188. [Google Scholar]
- Loon, S.C.; Lun, K. SARS: A timely reminder. Br. J. Ophthalmol. 2013, 97, 1217–1218. [Google Scholar] [CrossRef] [PubMed]
- WHO. Summary Table of SARS Cases by Country N–A; World Health Organisation: Geneva, Switzerland, 1 November 2002. [Google Scholar]
- WHO. Organisation WH Coronavirus Disease 2019 (COVID-19); Situation Report 32; World Health Organisation: Geneva, Switzerland, March 2020. [Google Scholar]
- WHO. Organisation WH Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV); World Health Organisation: Geneva, Switzerland, March 2020. [Google Scholar]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19; World Health Organisation: Geneva, Switzerland, 29 June 2020. [Google Scholar]
- Martini, N.; Piccinni, C.; Pedrini, A.; Maggioni, A. CoViD-19 and chronic diseases: Current knowledge, future steps and the MaCroScopio project. Recenti Prog. Med. 2020, 111, 198–201. [Google Scholar] [PubMed]
- Amesty, M.A.; Del Barrio, J.L.A.; Alio, J.L. COVID-19 Disease and Ophthalmology: An Update. Ophthalmol. Ther. 2020, 1–12. Available online: https://doi.org/10.1007/s40123-020-00260-y (accessed on 13 July 2020). [CrossRef] [PubMed]
- Emparan, J.P.O.; Sardi-Correa, C.; López-Ulloa, J.A.; Viteri-Soria, J.; Penniecook, J.A.; Jimenez-Román, J.; Lansingh, V.C. COVID-19 and the eye: How much do we really know? A best evidence review. Arq. Bras. Oftalmol. 2020, 83. [Google Scholar] [CrossRef]
- Bayyoud, T.; Iftner, A.; Iftner, T.; Bartz-Schmidt, K.U.; Ueffing, M.; Schindler, M.; Thaler, S. Absence of Severe Acute Respiratory Syndrome-Coronavirus-2 RNA in ocular tissues. Am. J. Ophthalmol. Case Rep. 2020, 19, 100805. [Google Scholar] [CrossRef]
- Güemes-Villahoz, N.; Burgos-Blasco, B.; Vilela, A.A.; Arriola-Villalobos, P.; Luna, C.M.R.; Sardiña, R.C.; Delgado-Iribarren, A.; García-Feijoó, J.; García-Feijoó, J. Detecting SARS-CoV-2 RNA in conjunctival secretions: Is it a valuable diagnostic method of COVID-19? J. Med Virol. 2020. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.26219 (accessed on 13 July 2020). [CrossRef]
- Atum, M.; Boz, A.A.E.; Çakır, B.; Karabay, O.; Köroğlu, M.; Öğütlü, A.; Alagöz, G. Evaluation of Conjunctival Swab PCR Results in Patients with SARS-CoV-2 Infection. Ocul. Immunol. Inflamm. 2020, 22, 1–4. [Google Scholar] [CrossRef]
- Valente, P.; Iarossi, G.; Federici, M.; Petroni, S.; Palma, P.; Cotugno, N.; De Ioris, M.A.; Campana, A.; Buzzonetti, L. Ocular manifestations and viral shedding in tears of pediatric patients with coronavirus disease 2019: A preliminary report. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7282793/ (accessed on 13 July 2020). [CrossRef]
- Doherty, M.J. Ocular manifestations of feline infectious peritonitis. J. Am. Vet. Med. Assoc. 1971, 159, 417–424. [Google Scholar]
- Robbins, S.G.; Detrick, B.; Hooks, J.J. Retinopathy Following Intravitreal Injection of Mice with MHV Strain JHM. Adv. Exp. Med. Biol. 1990, 276, 519–524. [Google Scholar] [PubMed]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Provis, J. Development of the primate retinal vasculature. Prog. Retin. Eye Res. 2001, 20, 799–821. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Weinhaus, R.S.; Choi, J.C. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis). J. Neurosci. 1992, 12, 1169–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Huang, D.; Wilson, D.J.; Jia, Y. Reflectance-based projection-resolved optical coherence tomography angiography. Biomed. Opt. Express 2017, 8, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, E.; An, D.; McAllister, I.L.; Yu, D.-Y.; Balaratnasingam, C. Three-Dimensional Microscopy Demonstrates Series and Parallel Organization of Human Peripapillary Capillary Plexuses. Investig. Opthalmol. Vis. Sci. 2018, 59, 4327–4344. [Google Scholar] [CrossRef]
- Rabiolo, A.; Carnevali, A.; Bandello, F.; Querques, G. Optical coherence tomography angiography: Evolution or revolution? Expert Rev. Ophthalmol. 2016, 11, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 42201. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, F.J.; Staurenghi, G.; Gale, R.; Vision Academy Steering Committee; On behalf of the Vision Academy Steering Committee; Eldem, B. The role of OCT-A in retinal disease management. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2019–2026. [Google Scholar] [CrossRef]
- Rispoli, M.; Lumbroso, B.; Savastano, M.C. Incidence of Neovascularization in Central Serous Chorioretinopathy by OCT Angiography. Retina 2020. Available online: https://www.practiceupdate.com/content/incidence-of-neovascularization-in-central-serous-chorioretinopathy-by-oct-angiography/102302 (accessed on 13 July 2020). [CrossRef]
- Chu, Z.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.; Chen, C.-L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Opt. 2016, 21, 66008. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4901200/ (accessed on 24 July 2020). [CrossRef] [PubMed] [Green Version]
- Nelson, A.J.; Chang, R.; LeTran, V.; Vu, B.; Burkemper, B.; Chu, Z.; Fard, A.; Kashani, A.; Xu, B.; Wang, R.; et al. Ocular Determinants of Peripapillary Vessel Density in Hea nal RNFL and Fundus Vasculature by OCTA in Healthy African Americans: The African American Eye Disease Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3368–3373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miara, H.; Ouyang, P.; Jiang, B. The comparison of regional RNFL and fundus vasculature by OCTA in Chinese myopia population. J. Ophthalmol. 2018. [Google Scholar] [CrossRef]
- Giannis, D.; Ziogas, I.A.; Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020, 127, 104362. [Google Scholar] [CrossRef]
- Jung, F.; Krieger, V.; Hufert, F.T.; Küpper, J.-H. How we should respond to the Coronavirus SARS-CoV-2 outbreak: A German perspective. Clin. Hemorheol. Microcirc. 2020, 74, 1–10. [Google Scholar] [CrossRef]
- Oudkerk, M.; Büller, H.R.; Kuijpers, D.; Van Es, N.; Oudkerk, S.F.; McLoud, T.C.; Gommers, D.; Van Dissel, J.; Cate, H.T.; Van Beek, E.J.R. Diagnosis, Prevention, and Treatment of Thromboembolic Complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology 2020, 201629. [Google Scholar] [CrossRef]
- Marchetti, M. COVID-19-driven endothelial damage: Complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann. Hematol. 2020, 99, 1701–1707. [Google Scholar] [CrossRef]
- Jung, F.; Krüger-Genge, A.; Franke, R.; Hufert, F.; Küpper, J.H. COVID-19 and the endothelium. Clin. Hemorheol. Microcirc. 2020, 1–5. [Google Scholar] [CrossRef]
- Mammo, Z.; Heisler, M.; Balaratnasingam, C.; Lee, S.; Yu, D.-Y.; MacKenzie, P.; Schendel, S.; Merkur, A.; Kirker, A.; Albiani, D.; et al. Quantitative Optical Coherence Tomography Angiography of Radial Peripapillary Capillaries in Glaucoma, Glaucoma Suspect, and Normal Eyes. Am. J. Ophthalmol. 2016, 170, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Simonett, J.M.; Wang, J.; Hua, X.; Liu, L.; Hwang, T.S.; Huang, D. Wide-Field OCT Angiography Investigation of the Relationship Between Radial Peripapillary Capillary Plexus Density and Nerve Fiber Layer Thickness. Investig. Opthalmol. Vis. Sci. 2017, 58, 5188–5194. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, T.; Sivaswamy, J.; Gamalapati, J.S.; Balakrishna, N. Radial Peripapillary Capillary Density Measurement Using Optical Coherence Tomography Angiography in Early Glaucoma. J. Glaucoma 2017, 26, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Cerdà-Ibáñez, M.; Duch-Samper, A.; Clemente-Tomás, R.; Torrecillas-Picazo, R.; Del Río, N.R.; Manfreda-Dominguez, L. Correlation Between Ischemic Retinal Accidents and Radial Peripapillary Capillaries in the Optic Nerve Using Optical Coherence Tomographic Angiography: Observations in 6 Patients. Ophthalmol. Eye Dis. 2017, 9. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5397294/ (accessed on 13 July 2020). [CrossRef] [Green Version]
- Mansoori, T.; Sivaswamy, J.; Gamalapati, J.S.; Agraharam, S.G.; Balakrishna, N. Measurement of Radial Peripapillary Capillary Density in the Normal Human Retina Using Optical Coherence Tomography Angiography. J. Glaucoma 2017, 26, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Augstburger, E.; Zéboulon, P.; Keilani, C.; Baudouin, C.; Labbé, A. Retinal and Choroidal Microvasculature in Nonarteritic Anterior Ischemic Optic Neuropathy: An Optical Coherence Tomography Angiography Study. Investig. Opthalmol. Vis. Sci. 2018, 59, 870–877. [Google Scholar] [CrossRef]
- Mazzaccaro, D.; Giacomazzi, F.; Giannetta, M.; Varriale, A.; Scaramuzzo, R.; Modafferi, A.; Malacrida, G.; Righini, P.; Marrocco-Trischitta, M.M.; Nano, G. Non-Overt Coagulopathy in Non-ICU Patients with Mild to Moderate COVID-19 Pneumonia. J. Clin. Med. 2020, 9, 1781. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Romacho, T.; Valencia, I.; Ramos-González, M.; Vallejo, S.; López-Esteban, M.; Lorenzo, O.; Cannata, P.; Romero, A.; Hipólito-Luengo, A.S.; Gómez-Cerezo, J.F.; et al. Visfatin/eNampt induces endothelial dysfunction in vivo: A role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci. Rep. 2020, 10, 5386. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; He, G. Hematological findings in coronavirus disease 2019: Indications of progression of disease. Ann. Hematol. 2020, 99, 1421–1428. [Google Scholar] [CrossRef]
- Yin, S.; Huang, M.; Li, D.; Tang, N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis 2020, 3, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, D.; Gerbelli, J.; Chew, A.-N.; Cooley-Andrade, O.; Goonawardhana, D.; Cheung, K.; Parsi, K. Sirolimus and propranolol inhibit endothelial proliferation while detergent sclerosants induce endothelial activation, microparticle release and apoptosis in vitro. Phlebol. J. Venous Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhao, J.; Fan, T.-J. Dose- and Time-Dependent Cytotoxicity of Carteolol in Corneal Endothelial Cells and the Underlying Mechanisms. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hu, B.; Qu, H.; Wang, L.; Huang, X.; Li, M.; Zhang, M. Upregulation of angiotensin converting enzyme 2 by shear stress reduced inflammation and proliferation in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2020, 525, 812–818. [Google Scholar] [CrossRef]
| Investigated Anamnestic Data | |
|---|---|
| Post-COVID-19 Group | Control Group (Healthy Patients) |
| Age | Age |
| Sex | Sex |
| Date of birth | Date of birth |
| Height (cm) | Height (cm) |
| Weight (Kg) | Weight (Kg) |
| Sanitary code | Sanitary code |
| Preexisting systemic diseases | Preexisting systemic diseases |
| Preexisting ocular diseases | Preexisting ocular diseases |
| Familiar diseases | Familiar diseases |
| Date and findings of the oropharyngeal swabs | Date and findings of the oropharyngeal swabs |
| Results from the serologic exam | Results from the serologic exam |
| Reported ocular and systemic symptoms | Reported ocular and systemic symptoms |
| Duration of hospital stay | |
| Hosting hospital department | |
| Administered treatment | |
| Supportive treatment | |
| Complications occurred during hospital stay | |
| Variable | Post-COVID-19 | Controls | p |
|---|---|---|---|
| Age (years) | 52.9 ± 13.5 | 48.5 ± 13.4 | 0.71 |
| Sex | M = 46/80 (57.5%) F = 34/80 (42.5%) | M = 13/30 (43.3%) F = 17/30 (56.6%) | 0.26 |
| Systemic arterial hypertension | 19/80 (23.8%) | 3/30 (10%) | 0.03 |
| Diabetes | 34/80 (42.5%) | 0/30 (0%) | <0.001 |
| Autoimmune or inflammatory diseases | 19/80 (23.8%) | 0/30 (0%) | <0.001 |
| Myopia > 1D | 11/80 (13.8%) | (13.3%) | 0.87 |
| IOP | 16.2 ± 1.5 mmHg | 14.4 ± 2.1 mmHg | 0.34 |
| Red/dry eye during infection | 36/80 (45%) | ||
| Days since symptoms onset | 60.3 ± 13.6 | ||
| Days since hospital discharge | 36.1 ± 12.9 | ||
| ICU admission | 5/80 (6.25%) | ||
| Oxygen therapy | 33/80 (41.25%) | ||
| Noninvasive ventilation | 7/80 (8.8%) | ||
| Pulmonary embolism | 2/80 (2.5%) | ||
| Venous thrombosis | 2/80 (2.5%) | ||
| Hydroxychloroquine | 55/80 (68.8%) | ||
| Lopinavir + ritonavir | 27/80 (33.8%) | ||
| Darunavir + ritonavir | 35/80 (43.8%) | ||
| Azithromycin | 28/80 (35%) | ||
| Heparin | 33/80 (41.3%) | ||
| Antiplatelet therapy | 6/80 (7.5%) | ||
| Corticosteroids | 4/80 (5%) |
| Outcomes | Variable | Post-COVID-19 Mean ± SD (CI) | Control Mean ± SD (CI) | p t Test |
|---|---|---|---|---|
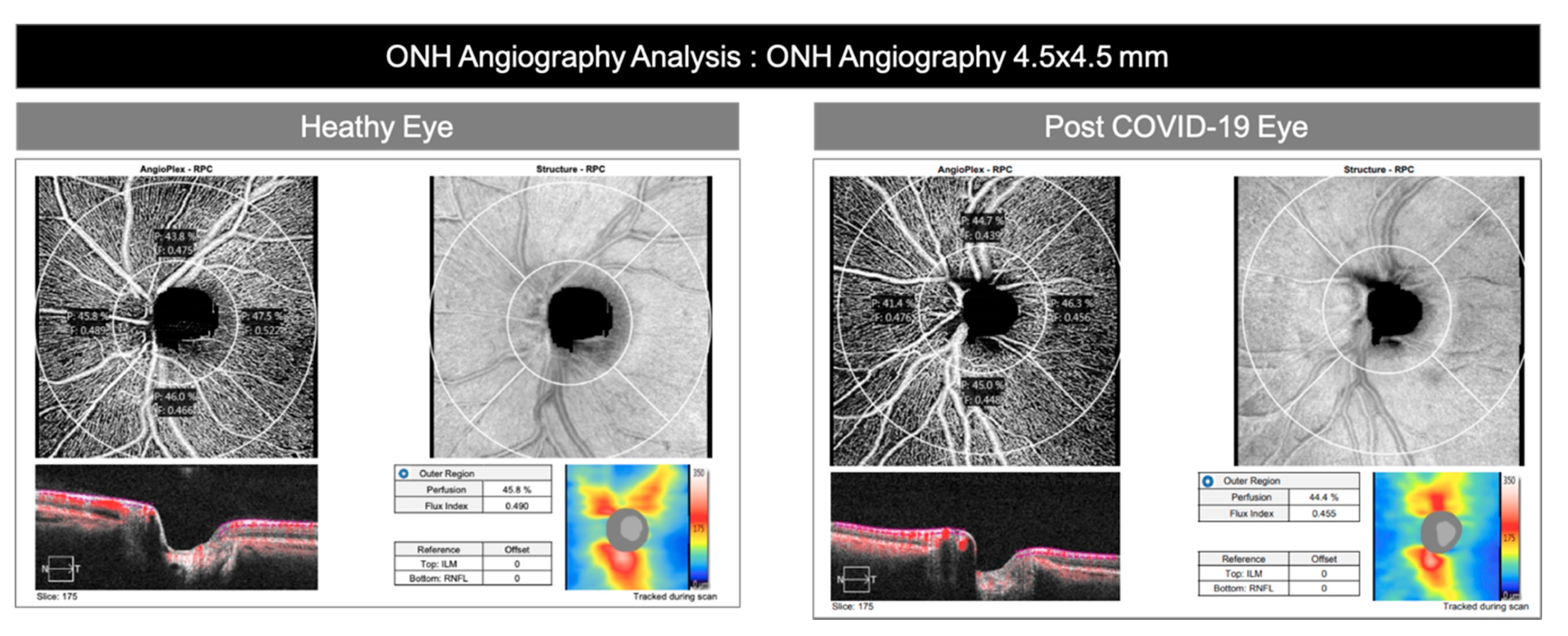
| Primary Outcomes | RPCP flow index | 0.454 ± 0.017 (0.450–0.457) | 0.456 ± 0.012 (0.452–0.461) | 0.42 |
| RPCP perfusion density | 0.437 ±0.031 (0.430–0.444) | 0.450 ± 0.025 (0.441–0.459) | 0.041 | |
| Secondary Outcomes | RNFL average thickness | 94.09 ± 10.77 (91.77–96.43) | 96.50 ± 7.78 (93.72–99.28) | 0.26 |
| GCC average thickness | 81.21 ± 8.67 (79.33–83.09) | 80.87 ± 5.80 (78.791–82.94) | 0.84 | |
| CD ratio | 0.43 ± 0.18 (0.39–0.47) | 0.43 ± 0.14 (0.38–0.48) | 0.90 | |
| Disc area | 1.76 ± 0.33 (1.69–1.83) | 1.688 ± 0.36 (1.56–1.82) | 0.31 | |
| Central foveal thickness | 263.83 ± 24.28 (258.57–269.08) | 260.1± 22.6 (252.0–268.2) | 0.46 | |
| Subfoveal choroidal thickness | 310.463 ± 81.60 (292.80–328.13) | 293.5 ± 86.56 (262.52–324.48) | 0.34 |
| Variable | RPCP Flow Index Means ± SD (CI) | p | RPCP Perfusion Index Means ± SD (CI) | p |
|---|---|---|---|---|
| Age | R = −0.421 Slope = −340.216 Intercept = 207.199 | <0.001 | R = −0.278 Slope = −145.568 Intercept = 116.528 | 0.01 |
| Sex | M = 0.453 ± 0.02 (0.448–0.458) F = 0.454 ± 0.02 (0.449–0.460) | 0.70 | M = 0.435 ± 0.03 (0.425–0.444) F = 0.441 ± 0.03 (0.432–0.450) | 0.39 |
| Systemic arterial hypertension | Absent = 0.457 ± 0.01 (0.453−0.461) Present = 0.442 ± 0.02 (0.433–0.451) | <0.001 | Absent = 0.439 ± 0.03 (0.432–0.446) Present = 0.431 ± 0.04 (0.413–0.449) | 0.31 |
| Systemic autoimmune or inflammatory diseases | Absent = 0.453 ± 0.02 (0.449–0.457) Present = 0.455 ± 0.02 (0.447–0.463) | 0.68 | Absent = 0.437± 0.03 (0.429–0.444) Present = 0.439 ± 0.03 (0.425–0.453) | 0.75 |
| Diabetes | Absent = 0.453 ± 0.02 (0.448–0.458) Present = 0.454 ± 0.02 (0.448–0.459) | 0.91 | Absent = 0.433 ± 0.03 (0.424–0.442) Present = 0.442 ± 0.03 (0.432–0.453) | 0.17 |
| Axial myopia > 1D | Absent = 0.453 ± 0.02 (0.449–0.458) Present = 0.454 ± 0.01 (0.447–0.462) | 0.87 | Absent = 0.438 ± 0.03 (0.430–0.446) Present = 0.434 ± 0.02 (0.422–0.446) | 0.68 |
| IOP in study examination | R = 1.28 Slope = 41.712 Intercept = 10.553 | 0.53 | R = 2.83 Slope = 57.931 Intercept = 4.006 | 0.61 |
| Red/dry eye during infection | Absent = 0.453 ± 0.02 (0.448–0.459) Present = 0.454 ± 0.02 (0.449–0.459) | 0.87 | Absent = 0.436 ± 0.03 (0.427–0.446) Present = 0.438 ± 0.03 (0.429–0.448) | 0.78 |
| Hydroxychloroquine | No = 0.455 ± 0.02 (0.449–0.462) Yes = 0.453 ± 0.02 (0.448–0.457) | 0.57 | No = 0.442 ± 0.03 (0.431–0.453) Yes = 0.435 ± 0.03 (0.427–0.444) | 0.36 |
| Lopinavir + ritonavir | No = 0.457 ± 0.01 (0.453–0.461) Yes = 0.447 ± 0.02 (0.439–0.455) | 0.01 | No = 0.443 ± 0.03 (0.435–0.450) Yes = 0.425 ± 0.03 (0.413–0.438) | 0.01 |
| Darunavir + ritonavir | No = 0.451 ± 0.02 (0.445–0.457) Yes = 0.457 ± 0.01(0.453–0.461) | 0.10 | No = 0.433 ± 0.03 (0.424–0.443) Yes = 0.442 ± 0.03 (0.433–0.452) | 0.19 |
| Heparin | No = 0.454 ± 0.02 (0.450–0.458) Yes = 0.453 ± 0.020(0.445–0.460) | 0.71 | No = 0.438 ± 0.03 (0.430–0.446) Yes = 0.435 ± 0.03 (0.422–0.448) | 0.68 |
| Antiplatelet therapy | No = 0.455 ± 0.02 (0.451–0.458) Yes = 0.43 ± 0.02 (0.409–0.450) | 0.0036 | No = 0.439 ± 0.03 (0.433–0.445) Yes = 0.404 ± 0.06 (0.343–0.464) | 0.0026 |
| Corticosteroids | No = 0.454 ± 0.02 (0.450–0.457) Yes = 0.453 ± 0.03 (0.419–0.486) | 0.89 | No = 0.438 ± 0.03(0.431–0.444) Yes = 0.425 ± 0.05 (0.374–0.476) | 0.43 |
| ICU admission | No = 0.454 ± 0.02 (0.450–0.458) 0.02 Yes = 0.449 ± 0.01(0.438–0.461) | 0.57 | No = 0.437 ± 0.03 (0.430–0.444) Yes = 0.44 ± 0.02 (0.424–0.456) | 0.84 |
| NIV treatment | No = 0.453 ± 0.02 (0.449–0.457) Yes = 0.461 ± 0.02 (0.444–0.479) | 0.22 | No = 0.438 ± 0.03 (0.431–0.445) Yes = 0.43 ± 0.02 (0.413–0.448) | 0.55 |
| Pulmonary embolism | No = 0.453 ± 0.02 (0.449–0.457) Yes = 0.47 ± 0.01 (0.450–0.490) | 0.16 | No = 0.438 ± 0.03 (0.431–0.445) Yes = 0.414 0.372–0.457 0.030 | 0.3 |
| Venous thrombosis | No = 0.453 ± 0.02 (0.449–0.457) Yes = 0.476 ± 0.005 (0.470–0.483) | 0.054 | No = 0.438 ± 0.03 (0.431–0.445) Yes = 0.417 ± 0.03 (0.369–0.466) | 0.37 |
| Linear Correlation Variables (Spearman’s Test) | Result | p |
|---|---|---|
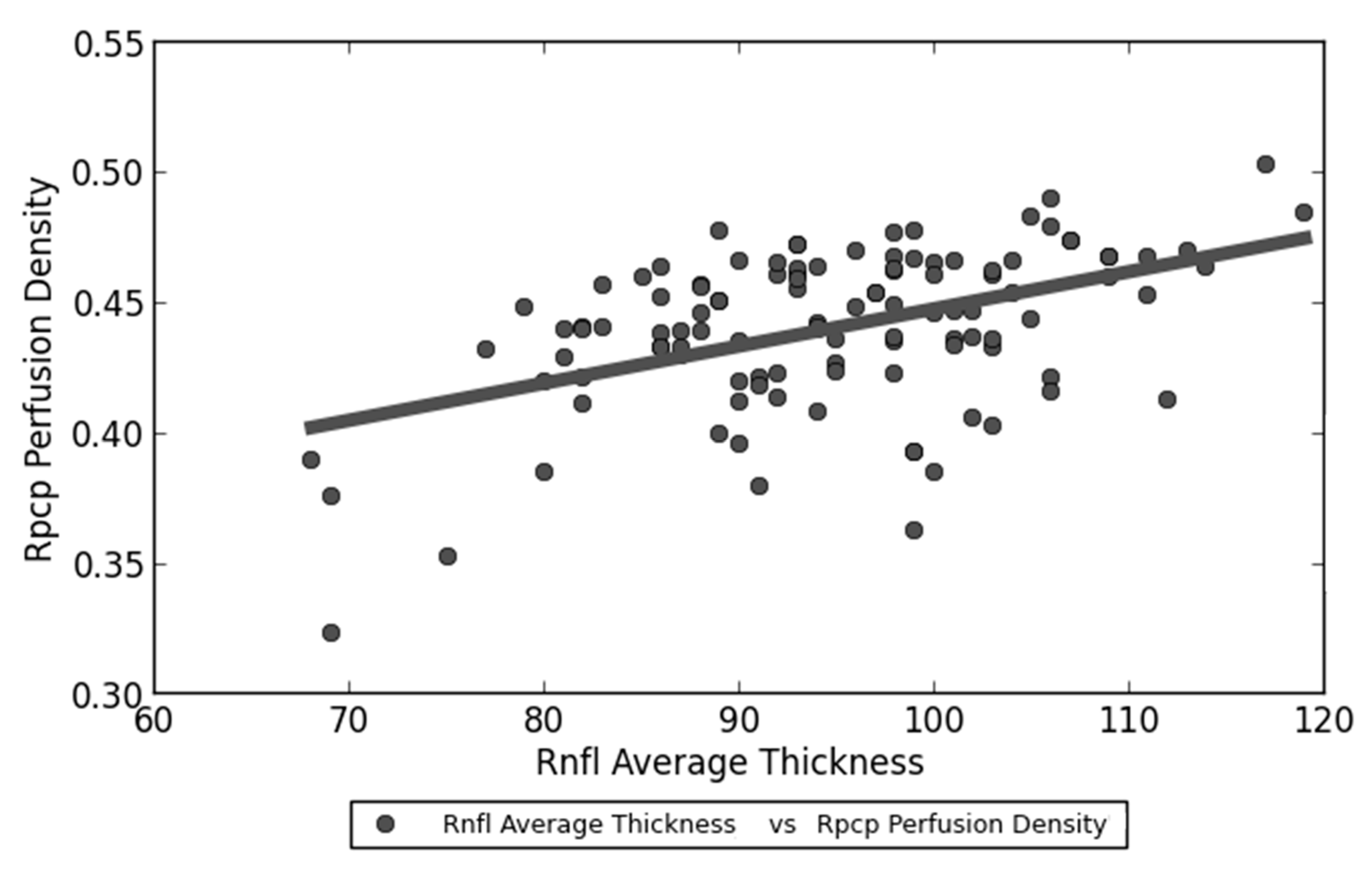
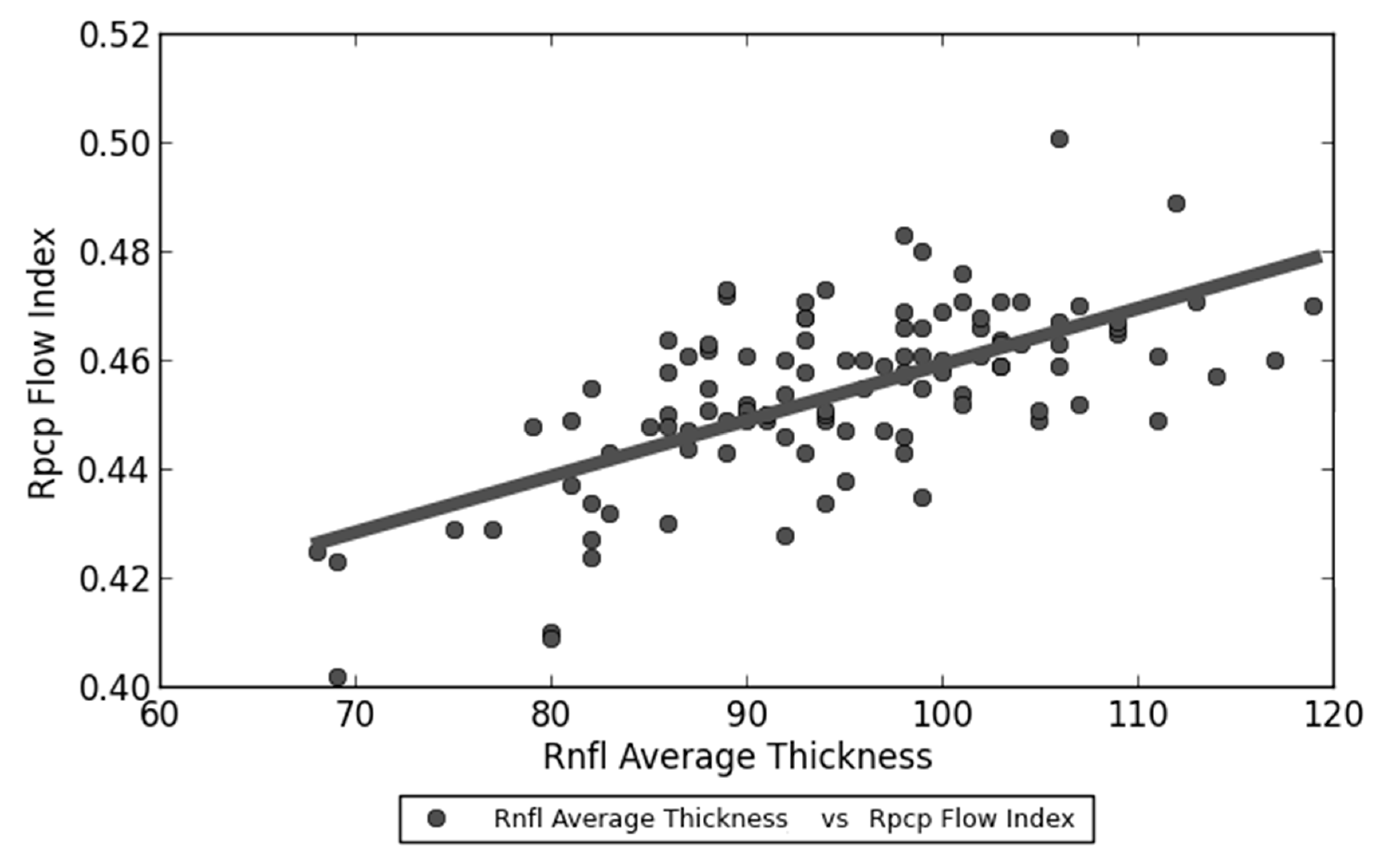
| RPCP Flow Index—RNFL Average Thickness | R = 0.633 Slope = 429.802 Intercept = −100.844 | <0.001 |
| RPCP Perfusion Density—RNFL Average Thickness | R = 0.366 Slope = 169.12 Intercept = 20.163 | <0.001 |
| RPCP Flow Index—Subfoveal Choroidal Thickness | R = 0.238 Slope = 1289.115 Intercept = −274.229 | 0.031 |
| RPCP Perfusion Density—Subfoveal Choroidal Thickness | R = 0.105 Slope: 353.021 Intercept: 156.133 | 0.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savastano, A.; Crincoli, E.; Savastano, M.C.; Younis, S.; Gambini, G.; De Vico, U.; Cozzupoli, G.M.; Culiersi, C.; Rizzo, S.; Gemelli Against COVID-19 Post-Acute Care Study Group. Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients. J. Clin. Med. 2020, 9, 2895. https://doi.org/10.3390/jcm9092895
Savastano A, Crincoli E, Savastano MC, Younis S, Gambini G, De Vico U, Cozzupoli GM, Culiersi C, Rizzo S, Gemelli Against COVID-19 Post-Acute Care Study Group. Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients. Journal of Clinical Medicine. 2020; 9(9):2895. https://doi.org/10.3390/jcm9092895
Chicago/Turabian StyleSavastano, Alfonso, Emanuele Crincoli, Maria Cristina Savastano, Saad Younis, Gloria Gambini, Umberto De Vico, Grazia Maria Cozzupoli, Carola Culiersi, Stanislao Rizzo, and Gemelli Against COVID-19 Post-Acute Care Study Group. 2020. "Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients" Journal of Clinical Medicine 9, no. 9: 2895. https://doi.org/10.3390/jcm9092895
APA StyleSavastano, A., Crincoli, E., Savastano, M. C., Younis, S., Gambini, G., De Vico, U., Cozzupoli, G. M., Culiersi, C., Rizzo, S., & Gemelli Against COVID-19 Post-Acute Care Study Group. (2020). Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients. Journal of Clinical Medicine, 9(9), 2895. https://doi.org/10.3390/jcm9092895






