Therapeutic Efficacy of Human Embryonic Stem Cell-Derived Multipotent Stem/Stromal Cells in Diabetic Detrusor Underactivity: A Preclinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. MSC Cell Culture and Establishment of GFP+ M-MSCs
2.3. Animal Model and Study Design
2.4. Evaluation of Bladder Function and Tissue Preparation
2.5. Histological Analysis and Immunofluorescent Staining
2.6. Gene Expression Analyses
2.7. Organ Bath Study
2.8. Statistical Analysis
3. Results
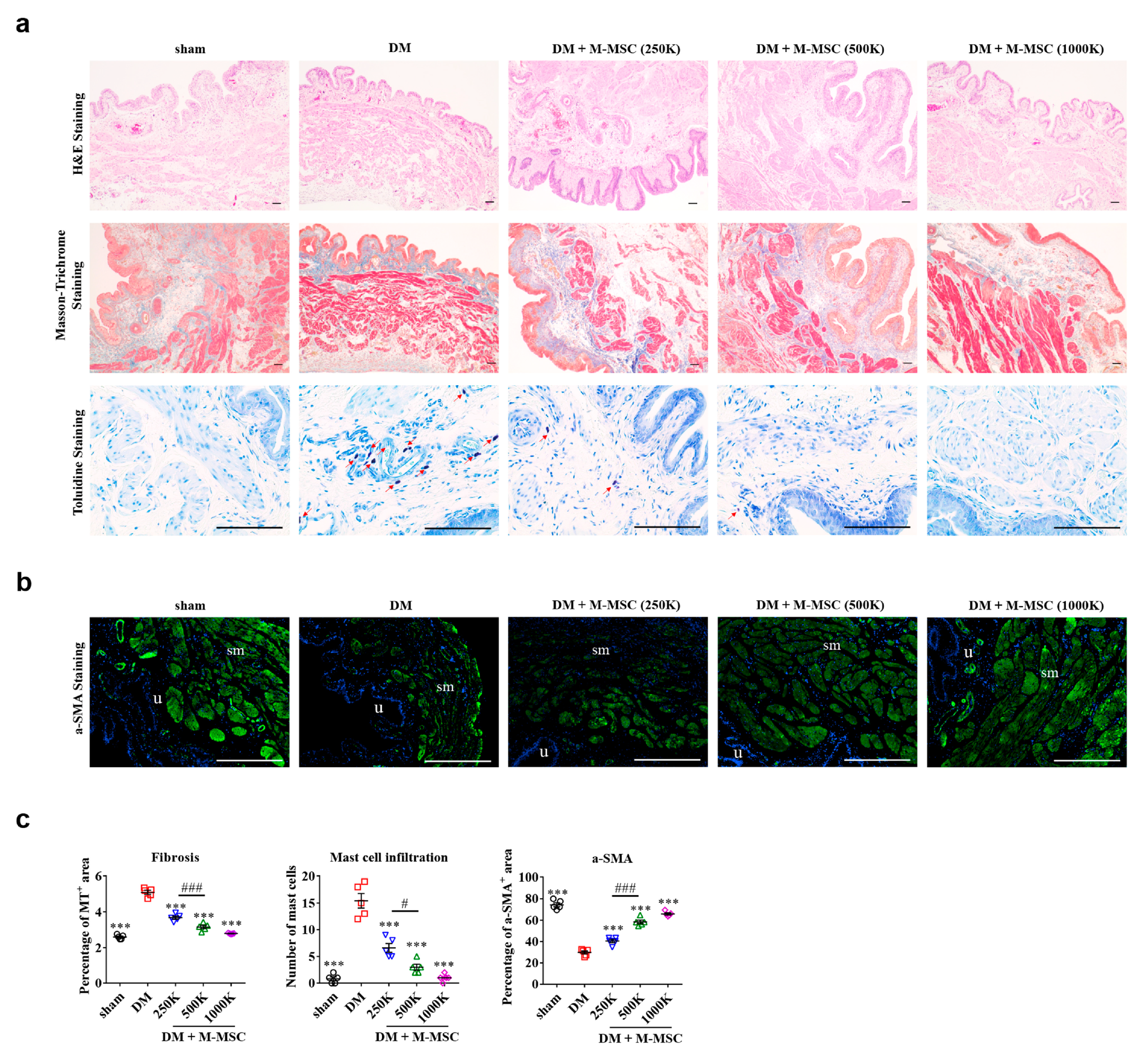
3.1. Therapeutic Efficacy of M-MSCs in an Animal Model of Diabetic DUA
3.2. Long-Term Therapeutic Effects of M-MSC Transplantation
3.3. Determining Optimal Cell Dosage for M-MSC Therapy
3.4. Therapeutic Efficacy of M-MSCs Relative to Umbilical Cord-Derived Stem Cells
3.5. Cellular Properties of Transplanted M-MSCs
3.6. Paracrine Effects of M-MSCs for Suppressing Apoptosis in Diabetic DUA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37. [Google Scholar] [CrossRef]
- Aldamanhori, R.; Chapple, C.R. Underactive bladder, detrusor underactivity, definition, symptoms, epidemiology, etiopathogenesis, and risk factors. Curr. Opin. Urol. 2017, 27, 293. [Google Scholar] [CrossRef] [PubMed]
- Gammie, A.; Kaper, M.; Dorrepaal, C.; Kos, T.; Abrams, P. Signs and Symptoms of Detrusor Underactivity: An Analysis of Clinical Presentation and Urodynamic Tests from a Large Group of Patients Undergoing Pressure Flow Studies. Eur. Urol. 2016, 69, 361. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R.; Osman, N.I.; Birder, L.; van Koeveringe, G.A.; Oelke, M.; Nitti, V.W.; Drake, M.J.; Yamaguchi, O.; Abrams, P.; Smith, P.P. The underactive bladder: A new clinical concept? Eur. Urol. 2015, 68, 351. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Chapple, C.R.; Abrams, P.; Dmochowski, R.; Haab, F.; Nitti, V.; Koelbl, H.; van Kerrebroeck, P.; Wein, A.J. Detrusor underactivity and the underactive bladder: A new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur. Urol. 2014, 65, 389. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Tang, Z.; He, C.; Tang, W. Diabetic cystopathy: A review. J. Diabetes 2015, 7, 442. [Google Scholar] [CrossRef]
- Arrellano-Valdez, F.; Urrutia-Osorio, M.; Arroyo, C.; Elena, S.-V. A comprehensive review of urologic complications in patients with diabetes. Springerplus 2014, 3, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Li, M.; Vasanji, A.; Daneshgari, F. Temporal diabetes and diuresis-induced alteration of nerves and vasculature of the urinary bladder in the rat. BJU Int. 2011, 107, 1988. [Google Scholar] [CrossRef] [PubMed]
- Deli, G.; Bosnyak, E.; Pusch, G.; Komoly, S.; Feher, G. Diabetic neuropathies: Diagnosis and management. Neuroendocrinology 2013, 98, 267. [Google Scholar] [CrossRef]
- Tarcan, T.; Rademakers, K.; Arlandis, S.; von Gontard, A.; van Koeveringe, G.A.; Abrams, P. Do the definitions of the underactive bladder and detrusor underactivity help in managing patients: International Consultation on Incontinence Research Society (ICI-RS) Think Tank 2017? Neurourol. Urodyn. 2018, 37, S60. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Esperto, F.; Chapple, C.R. Detrusor Underactivity and the Underactive Bladder: A Systematic Review of Preclinical and Clinical Studies. Eur. Urol. 2018, 74, 633. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.W.; Cannon, A.; Bartlett, E.; Ellis-Jones, J.; Abrams, P. The natural history of lower urinary tract dysfunction in men: Minimum 10-year urodynamic follow-up of untreated bladder outlet obstruction. BJU Int. 2005, 96, 1301. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Shin, D.M.; Choo, M.S. Stem Cell Therapy for Interstitial Cystitis/Bladder Pain Syndrome. Curr. Urol. Rep. 2016, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Ryu, C.M.; Yu, H.Y.; Shin, D.M.; Choo, M.S. Current and Future Directions of Stem Cell Therapy for Bladder Dysfunction. Stem Cell Rev. Rep. 2020, 16, 82. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, S.R.; Song, Y.S.; Lee, H.J. Stem cell therapy in bladder dysfunction: Where are we? And where do we have to go? Biomed. Res. Int. 2013, 2013, 930713. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lim, J.; Lee, J.H.; Ju, H.; Heo, J.; Kim, Y.H.; Kim, S.; Yu, H.Y.; Ryu, C.M.; Lee, S.Y.; et al. Ascorbic Acid 2-Glucoside Stably Promotes the Primitiveness of Embryonic and Mesenchymal Stem Cells Through Ten-Eleven Translocation- and cAMP-Responsive Element-Binding Protein-1-Dependent Mechanisms. Antioxid. Redox Signal. 2020, 32, 35. [Google Scholar] [CrossRef]
- Lim, J.; Heo, J.; Ju, H.; Shin, J.W.; Kim, Y.H.; Lee, S.; Yu, H.Y.; Ryu, C.M.; Yun, H.D.; Song, S.; et al. Glutathione dynamics determine the therapeutic efficacy of mesenchymal stem cells for graft-versus-host disease via CREB1-NRF2 pathway. Sci. Adv. 2020, 6, eaba1334. [Google Scholar] [CrossRef]
- Kim, Y.; Jin, H.J.; Heo, J.; Ju, H.; Lee, H.Y.; Kim, S.; Lee, S.; Lim, J.; Jeong, S.Y.; Kwon, J.; et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia 2018, 32, 2672. [Google Scholar] [CrossRef]
- Knaän-Shanzer, S. Concise Review: The Immune Status of Mesenchymal Stem Cells and Its Relevance for Therapeutic Application. Stem Cells 2014, 32, 603. [Google Scholar] [CrossRef]
- Hong, K.S.; Bae, D.; Choi, Y.; Kang, S.W.; Moon, S.H.; Lee, H.T.; Chung, H.M. A porous membrane-mediated isolation of mesenchymal stem cells from human embryonic stem cells. Tissue Eng. Part C Methods 2015, 21, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kate, E.H.; Michelangelo, C.; Kate, D.; Anna, M.R.; Filipa, V.; Kwan, L.H.; Avina, H.; Donald, P.; Pierre, G.; Henrik, H.; et al. Embryonic Stem Cell-Derived Mesenchymal Stem Cells (MSCs) Have a Superior Neuroprotective Capacity over Fetal MSCs in the Hypoxic-Ischemic Mouse Brain. Stem Cells Transl. Med. 2018, 7, 439. [Google Scholar]
- Sheyn, D.; Ben-David, S.; Shapiro, G.; Mel, S.D.; Bez, M.; Ornelas, L.; Sahabian, A.; Sareen, D.; Da, X.; Pelled, G.; et al. Human Induced Pluripotent Stem Cells Differentiate into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl. Med. 2016, 5, 1447. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Yu, H.Y.; Lim, J.; Ryu, C.M.; Kim, Y.H.; Heo, J.; Han, Y.H.; Lee, H.; Bae, Y.S.; Kim, J.Y.; et al. Improved efficacy and in vivo cellular properties of human embryonic stem cell derivative in a preclinical model of bladder pain syndrome. Sci. Rep. 2017, 7, 8872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Ryu, C.M.; Shin, J.H.; Choi, D.; Kim, A.; Yu, H.Y.; Han, J.Y.; Lee, H.Y.; Lim, J.; Kim, Y.H.; et al. The Therapeutic Effect of Human Embryonic Stem Cell-Derived Multipotent Mesenchymal Stem Cells on Chemical-Induced Cystitis in Rats. Int. Neurourol. J. 2018, 22, S34. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.M.Y.H.; Lee, H.Y.; Shin, J.H.; Lee, S.; Ju, H.; Paulson, B.; Lee, S.; Kim, S.; Lim, J.; Heo, J.; et al. Longitudinal intravital imaging of transplanted mesenchymal stem cells elucidates their functional integration and therapeutic potency in an animal model of interstitial cystitis/bladder pain syndrome. Theranostics 2018, 8, 5610. [Google Scholar] [CrossRef]
- Lim, J.; Lee, S.; Ju, H.; Kim, Y.H.; Heo, J.; Lee, H.Y.; Choi, K.C.; Son, J.; Oh, Y.M.; Kim, I.G.; et al. Valproic acid enforces the priming effect of sphingosine-1 phosphate on human mesenchymal stem cells. Int. J. Mol. Med. 2017, 40, 739–747. [Google Scholar] [CrossRef]
- Heo, J.; Lim, J.; Lee, S.; Jeong, J.; Kang, H.; Kim, Y.H.; Kang, J.K.; Yu, H.Y.; Jeong, E.M.; Kim, K.; et al. Sirt1 Regulates DNA Methylation and Differentiation Potential of Embryonic Stem Cells by Antagonizing Dnmt3l. Cell Rep. 2017, 18, 1930. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.; Noh, B.J.; Lee, S.; Lee, H.Y.; Kim, Y.H.; Lim, J.; Ju, H.; Yu, H.Y.; Ryu, C.-M.; Lee, P.C.W.; et al. Phosphorylation of TFCP2L1 by CDK1 is required for stem cell pluripotency and bladder carcinogenesis. EMBO Mol. Med. 2020, 12, e10880. [Google Scholar] [CrossRef]
- Jeong, E.M.; Yoon, J.H.; Lim, J.; Shin, J.W.; Cho, A.Y.; Heo, J.; Lee, K.B.; Lee, J.H.; Kim, H.J.; Son, Y.H.; et al. Real-Time Monitoring of Glutathione in Living Cells Reveals that High Glutathione Levels Are Required to Maintain Stem Cell Function. Stem Cell Rep. 2018, 10, 600. [Google Scholar] [CrossRef] [Green Version]
- Jeong, E.M.; Shin, J.W.; Lim, J.; Kim, J.H.; Kang, H.; Yin, Y.; Kim, H.M.; Kim, Y.H.; Kim, S.G.; Kang, H.S.; et al. Monitoring Glutathione Dynamics and Heterogeneity in Living Stem Cells. Int. J. Stem Cells 2019, 12, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.R.; Lin, J.T.; Pan, C.T.; Chan, T.C.; Liu, C.L.; Wu, W.J.; Sheu, J.J.-C.; Yeh, B.W.; Huang, S.K.; Jhung, J.Y.; et al. Amplification-driven BCL6-suppressed cytostasis is mediated by transrepression of FOXO3 and post-translational modifications of FOXO3 in urinary bladder urothelial carcinoma. Theranostics 2020, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Ying, G.; Danzer, B.; Perez, R.E.; Madar, Z.S.; Levenson, V.V.; Maki, C.G. The prolyl peptidases PRCP/PREP regulate IRS-1 stability critical for rapamycin-induced feedback activation of PI3K and AKT. J. Biol. Chem. 2014, 289, 21694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadakata, T.; Kakegawa, W.; Mizoguchi, A.; Washida, N.; Katoh-Semba, R.; Shutoh, F.; Okamoto, T.; Nakashima, H.; Kimura, K.; Tanaka, M.; et al. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J. Neurosci. 2007, 27, 2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varland, S.; Myklebust, L.M.; Goksoyr, S.O.; Glomnes, N.; Torsvik, J.; Varhaug, J.E.; Arnesen, T. Identification of an alternatively spliced nuclear isoform of human N-terminal acetyltransferase Naa30. Gene 2018, 644, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qiu, X.; Shindel, A.W.; Ning, H.; Ferretti, L.; Jin, X.; Lin, G.; Lin, C.-S.; Lue, T.F. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev. 2012, 21, 1391. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, L.; Zhao, Y.; Wang, M.; Jin, X.; Zhang, H. Endogenous Stem Cells Were Recruited by Defocused Low-Energy Shock Wave in Treating Diabetic Bladder Dysfunction. Stem Cell Rev. 2017, 13, 287. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145. [Google Scholar] [CrossRef] [Green Version]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.J.; Kim, S.Y.; Jeong, H.C.; Cheong, H.; Kim, D.; Park, S.J.; Choi, J.J.; Kim, H.; Chung, H.M.; Moon, S.H.; et al. Repair of Ischemic Injury by Pluripotent Stem Cell Based Cell Therapy without Teratoma through Selective Photosensitivity. Stem Cell Rep. 2015, 5, 1067. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713. [Google Scholar] [CrossRef]
- Jurisicova, A.; Casper, R.F.; MacLusky, N.J.; Mills, G.B.; Librach, C.L. HLA-G expression during preimplantation human embryo development. Proc. Natl. Acad. Sci. USA 1996, 93, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, P.E.; Ransohoff, J.D.; Nahid, A.; Wu, J.C. Immunogenicity of pluripotent stem cells and their derivatives. Circ. Res. 2013, 112, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liang, X.; Liao, S.; Wang, W.; Wang, J.; Li, X.; Ding, Y.; Liang, Y.; Gao, F.; Yang, M.; et al. Potent Paracrine Effects of human induced Pluripotent Stem Cell-derived Mesenchymal Stem Cells Attenuate Doxorubicin-induced Cardiomyopathy. Sci. Rep. 2015, 5, 11235. [Google Scholar] [CrossRef]
- Chin, C.J.; Li, S.; Corselli, M.; Casero, D.; Zhu, Y.; He, C.B.; Hardy, R.; Péault, B.; Crooks, G.M. Transcriptionally and Functionally Distinct Mesenchymal Subpopulations Are Generated from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 436. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Daneshgari, F. Diabetic bladder dysfunction. Chin. Med. J. (Engl.) 2014, 127, 1357. [Google Scholar]
- Wittig, L.; Carlson, K.V.; Andrews, J.M.; Crump, R.T.; Baverstock, R.J. Diabetic bladder dysfunction: A review. Urology 2018. [Google Scholar] [CrossRef]
- Van Koeveringe, G.A.; Vahabi, B.; Andersson, K.E.; Herrmans, R.K.; Oelke, M. Detrusor underactivity: A plea for new approaches to a common bladder dysfunction. Neurourol. Urodyn. 2011, 30, 723. [Google Scholar] [CrossRef]
- Cucchi, A.; Quaglini, S.; Rovereto, B. Development of idiopathic detrusor underactivity in women: From isolated decrease in contraction velocity to obvious impairment of voiding function. Urology 2008, 71, 844. [Google Scholar] [CrossRef]
- Osman, N.I.; Chapple, C.R. Contemporary concepts in the aetiopathogenesis of detrusor underactivity. Nat. Rev. Urol. 2014, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.; Mangera, A.; Hillary, C.; Inman, R.; Chapple, C. The underactive bladder: Detection and diagnosis. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Grundy, L.; Caldwell, A.; Garcia Caraballo, S.G.; Erickson, A.; Schober, G.; Castro, J.; Harrington, A.M.; Brierley, S.M. Histamine induces peripheral and central hypersensitivity to bladder distension via the histamine H1 receptor and TRPV1. Am. J. Physiol. Renal. Physiol. 2020, 318, F298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, P.; Smith, P.P.; Kuchel, G.A.; de Groat, W.C.; Birder, L.A.; Chermansky, C.J.; Adam, R.A.; Tse, V.; Chancellor, M.B.; Yoshimura, N. Pathophysiology and animal modeling of underactive bladder. Int. Urol. Nephrol. 2014, 46 (Suppl. S11), 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, N.; Chancellor, M.B.; Andersson, K.E.; Christ, G.J. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005, 95, 733. [Google Scholar] [CrossRef]
- Sasaki, K.; Chancellor, M.B.; Phelan, M.W.; Yokoyama, T.; Fraser, M.O.; Seki, S.; Kubo, K.; Kumon, H.; de Groat, W.C.; Yoshimura, N. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J. Urol. 2002, 168, 1259. [Google Scholar] [CrossRef]
- Colaco, M.; Osman, N.I.; Karakeci, A.; Artibani, W.; Andersson, K.-E.; Badlani, G.H. Current concepts of the acontractile bladder. BJU Int. 2018, 122, 195. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.H.; Ryu, C.-M.; Ju, H.; Yu, H.Y.; Song, S.; Hong, K.-S.; Chung, H.-M.; Park, J.; Shin, D.-M.; Choo, M.-S. Therapeutic Efficacy of Human Embryonic Stem Cell-Derived Multipotent Stem/Stromal Cells in Diabetic Detrusor Underactivity: A Preclinical Study. J. Clin. Med. 2020, 9, 2853. https://doi.org/10.3390/jcm9092853
Shin JH, Ryu C-M, Ju H, Yu HY, Song S, Hong K-S, Chung H-M, Park J, Shin D-M, Choo M-S. Therapeutic Efficacy of Human Embryonic Stem Cell-Derived Multipotent Stem/Stromal Cells in Diabetic Detrusor Underactivity: A Preclinical Study. Journal of Clinical Medicine. 2020; 9(9):2853. https://doi.org/10.3390/jcm9092853
Chicago/Turabian StyleShin, Jung Hyun, Chae-Min Ryu, Hyein Ju, Hwan Yeul Yu, Sujin Song, Ki-Sung Hong, Hyung-Min Chung, Juhyun Park, Dong-Myung Shin, and Myung-Soo Choo. 2020. "Therapeutic Efficacy of Human Embryonic Stem Cell-Derived Multipotent Stem/Stromal Cells in Diabetic Detrusor Underactivity: A Preclinical Study" Journal of Clinical Medicine 9, no. 9: 2853. https://doi.org/10.3390/jcm9092853
APA StyleShin, J. H., Ryu, C.-M., Ju, H., Yu, H. Y., Song, S., Hong, K.-S., Chung, H.-M., Park, J., Shin, D.-M., & Choo, M.-S. (2020). Therapeutic Efficacy of Human Embryonic Stem Cell-Derived Multipotent Stem/Stromal Cells in Diabetic Detrusor Underactivity: A Preclinical Study. Journal of Clinical Medicine, 9(9), 2853. https://doi.org/10.3390/jcm9092853




