Management of Cardiac Toxicity Induced by Chemotherapy
Abstract
1. Introduction
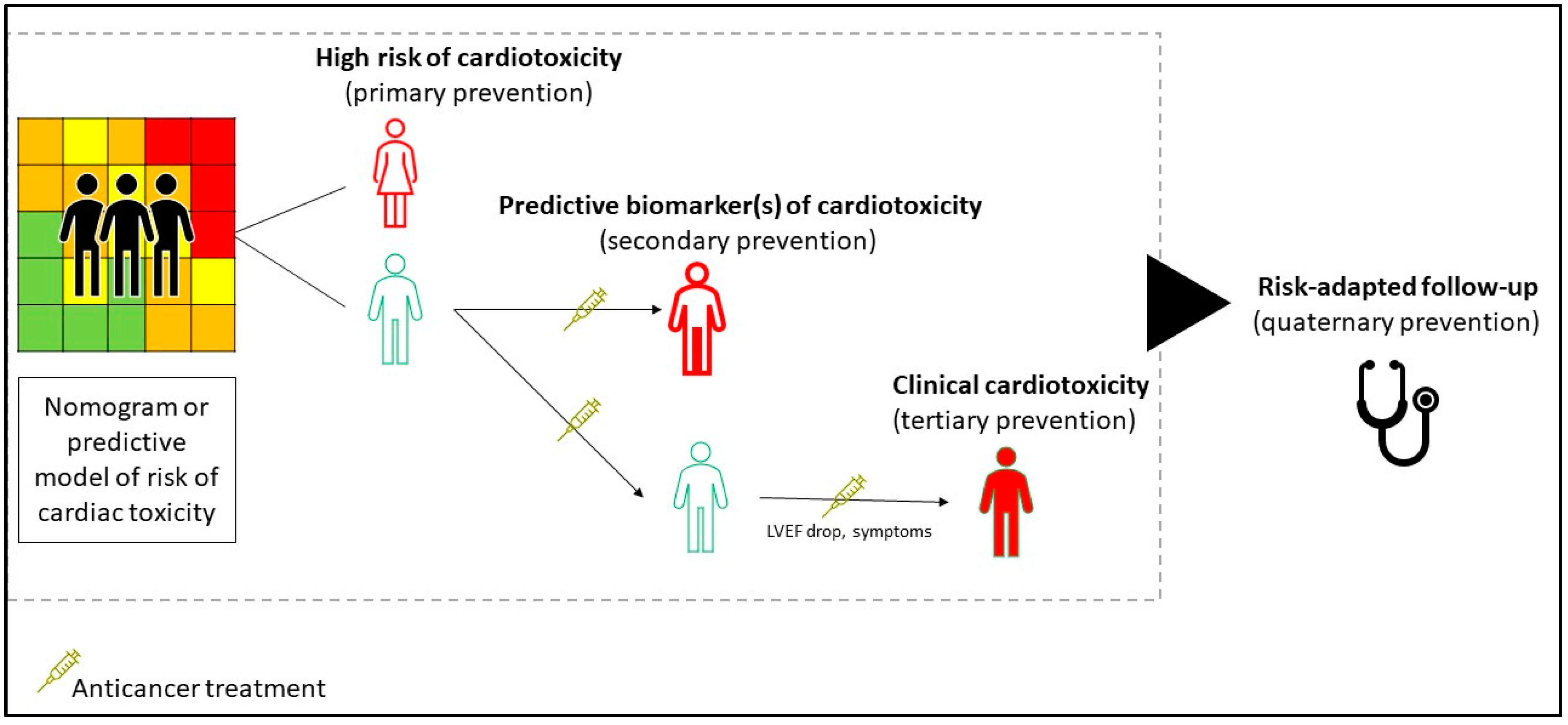
2. The Setting for the Management of Chemotherapy-Related Cardiotoxicity
3. Principles of Treatment of the Chemotherapy-Induced Cardiotoxicity
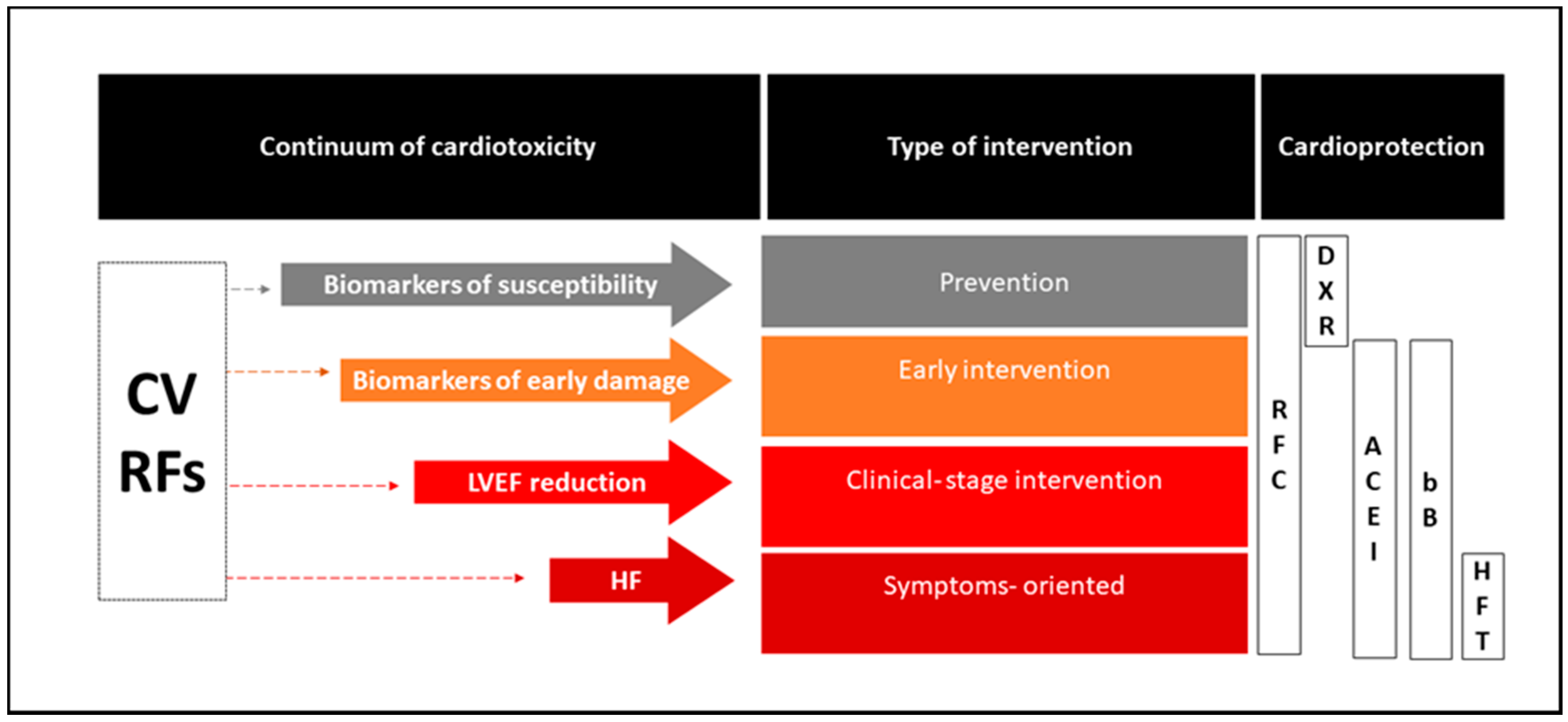
3.1. Prevention of Cardiovascular Damage
3.1.1. An Antidote to Reduce the Direct Damage of Cytotoxic Drugs: The Dexrazoxane Paradigm
3.1.2. Targeting the Key Pathogenetic Mechanisms of Chemotherapy-Induced Cardiotoxicity
3.2. The Management of Cardiotoxicity Occurring during the Cancer Treatments: The Clinical Scenarios
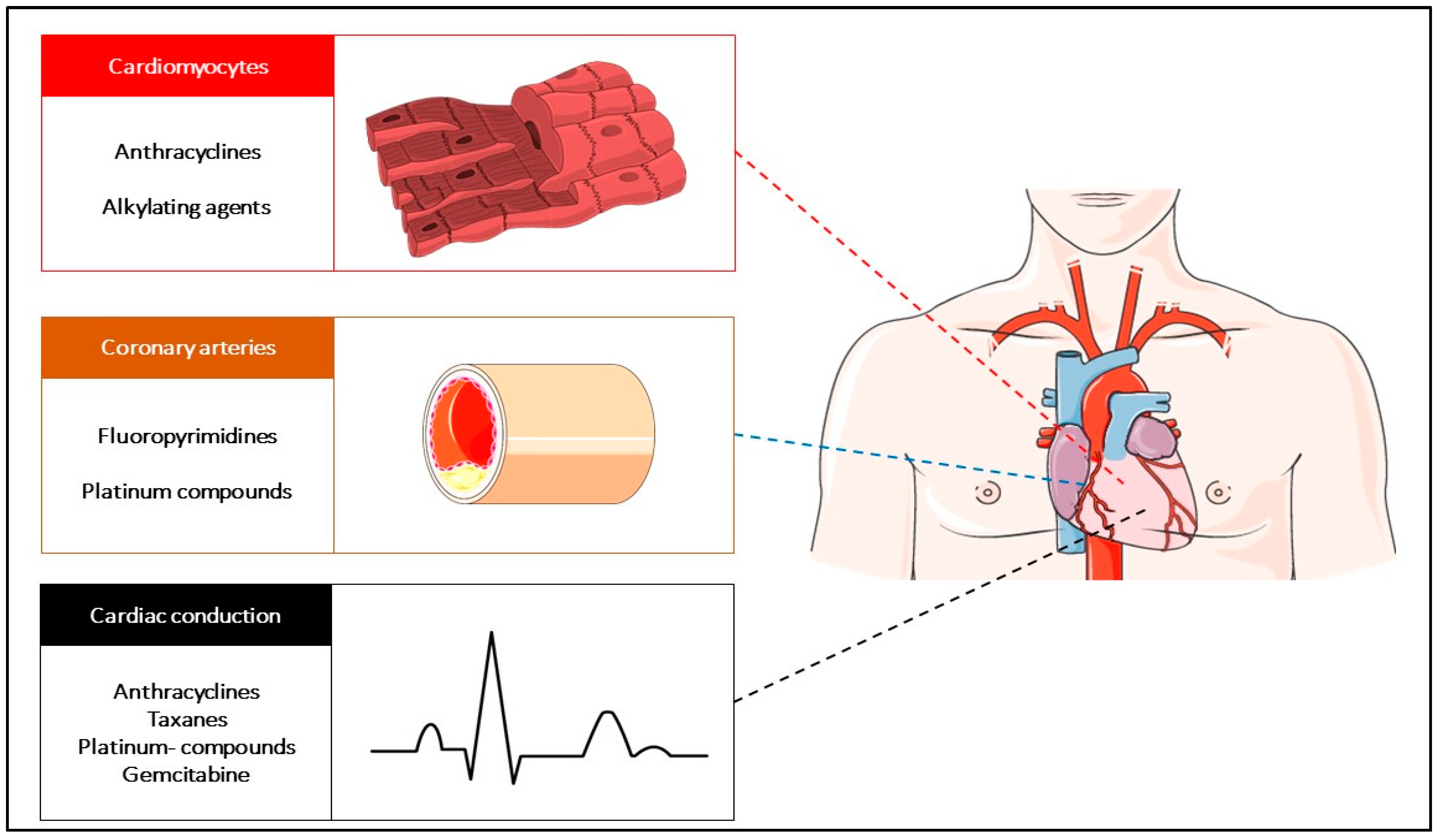
3.2.1. Anthracycline-Related Damage: Targeting the Direct Cardiomyocytes Toxic Agents
Clinical Scenario 1: Asymptomatic Patients Receiving Anthracycline
Clinical Scenario 2: Symptomatic Patients with a Significant Baseline Reduction in the LVEF
3.2.2. Fluoropyrimidine-Induced Cardiotoxicity: Targeting Vascular Coronary Artery Toxicity
3.2.3. Cardiac Arrhythmias Related to Cytotoxic Chemotherapy Agents
3.3. Management of Cardiotoxicity from Non-Chemotherapy Agents: An Overview
3.3.1. Trastuzumab and Other Anti-HER2 Agents
3.3.2. Immune-Related Cardiovascular Adverse Events
3.3.3. Tyrosine Kinase Inhibitors (TKIs) and Other Small Molecules
4. Post-Treatment Care for Long-Term Cardiotoxic Sequelae: Survivorship Care
5. Areas of Implementation and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Cheung, W.Y.; Atkinson, E.; Krzyzanowska, M.K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J. Clin. Oncol. 2011, 29, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Lenihan, D.J.; Oliva, S.; Chow, E.J.; Cardinale, D. Cardiac toxicity in cancer survivors. Cancer 2013, 119 (Suppl. 11), 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Oni, T.; Mogo, E.; Ahmed, A.; Davies, J.I. Breaking down the silos of Universal Health Coverage: Towards systems for the primary prevention of non-communicable diseases in Africa. BMJ Glob. Health 2019, 4, e001717. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan for Healthy Lives and Well-Being for All. Available online: https://www.who.int/sdg/global-action-plan/Global_Action_Plan_Phase_I.pdf (accessed on 11 August 2020).
- Tonorezos, E.S.; Conigliaro, J. Integration of cancer survivorship care and primary care practice. JAMA Intern. Med. 2017, 177, 1732–1734. [Google Scholar] [CrossRef]
- Mc Cabe, M.S.; Partridge, A.H.; Grunfeld, E.; Hudson, M.M. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Semin. Oncol. 2013, 40, 804–812. [Google Scholar] [CrossRef]
- Yoon, G.J.; Telli, M.L.; Kao, D.P.; Matsuda, K.Y.; Carlson, R.W.; Witteles, R.M. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally? J. Am. Coll. Cardiol. 2010, 56, 1644–1650. [Google Scholar] [CrossRef]
- WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Lee, K.; Brumme, Z.L. Operationalizing the One Health approach: The global governance challenges. Health Policy Plan. 2013, 28, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Parent, S.; Pituskin, E.; Paterson, D.I. The Cardio-oncology Program: A Multidisciplinary Approach to the Care of Cancer Patients With Cardiovascular Disease. Can. J. Cardiol. 2016, 32, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B. The anthracyclines: Will we ever find a better doxorubicin? Semin. Oncol. 1992, 19, 670–686. [Google Scholar] [PubMed]
- Jimenez Hernandez, R.M.; Antolín, J.M.S.; Hernandez, R.M.J.; Calle, P.T.; Ruigomez, A.C.; Arrojo, S.D.C.; Abad, C.G.; Varela, C.C.; Martín, J.J.A. Incidence of long-term cardiotoxicity and evolution of the systolic function in patients with breast cancer treated with anthracyclines. Cardiol. J. 2020. [Google Scholar] [CrossRef]
- Zeeneldin, A.A.; Sallam, Y.A.; Gaber, A.A.; Shaheen, A.A. Non-anthracycline chemotherapy associated with a poor outcome in elderly Egyptian patients with diffuse large B-cell non-Hodgkin lymphoma. J. Cancer Metastasis Treat. 2015, 1, 76–83. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; LaMantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Jones, R.L. Utility of dexrazoxane for the reduction of anthracycline-induced cardiotoxicity. Expert Rev. Cardiovasc. Ther. 2008, 6, 1311–1317. [Google Scholar] [CrossRef]
- Reichardt, P.; Tabone, M.D.; Mora, J.; Morland, B.; Jones, R.L. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: Re-evaluating the European labeling. Future Oncol. 2018, 14, 2663–2676. [Google Scholar] [CrossRef]
- van Dalen, E.C.; Caron, H.N.; Dickinson, H.O.; Kremer, L.C. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst. Rev. 2011, 15, CD003917. [Google Scholar] [CrossRef]
- Asselin, B.L.; Devidas, M.; Chen, L.; Franco, V.I.; Pullen, J.; Borowitz, M.J.; Hutchison, R.E.; Ravindranath, Y.; Armenian, S.H.; Camitta, B.M.; et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute Lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin Lymphoma: A Report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J. Clin. Oncol. 2016, 34, 854–862. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Committee for Medicinal Products for Human Use (EMA/398612/2017, 18th May 2017). Assessment Report for Dexrazoxane. Available online: https://www.ema.europa.eu/en/documents/referral/cardioxane-article-13-referral-chmp-assessment-rep. (accessed on 10 August 2020).
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Haber, E. The renin-angiotensin system. N. Engl. J. Med. 1974, 291, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Kwanten, W.J.; Jain, R.K. Renin-Angiotensin System Inhibitors to Mitigate Cancer Treatment-Related Adverse Events. Clin. Cancer Res. 2018, 24, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Akpek, M.; Ozdogru, I.; Sahin, O.; Inanc, M.; Dogan, A.; Yazici, C.; Berk, V.; Karaca, H.; Kalay, N.; Oguzhan, A.; et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur. J. Heart Fail. 2015, 17, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; Von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef]
- Bosch, X.; Rovira, M.; Sitges, M.; Domènech, A.; Ortiz-Pérez, J.T.; De Caralt, T.M.; Morales-Ruiz, M.; Perea, R.J.; Monzo, M.; Esteve, J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J. Am. Coll. Cardiol. 2013, 61, 2355–2362. [Google Scholar] [CrossRef]
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R., Jr.; das Dores Cruz, F.; Gonçalves Brandão, S.M.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Filho, R.K.; Hoff, P.M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J. Am. Coll. Cardiol. 2018, 71, 228. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2017, 135, 135. [Google Scholar] [CrossRef]
- Getz, K.D.; Sung, L.; Alonzo, T.A.; Leger, K.J.; Gerbing, R.B.; Pollard, J.A.; Cooper, T.; Kolb, E.A.; Gamis, A.S.; Ky, B.; et al. Effect of Dexrazoxane on Left Ventricular Systolic Function and Treatment Outcomes in Patients With Acute Myeloid Leukemia: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 2398–2406. [Google Scholar] [CrossRef]
- Jensen, B.V.; Skovsgaard, T.; Nielsen, S.L. Functional monitoring of anthracycline cardiotoxicity: A prospective, blinded, long-term observational study of outcome in 120 patients. Ann. Oncol. 2002, 13, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.H.; Cnaan, A.; Clark, B.J.; Paridon, S.M.; Chin, A.J.; Rychik, J.; Hogarty, A.N.; Cohen, M.I.; Barber, G.; Rutkowski, M.; et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J. Clin. Oncol. 2004, 22, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Cardinale, D.; Ciceri, F.; Latini, R.; Franzosi, M.G.; Sandri, M.T.; Civelli, M.; Cucchi, G.; Menatti, E.; Mangiavacchi, M.; Cavina, R.; et al. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur. J. Cancer 2018, 94, 126–137. [Google Scholar] [CrossRef]
- Meessen, J.M.T.A.; Cardinale, D.; Ciceri, F.; Sandri, M.T.; Civelli, M.; Bottazzi, B.; Cucchi, G.; Menatti, E.; Mangiavacchi, M.; Condorelli, G.; et al. Circulating biomarkers and cardiac function over 3 years after chemotherapy with anthracyclines: The ICOS-ONE trial. ESC Heart Fail. 2020, 7, 1452–1466. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2016 Dec 30]. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Lamberti, M.; Porto, S.; Zappavigna, S.; Addeo, E.; Marra, M.; Miraglia, N.; Sannolo, N.; Vanacore, D.; Stiuso, P.; Caraglia, M.; et al. A mechanistic study on the cardiotoxicity of 5-fluorouracil in vitro and clinical and occupational perspectives. Toxicol. Lett. 2014, 227, 151–156. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Hoff, P.M.; Blum, J.L.; Abt, M.; Osterwalder, B. Incidence of cardiotoxicity with the oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil. Ann. Oncol. 2002, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Adel, N.; Riedel, E.; Bhutani, M.; Feldman, D.R.; Tabbara, N.E.; Soff, G.A.; Parameswaran, R.; Hassoun, H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J. Clin. Oncol. 2011, 29, 3466–3473. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.C.; Touyz, R.M.; Lang, N.N. Vascular Complications of Cancer Chemotherapy. Can. J. Cardiol. 2016, 32, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Horacek, J.M.; Jakl, M.; Horackova, J.; Pudil, R.; Jebavy, L.; Maly, J. Assessment of anthracycline-induced cardiotoxicity with electrocardiography. Exp. Oncol. 2009, 31, 115–117. [Google Scholar] [PubMed]
- Simůnek, T.; Stérba, M.; Popelová, O.; Adamcová, M.; Hrdina, R.; Gersl, V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Milberg, P.; Fleischer, D.; Stypmann, J.; Osada, N.; Mönnig, G.; Engelen, M.A.; Bruch, C.; Breithardt, G.; Haverkamp, W.; Eckardt, L. Reduced repolarization reserve due to anthracycline therapy facilitates torsade de pointes induced by IKr blockers. Basic Res. Cardiol. 2007, 102, 42–51. [Google Scholar] [CrossRef]
- Gridelli, C.; Cigolari, S.; Gallo, C.; Manzione, L.; Ianniello, G.P.; Frontini, L.; Ferraù, F.; Robbiati, S.F.; Adamo, V.; Gasparini, G.; et al. Activity and toxicity of gemcitabine and gemcitabine + vinorelbine in advanced non-small-cell lung cancer elderly patients: Phase II data from the Multicenter Italian Lung Cancer in the Elderly Study (MILES) randomized trial. Lung Cancer 2001, 31, 277–284. [Google Scholar] [CrossRef]
- McGuire, W.P.; Rowinsky, E.K.; Rosenshein, N.B.; Grumbine, F.C.; Ettinger, D.S.; Armstrong, D.K.; Donehower, R.C. Taxol: A unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann. Intern. Med. 1989, 111, 273–279. [Google Scholar] [CrossRef]
- Nault, M.A.; Milne, B.; Parlow, J.L. Effects of the Selective H1 and H2Histamine Receptor Antagonists Loratadine and Ranitidine on Autonomic Control of the Heart. Anesthesiology 2002, 96, 336–341. [Google Scholar] [CrossRef]
- D’Uva, G.; Aharonov, A.; Lauriola, M.; Kain, D.; Yahalom-Ronen, Y.; Carvalho, S.; Weisinger, K.; Bassat, E.; Rajchman, D.; Yifa, O.; et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 2015, 17, 627–638. [Google Scholar] [CrossRef]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef]
- Perez, E.A.; Koehler, M.; Byrne, J.; Preston, A.J.; Rappold, E.; Ewer, M.S. Cardiac safety of lapatinib: Pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin. Proc. 2008, 83, 679. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Cortés, J.; Amadori, D.; Miles, D.; Knott, A.; Clark, E.; Benyunes, M.C.; Ross, G.; Baselga, J. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: A randomized, double-blind, placebo-controlled phase III study. Oncologist 2013, 18, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Pondé, N.; Ameye, L.; Lambertini, M.; Paesmans, M.; Piccart, M.; De Azambuja, E. Trastuzumab emtansine (T-DM1)-associated cardiotoxicity: Pooled analysis in advanced HER2-positive breast cancer. Eur. J. Cancer 2020, 126, 65. [Google Scholar] [CrossRef]
- Guglin, M.; Krischer, J.; Tamura, R.; Fink, A.; Bello-Matricaria, L.; McCaskill-Stevens, W.; Munster, P.N. Randomized Trial of Lisinopril Versus Carvedilol to Prevent Trastuzumab Cardiotoxicity in Patients With Breast Cancer. J. Am. Coll. Cardiol. 2019, 73, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observationalretrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Heinzerling, L.; Ott, P.A.; Hodi, F.S.; Husain, A.N.; Tajmir-Riahi, A.; Tawbi, H.; Pauschinger, M.; Gajewski, T.F.; Lipson, E.J.; Luke, J.J. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Cancer 2016, 4, 50. [Google Scholar] [CrossRef]
- Santos, R.C.; Figueiredo, V.N.; Martins, L.C.; de Haro Moraes, C.; Quinaglia, T.; Boer-Martins, L.; Ferreira-Melo, S.E.; Yazbek, M.A.; Bertolo, M., Jr. Heitor Moreno Infliximab reduces cardiac output in rheumatoid arthritis patients without heart failure. Rev. Assoc. Med. Bras. 2012, 58, 698–702. [Google Scholar] [CrossRef]
- Esfahani, K.; Buhlaiga, N.; Thébault, P.; Lapointe, R.; Johnson, N.A.; Miller, W.H. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N. Engl. J. Med. 2019, 380, 2375–2376. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Criscuolo, G.; Triassi, M.; Bonaduce, D.; Marone, G.; Tocchetti, C.G. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017, 2, e000247. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; Shen, Z.; Tang, L.N.; Yao, Y. Congestive heart failure risk in cancer patients treated with vascular endothelial growth factor tyrosine kinase inhibitors: A systematic review and meta-analysis of 36 clinical trials. Br. J. Clin. Pharmacol. 2014, 78, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Mincu, R.I.; Mahabadi, A.A.; Michel, L.; Mrotzek, S.M.; Schadendorf, D.; Rassaf, T.; Totzeck, M. Cardiovascular adverse events associated with BRAF and MEK inhibitors: A systematic review and meta-analysis. JAMA Netw. Open 2019, 2, e198890. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Martin, T.; Nooka, A.; Harvey, R.D.; Vij, R.; Niesvizky, R.; Badros, A.Z.; Jagannath, S.; McCulloch, L.; Rajangam, K.; et al. Integrated safety profile of single-agent carfilzomib: Experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica 2013, 98, 1753–1761. [Google Scholar] [CrossRef]
- Ou, S.H.; Tang, Y.; Polli, A.; Wilner, K.D.; Schnell, P. Factors associated with sinus bradycardia during crizotinib treatment: A retrospective analysis of two large-scale multinational trials (PROFILE 1005 and 1007). Cancer Med. 2016, 5, 617–622. [Google Scholar] [CrossRef]
- Ghatalia, P.; Je, Y.; Kaymakcalan, M.D. QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br. J. Cancer 2015, 112, 296–305. [Google Scholar] [CrossRef]
- Leong, D.P.; Caron, F.; Hillis, C.; Duan, A.; Healey, J.S.; Fraser, G.; Siegal, D.M. The risk of atrial fibrillation with ibrutinib use: A systematic review and meta-analysis. Blood 2016, 128, 138–140. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Parikh, S.A.; Noseworthy, P.A.; Goede, V.; Chaffee, K.G.; Bahlo, J.; Call, T.G.; Schwager, S.M.; Ding, W.; Eichhorst, B.; et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma 2017, 58, 1630–1639. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Deininger, M. Tyrosine Kinase Inhibitor-Associated Cardiovascular Toxicity in Chronic Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 4210–4218. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H.M.; Saglio, G.; Steegmann, J.L.; Shah, N.P.; Boqué, C.J.; Chuah, C.; Pavlovsky, C.; Mayer, J.; Cortes, J.; et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014, 123, 494–500. [Google Scholar] [CrossRef]
- Le Coutre, P.; Rea, D.; Abruzzese, E.; Dombret, H.; Trawinska, M.M.; Herndlhofer, S.; Dörken, B.; Valent, P. Severe peripheral arterial disease during nilotinib therapy. J. Natl. Cancer Inst. 2011, 103, 1347–1348. [Google Scholar] [CrossRef] [PubMed]
| Population (n) | Study Type | Cardioprotective Intervention | Outcome Measured | Benefit of the Intervention | Reference |
|---|---|---|---|---|---|
| Patients with a solid tumor or leukemia receiving Ant (n = 1619) | Meta-analysis | Dexrazoxane | Heart failure (clinical and subclinical) | Statistically significant benefit in favor of dexrazoxane for the occurrence of heart failure (RR: 0.29, 95% CI: 0.20–0.41, p < 0.00001). | Van Dalen et al., Cochrane Database Syst Rev 2011 [21] |
| Pediatric patients receiving Ant for AML (n = 1014) | Prospective, observational | Dexrazoxane | LVSD using TTE (defined as SF < 28% or EF < 55%) | Smaller EF and SF declines with dexrazoxane compared to unexposed patients across courses and a lower risk for LVSD (26.5% vs. 42.2%; HR: 0.55; 95% CI: 0.36–0.86; p = 0.009). | Getz et al., J. Clin. Oncol. 2020 [32] |
| BC patients receiving Ant (n = 83) | Prospective, randomized, placebo-controlled, double-blind | Spironolactone | LVEF using TTE | LVEF decreased from 67.0 ± 6.1% to 65.7 ± 7.4% (p = 0.094) in the spironolactone group, and from 67.7 ± 6.3% to 53.6 ± 6.8% in the control group (p < 0.001). | Akpek et al., Eur J Heart Fail 2015 [27] |
| Early BC patients receiving adjuvant Ant +/- trastuzumab and RT (n = 120) | 2 × 2 factorial, randomized, placebo-controlled, double-blind | Candesartan, metoprolol, or matching placebos | LVEF using cardiac MRI | The overall decline in LVEF was 2.6% (95% CI: 1.5–3.8%) in the placebo group and 0.8% (95% CI: 0.4–1.9%) in the candesartan group in the intention-to-treat analysis (p = 0.026). No effect of metoprolol on the overall decline in LVEF. | Gulati et al., Eur Heart J 2016 [28] |
| Patients with malignant memopathies that were eligible for intensive chemotherapy (n = 90) | Randomized, controlled | Enalapril and carvedilol | LVEF using TTE and MRI | LVEF did not change in the intervention group but significantly decreased in the controls, resulting in a −3.1% absolute difference based on echocardiography (p = 0.035) and −3.4% (p = 0.09) in the 59 patients who underwent MRI. | Bosch et al., Jam Coll Cardiol 2013 [29] |
| Patients with HER2-negative early BC receiving Ant (n = 200) | Prospective, randomized, placebo-controlled, double-blind | Carvedilol | Early-onset decrease in LVEF ≥ 10% at 6 months | Primary endpoint occurred in 14 patients (14.5%) in the carvedilol group and 13 patients (13.5%) in the placebo group (p = 1.0). No differences in changes in LVEF were noted between groups. | Avila et al., J Am Coll Cardiol. 2018 [30] |
| Patients with advanced BC treated with Ant (n = 120) | Prospective, blinded, observational | Digitalo-diuretic therapy + ACE inhibitor | Recovery of the LVEF after developing CHF | Cardiac function continued to deteriorate during digitalo-diuretic therapy for 3 months. Almost all LVEF values returned to normal after a median of 3 months of ACE inhibitor therapy and remained stable in the follow-up period (median of 33 months). | Jensen et al. Ann Oncol. 2002 [33] |
| Long-term survivors of pediatric cancer exposed to Ant, with a cardiac abnormality identified (n = 135) | Randomized, double-blind, controlled | Enalapril | Cardiac function deterioration (defined using MCI on an exercise test or an increase in LVESWS) | No difference in the rate of change in MCI between enalapril and the placebo groups. The rate of change in LVESWS was greater in the enalapril group than in the placebo group (−8.59 vs. 1.85 g/cm [2]; p = 0.033) during the first year and maintained over time, resulting in a 9% reduction in the estimated LVESWS by year 5 in the enalapril group. | Silber et al., J. Clin. Oncol. 2004 [34] |
| Patients with hematologic or solid tumors with LVEF ≤ 45% due to AC-CMP (n = 201) | Prospective study | Enalapril and, when possible, carvedilol | Recovery of the LVEF using TTE | 42% of patients were responders (LVEF ≥ 50%), 13% were partial responders (10% < LVEF ≤50%), and 45% were non-responders (LVEF increase ≤ 10%). Responders showed a lower rate of cumulative cardiac events than partial and non-responders (5%, 31%, and 29%, respectively; p < 0.001). | Cardinale et al., J Am Coll Cardiol. 2010 [35] |
| Adult patients treated with Ant (n = 273) | Controlled, open-label, phase III | Enalapril started before Ant (prevention arm) or at troponin increase (troponin-triggered arm) | Incidence of troponin elevation | No difference in the proportion of patients with a first high troponin level: 23% in the prevention group and 26% in the troponin-triggered group (p = 0.50), or in the time to the first troponin elevation (HR: 1.13, 95% CI: 0.70–1.83; p = 0.61). The median level of the first elevation of troponin was 40% (22–90%) above the ULN in the prevention group and 33% (18–50%) in the troponin-triggered arm (p = 0.17). | Cardinale et al., Eur J Cancer. 2018 [36] |
| Population | Intervention | Pharmacological Class | Phase | Primary Outcome | NCT Identifier |
|---|---|---|---|---|---|
| Patients with ES, OS, and AML scheduled for chemotherapy | Captopril | ACE-I | 3 | Effect of ACE-Is in preventing chemotherapy-related cardiotoxicity | NCT03389724 |
| Patients scheduled for anthracycline | Ivabradine | Selective inhibitor of If | NA | Reduction in the global longitudinal strain of at least 10% | NCT03650205 |
| Early breast cancer patients eligible for anthracycline +/- trastuzumab | Bisoprolol; ramipril | bB and ACE-I | 3 | Maximum change in the LVEF | NCT02236806 (SAFE) |
| Breast cancer patients eligible for anthracycline treatment | Sulforaphane | Nutritional supplement | 1/2 | Change in cardiac function after doxorubicin | NCT03934905 |
| NHL patients scheduled for (R)CHOP type treatments | Atorvastatin | Lipid- lowering statin | 2 | LVEF preservation at 12 months | NCT02943590 (STOP-CA) |
| Adolescent patients after a bone marrow transplantation for hematological malignancies | Sacubitril, valsartan | Neprilysin inhibitor and ACE-I | NA | Change in the left ventricular function | NCT04092309 |
| Breast cancer patients treated with doxorubicin | Alfacalcidol | Vitamin D | 2 | Change in the plasma levels of troponin-T | NCT04166253 |
| Breast cancer patients scheduled for anthracycline | Alpha-lipoic acid | Dietary supplement | NA | Serum brain natriuretic peptide, neurotensin, and TNF-α level plasma assessment | NCT03908528 |
| Early breast cancer patients eligible for anthracycline | Xinmailong | Bioactive fraction extracted from Periplaneta Americana (American cockroach) | 2 | Rate of no cardiac events during chemotherapy | NCT03785704 |
| Early breast cancer patients eligible for anthracycline | Atorvastatin | Lipid- lowering statin | 2 | LVEF preservation at 24 months | NCT01988571 (PREVENT) |
| Chemotherapy patients at risk of cardiotoxicity | ACE-I and bB | ACE-I and bB | NA | New LV dysfunction, as defined based on a 3D echo | ACTRN12614000341628 (SUCCOUR) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trapani, D.; Zagami, P.; Nicolò, E.; Pravettoni, G.; Curigliano, G. Management of Cardiac Toxicity Induced by Chemotherapy. J. Clin. Med. 2020, 9, 2885. https://doi.org/10.3390/jcm9092885
Trapani D, Zagami P, Nicolò E, Pravettoni G, Curigliano G. Management of Cardiac Toxicity Induced by Chemotherapy. Journal of Clinical Medicine. 2020; 9(9):2885. https://doi.org/10.3390/jcm9092885
Chicago/Turabian StyleTrapani, Dario, Paola Zagami, Eleonora Nicolò, Gabriella Pravettoni, and Giuseppe Curigliano. 2020. "Management of Cardiac Toxicity Induced by Chemotherapy" Journal of Clinical Medicine 9, no. 9: 2885. https://doi.org/10.3390/jcm9092885
APA StyleTrapani, D., Zagami, P., Nicolò, E., Pravettoni, G., & Curigliano, G. (2020). Management of Cardiac Toxicity Induced by Chemotherapy. Journal of Clinical Medicine, 9(9), 2885. https://doi.org/10.3390/jcm9092885






