Hematological Phenotype of COVID-19-Induced Coagulopathy: Far from Typical Sepsis-Induced Coagulopathy
Abstract
:1. Introduction
2. Experimental Section
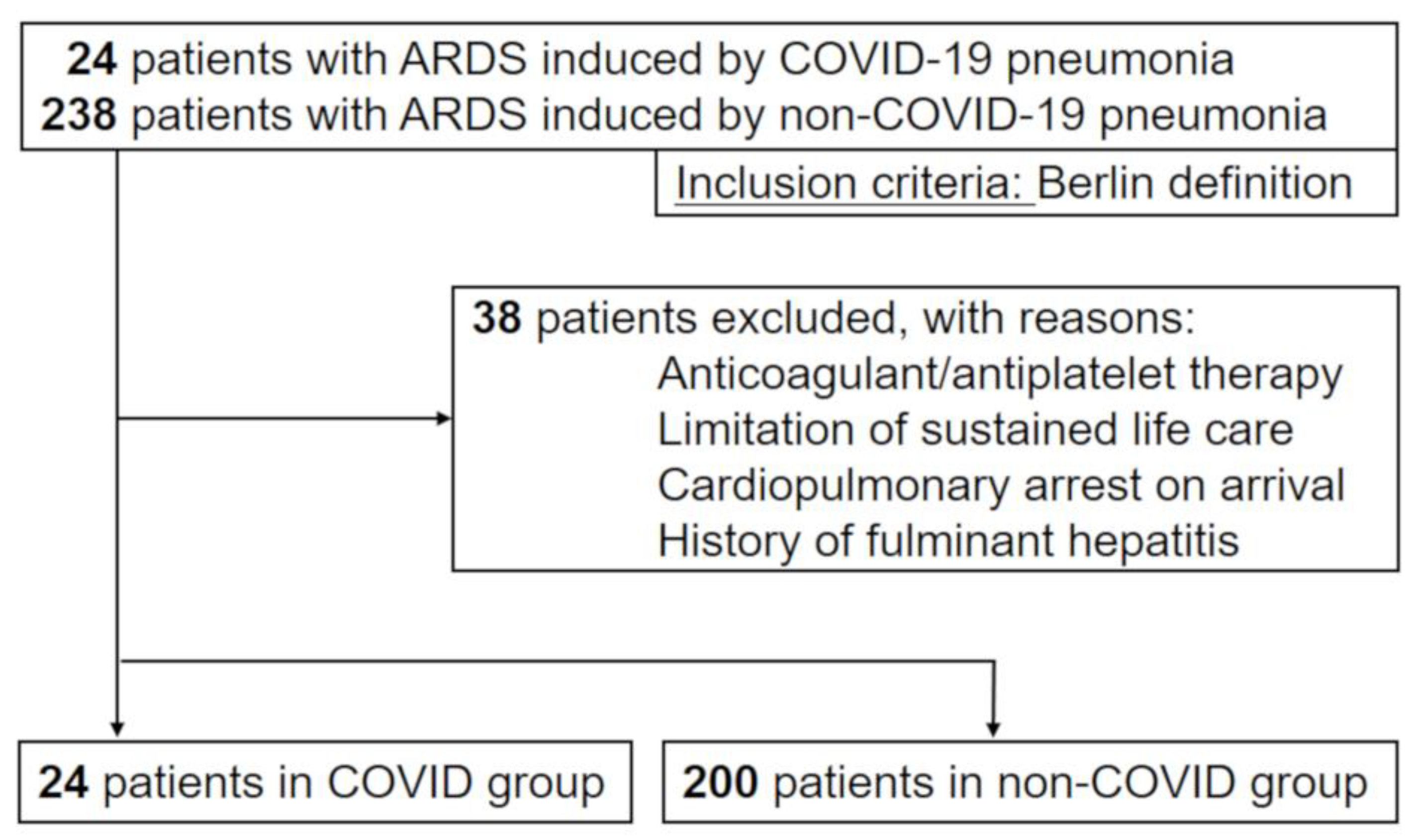
2.1. Study Design, Setting, and Participants
2.2. Diagnosis of COVID-19 and ARDS
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Hemostatic Parameters in COVID-19 Pneumonia
3.3. Differences in Clinical Manifestations over Time
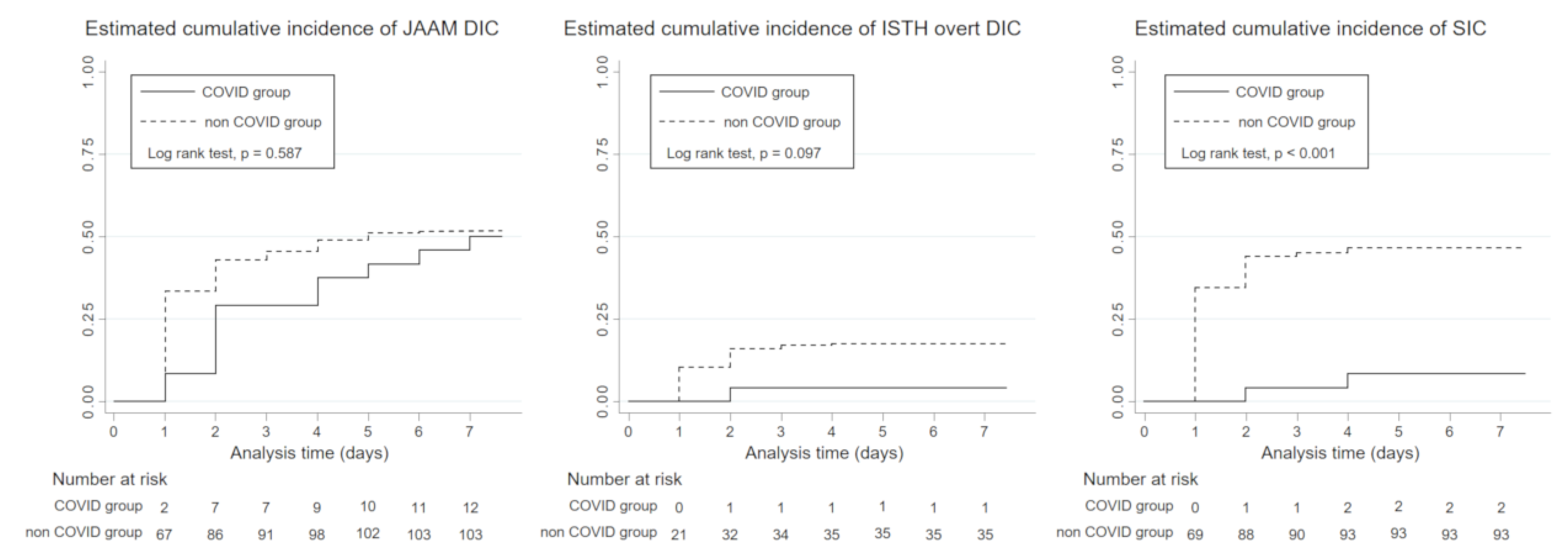
3.4. Incidence of DIC
4. Discussion
4.1. Specific Phenotype of Coagulopathy in COVID-19
4.2. Incidence of DIC in COVID-19
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019-Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. SSRN Electron. J. 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, H.; Townsend, L.; Ni Cheallaigh, C.; Bergin, C.; Martin-Loeches, I.; Browne, P.; Bacon, C.L.; Gaule, R.; Gillett, A.; Byrne, M.; et al. More on COVID-19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020, 189, 1060–1061. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Wang, J.; Hajizadeh, N.; Moore, E.E.; McIntyre, R.C.; Moore, P.K.; Veress, L.A.; Yaffe, M.B.; Moore, H.B.; Barrett, C.D. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Asakura, H. Classifying types of disseminated intravascular coagulation: Clinical and animal models. J. Intensiv. Care 2014, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Umemura, Y.; Yamakawa, K.; Ogura, H.; Yuhara, H.; Fujimi, S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: A meta-analysis of randomized controlled trials. J. Thromb. Haemost. 2016, 14, 518–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance. Published 28 January 2020. Available online: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed on 31 January 2020).
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Iba, T.; Levy, J.H.; Warkentin, T.E.; Thachil, J.; Van Der Poll, T.; Levi, M. Scientific and Standardization Committee on DIC, and the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019, 17, 1989–1994. [Google Scholar] [PubMed] [Green Version]
- Taylor, F.B., Jr.; Toh, C.-H.; Hooth, W.K.; Wada, H.; Levi, M. Scientific and Standardization Committee Communications: Towards a Definition, Clinical and Laboratory Criteria, and a Scoring System for Disseminated Intravascular Coagulation. Thromb Haemost. 2001, 86, 1327–1330. [Google Scholar] [CrossRef] [Green Version]
- Gando, S.; Saitoh, D.; Ogura, H.; Mayumi, T.; Koseki, K.; Ikeda, T.; Ishikura, H.; Iba, T.; Ueyama, M.; Eguchi, Y.; et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: Results of a multicenter, prospective survey*. Crit. Care Med. 2008, 36, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Gando, S.; Iba, T.; Eguchi, Y.; Ohtomo, Y.; Okamoto, K.; Koseki, K.; Mayumi, T.; Murata, A.; Ikeda, T.; Ishikura, H.; et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria*. Crit. Care Med. 2006, 34, 625–631. [Google Scholar] [CrossRef]
- Ramos, L.F.; Shintani, A.; Ikizler, T.A.; Himmelfarb, J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J. Am. Soc. Nephrol. 2008, 19, 593–599. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis); et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensiv. Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Schouten, M.; Van Der Poll, T. Sepsis, Coagulation, and Antithrombin: Old Lessons and New Insights. Semin. Thromb. Hemost. 2008, 34, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semeraro, F.; Colucci, M.; Caironi, P.; Masson, S.; Ammollo, C.T.; Teli, R.; Semeraro, N.; Magnoli, M.; Salati, G.; Isetta, M.; et al. Platelet Drop and Fibrinolytic Shutdown in Patients With Sepsis. Crit. Care Med. 2018, 46, e221–e228. [Google Scholar] [CrossRef] [PubMed]
- Gris, J.-C.; Bouvier, S.; Cochery-Nouvellon, E.; Faillie, J.-L.; Lissalde-Lavigne, G.; Lefrant, J.-Y. Fibrin-related markers in patients with septic shock: Individual comparison of D-dimers and fibrin monomers impacts on prognosis. Thromb. Haemost. 2011, 106, 1228–1230. [Google Scholar]
- Linkins, L.-A.; Takach Lapner, S. Review of D-dimer testing: Good, bad, and ugly. Int. J. Lab. Hematol. 2017, 39 (Suppl. 1), 98–103. [Google Scholar] [CrossRef] [Green Version]
- Bannow, B.T.S.; Konkle, B. Laboratory biomarkers for venous thromboembolism risk in patients with hematologic malignancies: A review. Thromb. Res. 2018, 163, 138–145. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.-P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Llitjos, J.; Leclerc, M.; Chochois, C.; Monsallier, J.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Raj, A.; Warkentin, T.E. Advance in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. J. Clin. Med. 2019, 8, 728. [Google Scholar] [CrossRef] [Green Version]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]



| COVID-19 Group | Non-COVID-19 Group | p Value | |
|---|---|---|---|
| n = 24 | n = 200 | ||
| Sex (male) | 15 (63%) | 138 (69%) | 0.518 |
| Age (year) | 71 (64–76) | 72 (66–79) | 0.336 |
| Pre-existing conditions | |||
| Hypertension | 10 (42%) | 51 (26%) | 0.093 |
| Diabetes mellitus | 10 (42%) | 59 (30%) | 0.223 |
| Chronic heart failure | 2 (8%) | 27 (14%) | 0.382 |
| Chronic kidney disease | 2 (8%) | 24 (12%) | 0.596 |
| Chronic liver failure | 2 (8%) | 17 (9%) | 0.978 |
| Chronic respiratory failure | 3 (13%) | 30 (15%) | 0.744 |
| Cerebrovascular disease | 4 (17%) | 29 (15%) | 0.777 |
| Cancer | 2(8%) | 12 (6%) | 0.317 |
| Pathogens | |||
| Gram-positive | - | 33 (16.5%) | |
| Gram-negative | - | 71 (35.5%) | |
| Polymicrobial infection | - | 63 (31.5%) | |
| Culture negative | - | 33 (16.5%) | |
| Laboratory tests | |||
| Platelet count (103/µL) | 177 (125–249) | 173 (117–246) | 0.905 |
| Prothrombin time (%) | 90.6 (83.4–98.3) | 65.7 (50.1–79.1) | <0.001 |
| Fibrinogen (mg/dL) | 577.5 (500.5–672.5) | 429 (304–558) | <0.001 |
| Antithrombin activity (%) | 85.1 (75–95.5) | 63 (51.6–77.7) | <0.001 |
| FDP (µg/mL) | 5.1 (3.8–7.8) | 10.6 (5.6–21.7) | 0.001 |
| D-dimer (µg/mL) | 2 (1.2–5) | 4.4 (2.1–11.5) | 0.006 |
| Serum creatinine (mg/dL) | 0.9 (0.6–1.1) | 1.3 (0.8–2.2) | 0.001 |
| Serum bilirubin (mg/dL) | 0.6 (0.5–0.9) | 0.8 (0.5–1.2) | 0.139 |
| CRP (mg/dL) | 14.2 (9.1–20.3) | 10.7 (4.4–19.9) | 0.157 |
| White blood cells (103/µL) | 6.3 (5.1-8) | 7.8 (4.1–11.8) | 0.154 |
| Severity of illness | |||
| Glasgow Coma Scale | 13 (6–15) | 11 (6–14) | 0.631 |
| P/F ratio | 181 (124–277) | 176 (121–256) | 0.417 |
| SOFA score | 7 (5–8) | 8 (6–11) | 0.012 |
| APACHE II score | 19 (12–25) | 22 (16–27) | 0.105 |
| JAAM DIC score | 0.5 (0–1) | 2 (1–4) | <0.001 |
| ISTH DIC score | 0 (0–1) | 2 (1–4) | <0.001 |
| SIC score | 2 (2–2) | 3 (2–4) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umemura, Y.; Yamakawa, K.; Kiguchi, T.; Nishida, T.; Kawada, M.; Fujimi, S. Hematological Phenotype of COVID-19-Induced Coagulopathy: Far from Typical Sepsis-Induced Coagulopathy. J. Clin. Med. 2020, 9, 2875. https://doi.org/10.3390/jcm9092875
Umemura Y, Yamakawa K, Kiguchi T, Nishida T, Kawada M, Fujimi S. Hematological Phenotype of COVID-19-Induced Coagulopathy: Far from Typical Sepsis-Induced Coagulopathy. Journal of Clinical Medicine. 2020; 9(9):2875. https://doi.org/10.3390/jcm9092875
Chicago/Turabian StyleUmemura, Yutaka, Kazuma Yamakawa, Takeyuki Kiguchi, Takeshi Nishida, Masahiro Kawada, and Satoshi Fujimi. 2020. "Hematological Phenotype of COVID-19-Induced Coagulopathy: Far from Typical Sepsis-Induced Coagulopathy" Journal of Clinical Medicine 9, no. 9: 2875. https://doi.org/10.3390/jcm9092875
APA StyleUmemura, Y., Yamakawa, K., Kiguchi, T., Nishida, T., Kawada, M., & Fujimi, S. (2020). Hematological Phenotype of COVID-19-Induced Coagulopathy: Far from Typical Sepsis-Induced Coagulopathy. Journal of Clinical Medicine, 9(9), 2875. https://doi.org/10.3390/jcm9092875






