Hypertensive Cardiotoxicity in Cancer Treatment—Systematic Analysis of Adjunct, Conventional Chemotherapy, and Novel Therapies—Epidemiology, Incidence, and Pathophysiology
Abstract
1. Introduction
2. Methods
Definition
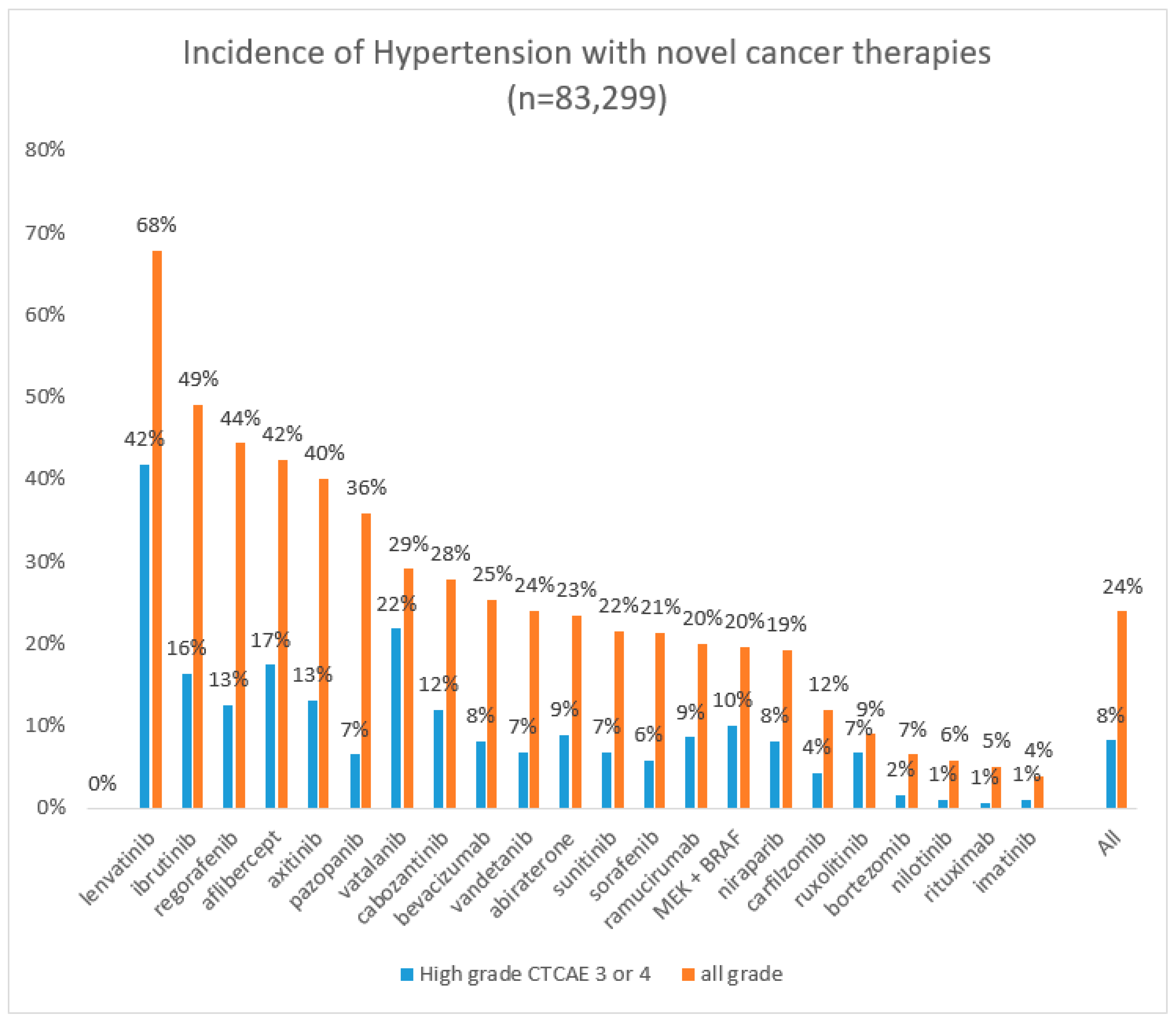
3. Epidemiology
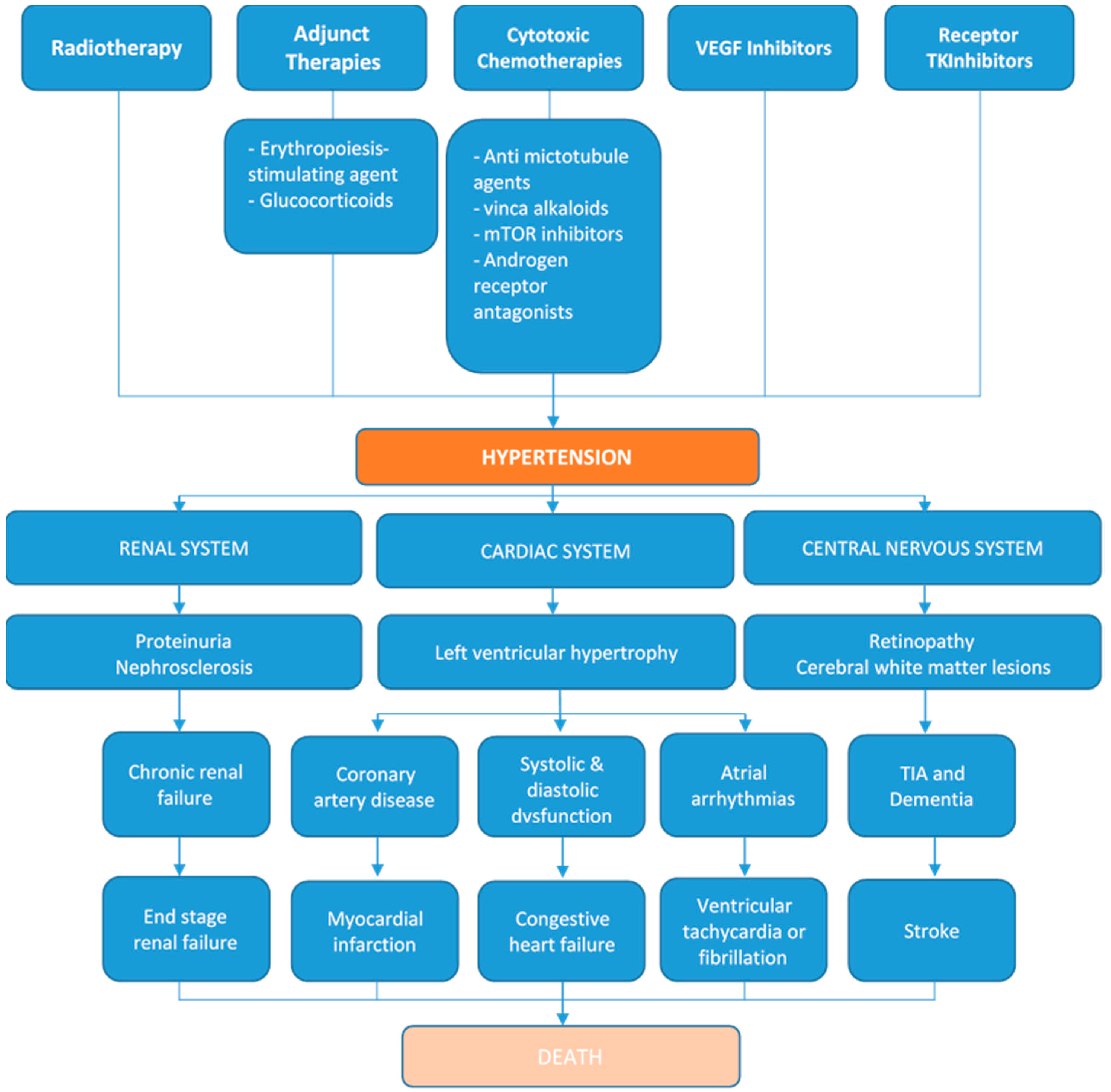
4. Hypertensive Cardiotoxicities of Cancer Therapies
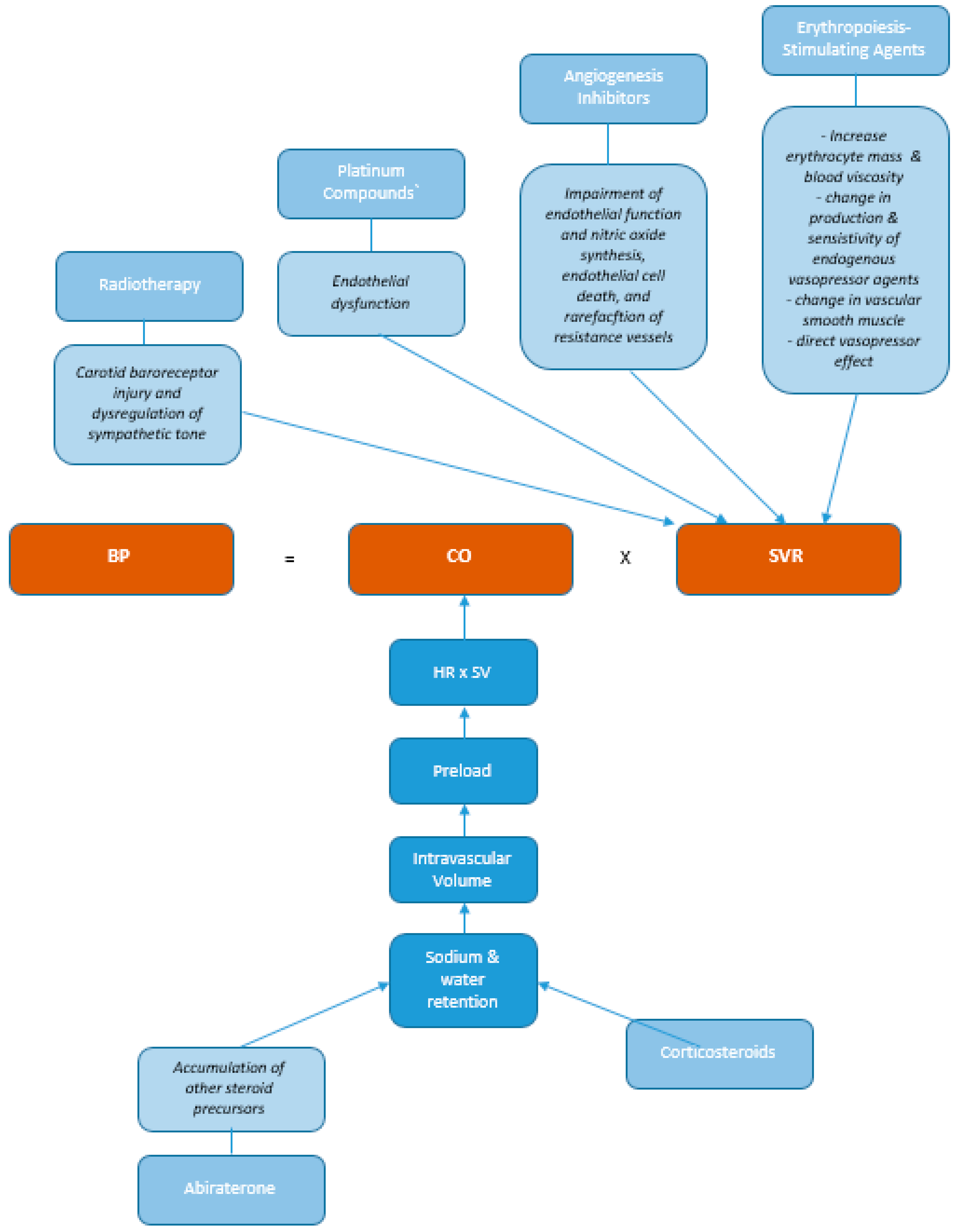
4.1. Pathophysiologic Mechanisms
4.2. Radiotherapy
4.3. Cytotoxic Chemotherapies
4.4. Alkylating Agents
4.5. Platinum Compounds
4.6. mTOR and Interferon Alpha
4.7. Abiraterone
4.8. Rituximab
4.9. Vascular Endothelial Growth Factor (VEGF) Inhibitors
4.10. Tyrosine Kinase Inhibitors (TKI)
4.11. Adjunct Treatments
5. Gaps in Evidence—Late Effects, Reversibility and Recurrence after Treatment
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| ASCO | American Society of Clinical Oncology |
| ASH | American Society of Hematology |
| BP | Blood pressure |
| BRAF | B-Rapid Accelerating Fibrosarcoma gene |
| CD | Cluster of Differentiation |
| CVD | Cardiovascular Disease |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CVD | Cardiovascular Disease |
| CUP | Cancer of Unknown Primary |
| DNA | de-oxyribonucleic acid |
| ET-1 | Endothelian-1 |
| ESA | Erythropoeisis Stimulating Agents |
| ESC | European Society of Cardiology |
| HER2 | Human Epidural Growth Factor Receptor 2 |
| HMOD | Hypertension-Mediated Organ Damage |
| LVD | Left Ventricular Dysfunction |
| LVSD | Left Ventricular Systolic Dysfunction |
| MACE | Major Adverse Cardiovascular Event |
| MEK | Mitogen extracellular signal-regulated kinase |
| mTOR | mammalian Target of Rapamycin |
| NCI | National Cancer Institute |
| NO | Nitric Oxide |
| NSAID | Non-steroidal anti-inflammatory drug |
| PDGFR | Plate derived growth factor receptor |
| RAS | Renin Angiotensin System |
| RNA | ribonucleic acid |
| SPRINT | Systolic Blood Pressure Intervention Trial |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| TKI | Tyrosine Kinase Inhibitors |
| VEGF | Vascular Endothelial Growth Factor |
Appendix A
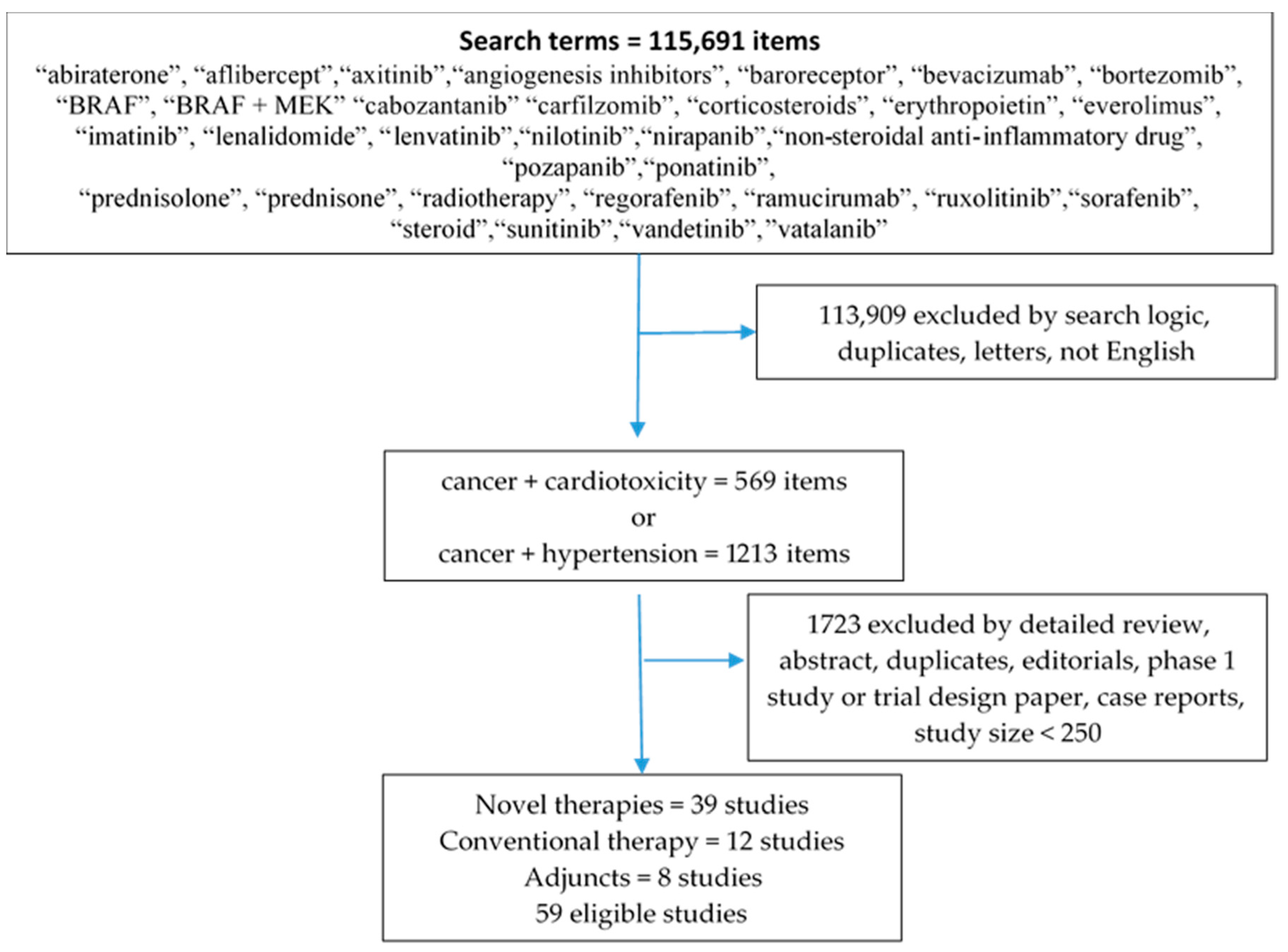
References
- Siegel, R.; Mph, K.D.M.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Maddams, J.; Utley, M.; Møller, H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br. J. Cancer 2012, 107, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Beutner, R. The cardiac toxicity of injectable local anesthetics. Fed. Proc. 1946, 5, 166. [Google Scholar] [PubMed]
- Chapman, D.W.; Shaffer, C.F. The mercurial diuretics; a comparison of acute cardiac toxicity in animals and the effect of ascorbic acid on detoxification in their intravenous administration. Proc. Annu. Meet. Cent. Soc. Clin. Res. US 1946, 19, 7. [Google Scholar]
- Kyser, F.A.; Ginsberg, H.; Gilbert, N.C. The effect of certain drugs upon the cardiotoxic lesions of digitalis in the dog. Am. Heart J. 1946, 31, 451–459. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P. Risk factors for doxorubicin-induced congestive heart failure. Am. Heart J. 1979, 91, 710–717. [Google Scholar]
- Chung, R.; Ghosh, A.K.; Banerjee, A. Cardiotoxicity: Precision medicine with imprecise definitions. Open Heart 2018, 5, e000774. [Google Scholar] [CrossRef]
- Guha, A.; Armanious, M.; Fradley, M.G. Update on cardio-oncology: Novel cancer therapeutics and associated cardiotoxicities. Trends Cardiovasc. Med. 2019, 29, 29–39. [Google Scholar] [CrossRef]
- Iarussi, D.; Pisacane, C.; Indolfi, P.; Casale, F.; Martino, V.; Di Tullio, M.T. Evaluation of left ventricular function in long-term survivors of childhood Hodgkin disease. Pediatr. Blood Cancer 2005, 45, 700–705. [Google Scholar] [CrossRef]
- Stoddard, M.F.; Seeger, J.; Liddell, N.E.; Hadley, T.J.; Sullivan, D.M.; Kupersmith, J. Prolongation of isovolumetric relaxation time as assessed by Doppler echocardiography predicts doxorubicin-induced systolic dysfunction in humans. J. Am. Coll. Cardiol. 1992, 20, 62–69. [Google Scholar] [CrossRef]
- Zhang, K.W.; Finkelman, B.S.; Gulati, G.; Narayan, H.K.; Upshaw, J.; Narayan, V.; Plappert, T.; Englefield, V.; Smith, A.M.; Zhang, C.; et al. Abnormalities in 3-Dimensional Left Ventricular Mechanics With Anthracycline Chemotherapy Are Associated With Systolic and Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Tierney, R.M.; Costas, I.; Grove, L.; Spitznagel, E.L., Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004, 291, 2441–2447. [Google Scholar] [CrossRef]
- Aggarwal, R.; Petrie, B.; Bala, W.; Chiu, N. Mortality Outcomes with Intensive Blood Pressure Targets in Chronic Kidney Disease Patients. Hypertension 2019, 73, 1275–1282. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics--2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef]
- Wright, J.T., Jr.; Williamson, J.D.; Whelton, P.K.; Snyder, J.K.; Sink, K.M.; Rocco, M.V.; Reboussin, D.M.; Rahman, M.; Oparil, S.; Lewis, C.E.; et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [CrossRef]
- Dorans, K.S.; Mills, K.T.; Liu, Y.; He, J. Trends in Prevalence and Control of Hypertension According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; N.I.o.H. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 6 January 2020).
- Fernandes, M.; Olde Rikkert, M.G.M. The new US and European guidelines in hypertension: A multi-dimensional analysis. Contemp. Clin. Trials 2019, 81, 44–54. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Herrmann, J.; Yang, E.H.; Iliescu, C.A.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.; Leesar, M.A.; Grines, C.L.; Marmagkiolis, K. Vascular Toxicities of Cancer Therapies: The Old and the New—An Evolving Avenue. Circulation 2016, 133, 1272–1289. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46, S7–S15. [Google Scholar] [CrossRef]
- Fraeman, K.H.; Nordstrom, B.L.; Luo, W.; Landis, S.H.; Shantakumar, S. Incidence of new-onset hypertension in cancer patients: A retrospective cohort study. Int. J. Hypertens. 2013, 2013, 379252. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.B.; Silva, E.N.; Ribeiro, M.L.; Martins Wde, A. Hypertension in patients with cancer. Arq. Bras. Cardiol. 2015, 104, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Tini, G.; Sarocchi, M.; Tocci, G.; Arboscello, E.; Ghigliotti, G.; Novo, G.; Brunelli, C.; Lenihan, D.; Volpe, M.; Spallarossa, P. Arterial hypertension in cancer: The elephant in the room. Int. J. Cardiol. 2019, 281, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Katsi, V.; Magkas, N.; Georgiopoulos, G.; Athanasiadi, E.; Virdis, A.; Masi, S.; Kliridis, P.; Hatziyanni, A.; Tsioufis, C.; Tousoulis, D. Arterial hypertension in patients under antineoplastic therapy: A systematic review. J. Hypertens. 2019, 37, 884–901. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, S. Risk of hypertension in Cancer patients treated with Abiraterone: A meta-analysis. Clin. Hypertens. 2019, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Ciccarese, C.; Bria, E.; Romano, M.; Fantinel, E.; Bimbatti, D.; Muraglia, A.; Porcaro, A.B.; Siracusano, S.; Brunelli, M.; et al. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, e645–e653. [Google Scholar] [CrossRef]
- Qi, W.X.; Shen, Z.; Tang, L.N.; Yao, Y. Risk of hypertension in cancer patients treated with aflibercept: A systematic review and meta-analysis. Clin. Drug Investig. 2014, 34, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; He, A.N.; Shen, Z.; Yao, Y. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2013, 76, 348–357. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, X.; Xu, T.; Xu, X.; Liu, Z. Bevacizumab significantly increases the risks of hypertension and proteinuria in cancer patients: A systematic review and comprehensive meta-analysis. Oncotarget 2017, 8, 51492–51506. [Google Scholar] [CrossRef] [PubMed]
- Laubach, J.P.; Moslehi, J.J.; Francis, S.A.; San Miguel, J.F.; Sonneveld, P.; Orlowski, R.Z.; Moreau, P.; Rosiñol, L.; Faber, E.A., Jr.; Voorhees, P.; et al. A retrospective analysis of 3954 patients in phase 2/3 trials of bortezomib for the treatment of multiple myeloma: Towards providing a benchmark for the cardiac safety profile of proteasome inhibition in multiple myeloma. Br. J. Haematol. 2017, 178, 547–560. [Google Scholar] [CrossRef]
- Mincu, R.I.; Mahabadi, A.A.; Michel, L.; Mrotzek, S.M.; Schadendorf, D.; Rassaf, T.; Totzeck, M. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198890. [Google Scholar] [CrossRef]
- Heinzerling, L.; Eigentler, T.K.; Fluck, M.; Hassel, J.C.; Heller-Schenck, D.; Leipe, J.; Pauschinger, M.; Vogel, A.; Zimmer, L.; Gutzmer, R. Tolerability of BRAF/MEK inhibitor combinations: Adverse event evaluation and management. ESMO Open 2019, 4, e000491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shao, Y.; Wang, K. Incidence and risk of hypertension associated with cabozantinib in cancer patients: A systematic review and meta-analysis. Expert Rev. Clin. Pharmacol. 2016, 9, 1109–1115. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hájek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Waxman, A.J.; Clasen, S.; Hwang, W.T.; Garfall, A.; Vogl, D.T.; Carver, J.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; et al. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, e174519. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016, 30, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, T.; Wiczer, T.; Waller, A.; Philippon, J.; Porter, K.; Haddad, D.; Guha, A.; Rogers, K.A.; Bhat, S.; Byrd, J.C.; et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019, 134, 1919–1928. [Google Scholar] [CrossRef]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Roeker, L.E.; Sarraf Yazdy, M.; Rhodes, J.; Goodfriend, J.; Narkhede, M.; Carver, J.; Mato, A. Hypertension in Patients Treated with Ibrutinib for Chronic Lymphocytic Leukemia. JAMA Netw. Open 2019, 2, e1916326. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Gustine, J.; Meid, K.; Yang, G.; Xu, L.; Liu, X.; Demos, M.; Kofides, A.; Tsakmaklis, N.; Chen, J.G.; et al. Ibrutinib Monotherapy in Symptomatic, Treatment-Naïve Patients With Waldenström Macroglobulinemia. J. Clin. Oncol. 2018, 36, 2755–2761. [Google Scholar] [CrossRef]
- Coutre, S.E.; Byrd, J.C.; Hillmen, P.; Barrientos, J.C.; Barr, P.M.; Devereux, S.; Robak, T.; Kipps, T.J.; Schuh, A.; Moreno, C.; et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019, 3, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Suh, K.J.; Lee, J.Y.; Shin, D.Y.; Koh, Y.; Bang, S.M.; Yoon, S.S.; Park, S.; Kim, I.; Lee, J.O. Analysis of adverse events associated with dasatinib and nilotinib treatments in chronic-phase chronic myeloid leukemia patients outside clinical trials. Int. J. Hematol. 2017, 106, 229–239. [Google Scholar] [CrossRef]
- Blay, J.Y.; Shen, L.; Kang, Y.K.; Rutkowski, P.; Qin, S.; Nosov, D.; Wan, D.; Trent, J.; Srimuninnimit, V.; Pápai, Z.; et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): A randomised phase 3 trial. Lancet Oncol. 2015, 16, 550–560. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Zhao, Y. Incidence and risk of hypertension with ramucirumab in cancer patients: A meta-analysis of published studies. Clin. Drug Investig. 2015, 35, 221–228. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Harrison, C.N.; Vannucchi, A.M.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Knoops, L.; Cervantes, F.; Jones, M.M.; Sun, K.; McQuitty, M.; et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia 2016, 30, 1701–1707. [Google Scholar] [CrossRef]
- Passamonti, F.; Griesshammer, M.; Palandri, F.; Egyed, M.; Benevolo, G.; Devos, T.; Callum, J.; Vannucchi, A.M.; Sivgin, S.; Bensasson, C.; et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): A randomised, open-label, phase 3b study. Lancet Oncol. 2017, 18, 88–99. [Google Scholar] [CrossRef]
- Yang, X.; Pan, X.; Cheng, X.; Kuang, Y.; Cheng, Y. Risk of Hypertension with Sorafenib Use in Patients with Cancer: A Meta-Analysis From 20,494 Patients. Am. J. Ther. 2017, 24, e81–e101. [Google Scholar] [CrossRef]
- Zhu, X.; Stergiopoulos, K.; Wu, S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: Systematic review and meta-analysis. Acta Oncol. 2009, 48, 9–17. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bajetta, E.; Valle, J.; Köhne, C.H.; Hecht, J.R.; Moore, M.; Germond, C.; Berg, W.; Chen, B.L.; Jalava, T.; et al. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J. Clin. Oncol. 2011, 29, 2004–2010. [Google Scholar] [CrossRef]
- Qi, W.X.; Shen, Z.; Lin, F.; Sun, Y.J.; Min, D.L.; Tang, L.N.; He, A.N.; Yao, Y. Incidence and risk of hypertension with vandetanib in cancer patients: A systematic review and meta-analysis of clinical trials. Br. J. Clin. Pharmacol. 2013, 75, 919–930. [Google Scholar] [CrossRef]
- van den Born, B.H.; Lip, G.Y.H.; Brguljan-Hitij, J.; Cremer, A.; Segura, J.; Morales, E.; Mahfoud, F.; Amraoui, F.; Persu, A.; Kahan, T.; et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Caletti, S.; Paini, A.; Coschignano, M.A.; De Ciuceis, C.; Nardin, M.; Zulli, R.; Muiesan, M.L.; Salvetti, M.; Rizzoni, D. Management of VEGF-Targeted Therapy-Induced Hypertension. Curr. Hypertens. Rep. 2018, 20, 68. [Google Scholar] [CrossRef]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.W.; Wang, Z.; Macaulay, E.; Jagsi, R.; Duane, F.; Darby, S.C. Exposure of the Heart in Breast Cancer Radiation Therapy: A Systematic Review of Heart Doses Published During 2003 to 2013. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Geara, A.S.; Hogan, J.J.; Townsend, R.R. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC Cardio Oncol. 2019, 1, 238–251. [Google Scholar] [CrossRef]
- Timmers, H.J.; Karemaker, J.M.; Wieling, W.; Kaanders, J.H.; Folgering, H.T.; Marres, H.A.; Lenders, J.W. Arterial baroreflex and peripheral chemoreflex function after radiotherapy for laryngeal or pharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1203–1210. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Sagstuen, H.; Aass, N.; Fosså, S.D.; Dahl, O.; Klepp, O.; Wist, E.A.; Wilsgaard, T.; Bremnes, R.M. Blood pressure and body mass index in long-term survivors of testicular cancer. J. Clin. Oncol. 2005, 23, 4980–4990. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Halabi, S.; Eisen, T.; Broderick, S.; Stadler, W.M.; Jones, R.J.; Garcia, J.A.; Vaishampayan, U.N.; Picus, J.; Hawkins, R.E.; et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016, 17, 378–388. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 917–927. [Google Scholar] [CrossRef]
- De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Saliba, F.; Jonas, S.; Sudan, D.; Fung, J.; Fischer, L.; et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial. Am. J. Transplant. 2012, 12, 3008–3020. [Google Scholar] [CrossRef]
- Langer, R.M.; Hené, R.; Vitko, S.; Christiaans, M.; Tedesco-Silva, H., Jr.; Ciechanowski, K.; Cassuto, E.; Rostaing, L.; Vilatoba, M.; Machein, U.; et al. Everolimus plus early tacrolimus minimization: A phase III, randomized, open-label, multicentre trial in renal transplantation. Transpl. Int. 2012, 25, 592–602. [Google Scholar] [CrossRef]
- Pavel, M.; Unger, N.; Borbath, I.; Ricci, S.; Hwang, T.L.; Brechenmacher, T.; Park, J.; Herbst, F.; Beaumont, J.L.; Bechter, O. Safety and QOL in Patients with Advanced NET in a Phase 3b Expanded Access Study of Everolimus. Target. Oncol. 2016, 11, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009, 27, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, S.E.; Sandler, A.B.; Brahmer, J.R.; Schiller, J.H.; Johnson, D.H. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J. Clin. Oncol. 2010, 28, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Spano, J.P.; Falandry, C.; Chaibi, P.; Freyer, G. Current targeted therapies in breast cancer: Clinical applications in the elderly woman. Oncologist 2011, 16, 1144–1153. [Google Scholar] [CrossRef][Green Version]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Jones, R.L.; Ewer, M.S. Cardiac and cardiovascular toxicity of nonanthracycline anticancer drugs. Expert Rev. Anticancer Ther. 2006, 6, 1249–1269. [Google Scholar] [CrossRef]
- Wolf, V.L.; Taylor, E.B.; Ryan, M.J. Cyclophosphamide treatment for hypertension and renal injury in an experimental model of systemic lupus erythematosus. Physiol. Rep. 2019, 7, e14059. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.R.; Harel-Sterling, M.; Pizzi, M.; Huynh, L.; Hessey, E.; Zappitelli, M. Long-term renal follow-up of children treated with cisplatin, carboplatin, or ifosfamide: A pilot study. Pediatr. Nephrol. 2018, 33, 2311–2320. [Google Scholar] [CrossRef]
- Gietema, J.A.; Meinardi, M.T.; Messerschmidt, J.; Gelevert, T.; Alt, F.; Uges, D.R.; Sleijfer, D.T. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 2000, 355, 1075–1076. [Google Scholar] [CrossRef]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, M.A.F.; Grimm, D.; Bauer, J.; Wehland, M.; Wise, P.; Magnusson, N.E.; Infanger, M.; Krüger, M. Hypertension Caused by Lenvatinib and Everolimus in the Treatment of Metastatic Renal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 1736. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Grillo-López, A.; Varns, C.; Chambers, K.S.; Hanna, N. Targeted anti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin’s B-cell lymphoma. Biochem. Soc. Trans. 1997, 25, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D.R. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Ranpura, V.; Pulipati, B.; Chu, D.; Zhu, X.; Wu, S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: A meta-analysis. Am. J. Hypertens. 2010, 23, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, C.; Wong, S.J.; Hari, P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N. Engl. J. Med. 2006, 354, 980–982. [Google Scholar] [PubMed]
- Moslehi, J.J.; Deininger, M. Tyrosine Kinase Inhibitor-Associated Cardiovascular Toxicity in Chronic Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Fazio, N.; Ferrero, A.; Brizzi, M.P.; Volante, M.; Nobili, E.; Tozzi, L.; Bodei, L.; Torta, M.; D’Avolio, A.; et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: The XELBEVOCT study. BMC Cancer 2014, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Tomczak, P.; Hutson, T.E.; Michaelson, M.D.; Negrier, S.; Oudard, S.; Gore, M.E.; Tarazi, J.; Hariharan, S.; et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013, 14, 552–562. [Google Scholar] [CrossRef]
- Toblli, J.E.; Bevione, P.; Di Gennaro, F.; Madalena, L.; Cao, G.; Angerosa, M. Understanding the mechanisms of proteinuria: Therapeutic implications. Int. J. Nephrol. 2012, 2012, 546039. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Robinson, E.S.; Khankin, E.V.; Choueiri, T.K.; Dhawan, M.S.; Rogers, M.J.; Karumanchi, S.A.; Humphreys, B.D. Suppression of the nitric oxide pathway in metastatic renal cell carcinoma patients receiving vascular endothelial growth factor-signaling inhibitors. Hypertension 2010, 56, 1131–1136. [Google Scholar] [CrossRef]
- Mourad, J.J.; des Guetz, G.; Debbabi, H.; Levy, B.I. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann. Oncol. 2008, 19, 927–934. [Google Scholar] [CrossRef]
- Zou, A.P.; Cowley, A.W., Jr. Role of nitric oxide in the control of renal function and salt sensitivity. Curr. Hypertens. Rep. 1999, 1, 178–186. [Google Scholar] [CrossRef]
- Chen, D.D.; Dong, Y.G.; Yuan, H.; Chen, A.F. Endothelin 1 activation of endothelin A receptor/NADPH oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension. Hypertension 2012, 59, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Neves, K.B.; Rios, F.J.; van der Mey, L.; Alves-Lopes, R.; Cameron, A.C.; Volpe, M.; Montezano, A.C.; Savoia, C.; Touyz, R.M. VEGFR (Vascular Endothelial Growth Factor Receptor) Inhibition Induces Cardiovascular Damage via Redox-Sensitive Processes. Hypertension 2018, 71, 638–647. [Google Scholar] [CrossRef]
- Curwen, J.O.; Musgrove, H.L.; Kendrew, J.; Richmond, G.H.; Ogilvie, D.J.; Wedge, S.R. Inhibition of vascular endothelial growth factor-a signaling induces hypertension: Examining the effect of cediranib (recentin; AZD2171) treatment on blood pressure in rat and the use of concomitant antihypertensive therapy. Clin. Cancer Res. 2008, 14, 3124–3131. [Google Scholar] [CrossRef]
- Kappers, M.H.; van Esch, J.H.; Sluiter, W.; Sleijfer, S.; Danser, A.H.; van den Meiracker, A.H. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010, 56, 675–681. [Google Scholar] [CrossRef]
- Advani, A.; Kelly, D.J.; Advani, S.L.; Cox, A.J.; Thai, K.; Zhang, Y.; White, K.E.; Gow, R.M.; Marshall, S.M.; Steer, B.M.; et al. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc. Natl. Acad. Sci. USA 2007, 104, 14448–14453. [Google Scholar] [CrossRef]
- Eremina, V.; Jefferson, J.A.; Kowalewska, J.; Hochster, H.; Haas, M.; Weisstuch, J.; Richardson, C.; Kopp, J.B.; Kabir, M.G.; Backx, P.H.; et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 2008, 358, 1129–1136. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef]
- Kappers, M.H.; Smedts, F.M.; Horn, T.; van Esch, J.H.; Sleijfer, S.; Leijten, F.; Wesseling, S.; Strevens, H.; Jan Danser, A.H.; van den Meiracker, A.H. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension 2011, 58, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Alivon, M.; Giroux, J.; Briet, M.; Goldwasser, F.; Laurent, S.; Boutouyrie, P. Large artery stiffness and hypertension after antiangiogenic drugs: Influence on cancer progression. J. Hypertens. 2015, 33, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Schiller, J.H.; Fruehauf, J.P.; Cohen, E.E.; Tarazi, J.C.; Rosbrook, B.; Bair, A.H.; Ricart, A.D.; Olszanski, A.J.; Letrent, K.J.; et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin. Cancer Res. 2011, 17, 3841–3849. [Google Scholar] [CrossRef]
- Rizzo, J.D.; Somerfield, M.R.; Hagerty, K.L.; Seidenfeld, J.; Bohlius, J.; Bennett, C.L.; Cella, D.F.; Djulbegovic, B.; Goode, M.J.; Jakubowski, A.A.; et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update. Blood 2008, 111, 25–41. [Google Scholar] [CrossRef]
- Aw, T.J.; Haas, S.J.; Liew, D.; Krum, H. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch. Intern. Med. 2005, 165, 490–496. [Google Scholar] [CrossRef]
- Farkouh, M.E.; Kirshner, H.; Harrington, R.A.; Ruland, S.; Verheugt, F.W.; Schnitzer, T.J.; Burmester, G.R.; Mysler, E.; Hochberg, M.C.; Doherty, M.; et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: Randomised controlled trial. Lancet 2004, 364, 675–684. [Google Scholar] [CrossRef]
- MacDonald, T.M.; Reginster, J.Y.; Littlejohn, T.W.; Richard, D.; Lheritier, K.; Krammer, G.; Rebuli, R. Effect on blood pressure of lumiracoxib versus ibuprofen in patients with osteoarthritis and controlled hypertension: A randomized trial. J. Hypertens. 2008, 26, 1695–1702. [Google Scholar] [CrossRef]
- Mangray, M.; Vella, J.P. Hypertension after kidney transplant. Am. J. Kidney Dis. 2011, 57, 331–341. [Google Scholar] [CrossRef]
- Pope, J.E.; Anderson, J.J.; Felson, D.T. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch. Intern. Med. 1993, 153, 477–484. [Google Scholar] [CrossRef]
- Ruschitzka, F.; Borer, J.S.; Krum, H.; Flammer, A.J.; Yeomans, N.D.; Libby, P.; Lüscher, T.F.; Solomon, D.H.; Husni, M.E.; Graham, D.Y.; et al. Differential blood pressure effects of ibuprofen, naproxen, and celecoxib in patients with arthritis: The PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement) Trial. Eur. Heart J. 2017, 38, 3282–3292. [Google Scholar] [CrossRef]
- Snowden, S.; Nelson, R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol. Rev. 2011, 19, 184–191. [Google Scholar] [CrossRef]
- Wehling, M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: Management and mitigation of risks and adverse effects. Eur. J. Clin. Pharmacol. 2014, 70, 1159–1172. [Google Scholar] [CrossRef]
- Foy, M.C.; Vaishnav, J.; Sperati, C.J. Drug-Induced Hypertension. Endocrinol. Metab. Clin. N. Am. 2019, 48, 859–873. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Johnson, M.; Wagh, A.; Qin, Y.; Bartels-Peculis, L.; Ciepielewska, G.; Nelson, W.W. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr. Med. Res. Opin. 2018, 34, 1519–1527. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef]
- Chari, A.; Mezzi, K.; Zhu, S.; Werther, W.; Felici, D.; Lyon, A.R. Incidence and risk of hypertension in patients newly treated for multiple myeloma: A retrospective cohort study. BMC Cancer 2016, 16, 912. [Google Scholar] [CrossRef]
- Rini, B.I.; Cohen, D.P.; Lu, D.R.; Chen, I.; Hariharan, S.; Gore, M.E.; Figlin, R.A.; Baum, M.S.; Motzer, R.J. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl. Cancer Inst. 2011, 103, 763–773. [Google Scholar] [CrossRef]
| Classification | |||||
|---|---|---|---|---|---|
| CTCAE Qualitative description | Asymptomatic or mild symptoms | Minimal or moderate symptoms limiting activities of daily living | Severe or medically significant, may require hospitalization not life threatening | Life threatening or urgent intervention indicated | Death related to adverse effects |
| CTCAE grade Hypertension | CTCAE grade 1 Adult SBP 120–139 or DBP 80–89 | CTCAE grade 2 SBP 140–159 or DBP 90–99 if previously normal. Symptomatic increase DBP 20 mmHg or > 140/90 | CTCAE grade 3 SBP ≥ 160 mmHg or DBP ≥ 100 mmHg | CTCAE grade 4 Life-threatening consequences: Malignant hypertension (retinopathy with BP > 200/120), hypertensive crisis, permanent neurologic deficit | CTCAE grade 5 Death |
| CTCAE Indicated Treatment | None | Drug monotherapy | More than 1 drug, or increase current therapy | urgent intervention | |
| ACC/AHA | Normal SBP < 120 and DBP < 80 | Elevated SBP 120–129 and DBP < 80 | Stage 1 SBP 130–139, or DBP 80–89 | Stage 2 SBP ≥ 140 or DBP ≥ 90 | |
| ESC 2018 grade | Normal SBP 120–129, and/or DBP 80–84 | High normal SBP 130–139 and/or DBP 85–89 | Grade 1 SBP 140–159 and/or DBP 90–99 | Grade 2 SBP 160–179, and/or DBP 100–109 | Grade 3 SBP ≥ 180, and/or DBP ≥ 110 |
| Drug | Number of Patients | All Grades Hypertension % | CTCAE 3–4 Hypertension % |
|---|---|---|---|
| Abiraterone [29,30,31,32,33] | 8323 | 23.4% | 8.9% |
| Aflibercept [34] | 4451 | 42.4% | 17.4% |
| Axitinib [35] | 1908 | 40% | 13.1% |
| Bevacizumab [36] | 21,902 | 25% | 8% |
| Bortezomib [37] | 2509 | 6.5% | 1.6% |
| BRAF + MEK inhibitors [38,39] | 791 | 20.6% | 10.1% |
| Cabozantinib [40] | 1514 | 28% | 7% |
| Carfilzomib [41,42,43] | 2594 | 12% | 4.3% |
| Imatinib [22,44] | 280 | 4% | 0.4% |
| Ibrutinib [45,46,47,48,49] | 1364 | 49.1% | 16.3% |
| Lenvatinib [50] | 261 | 67.8% | 42.9% |
| Nilotinib [44,51,52] | 997 | 5.9% | 1.1% |
| Niraparib [53] | 367 | 19.3% | 8.2% |
| Pozapanib [54] | 1651 | 36% | 7% |
| Ramucirumab [55] | 3851 | 20% | 9% |
| Regorafenib [56] | 1069 | 44% | 12.5% |
| Ruxolitinib [57,58] | 220 | 9.3% | 6.7% |
| Sorafinib [59] | 20,494 | 21% | 6% |
| Sunitinib [60] | 4999 | 22% | 7.9% |
| Vatalanib [61] | 422 | 29% | 22% |
| Vandetanib [62] | 3154 | 24% | 6.8% |
| Drug | N | All Grades Hypertension | CTCAE Grade 3 or 4 Hypertension |
|---|---|---|---|
| Cisplatin [71] | 500 | 50–53% | 8.1–11.8% on anti-hypertensive medication |
| Everolimus [72,73,74,75,76] | 985 | 8.6–10% | 0.4–2% |
| Interferon alpha [77] | 360 | 4% | 1% |
| Paclitaxel [78,79,80] | 717 | 0.8% | 0.7% |
| Incidence of Hypertension | Magnitude | Mechanism | Indication | |
|---|---|---|---|---|
| Erythroypoeisis stimulating agents [26] | 33% | SBP + 5 to +8 mmHg | Increased systemic vascular resistance due to direct vasopressor, increased blood viscosity and nitric oxide vasodilator resistance | Anemia of chemotherapy, Hb < 10 g/dL. Black box warning—contra-indicated in non-chemotherapy cancer anemia |
| Glucocorticoids [122,123,124] | 20–30% | Sodium and water retention Upregulation of AT1 receptors | Immunosuppression in combination with CVP or R-CHOP regimens |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, R.; Tyebally, S.; Chen, D.; Kapil, V.; Walker, J.M.; Addison, D.; Ismail-Khan, R.; Guha, A.; Ghosh, A.K. Hypertensive Cardiotoxicity in Cancer Treatment—Systematic Analysis of Adjunct, Conventional Chemotherapy, and Novel Therapies—Epidemiology, Incidence, and Pathophysiology. J. Clin. Med. 2020, 9, 3346. https://doi.org/10.3390/jcm9103346
Chung R, Tyebally S, Chen D, Kapil V, Walker JM, Addison D, Ismail-Khan R, Guha A, Ghosh AK. Hypertensive Cardiotoxicity in Cancer Treatment—Systematic Analysis of Adjunct, Conventional Chemotherapy, and Novel Therapies—Epidemiology, Incidence, and Pathophysiology. Journal of Clinical Medicine. 2020; 9(10):3346. https://doi.org/10.3390/jcm9103346
Chicago/Turabian StyleChung, Robin, Sara Tyebally, Daniel Chen, Vikas Kapil, J. Malcolm Walker, Daniel Addison, Roohi Ismail-Khan, Avirup Guha, and Arjun K Ghosh. 2020. "Hypertensive Cardiotoxicity in Cancer Treatment—Systematic Analysis of Adjunct, Conventional Chemotherapy, and Novel Therapies—Epidemiology, Incidence, and Pathophysiology" Journal of Clinical Medicine 9, no. 10: 3346. https://doi.org/10.3390/jcm9103346
APA StyleChung, R., Tyebally, S., Chen, D., Kapil, V., Walker, J. M., Addison, D., Ismail-Khan, R., Guha, A., & Ghosh, A. K. (2020). Hypertensive Cardiotoxicity in Cancer Treatment—Systematic Analysis of Adjunct, Conventional Chemotherapy, and Novel Therapies—Epidemiology, Incidence, and Pathophysiology. Journal of Clinical Medicine, 9(10), 3346. https://doi.org/10.3390/jcm9103346






