Exosome: A New Player in Translational Nanomedicine
Abstract
1. Introduction
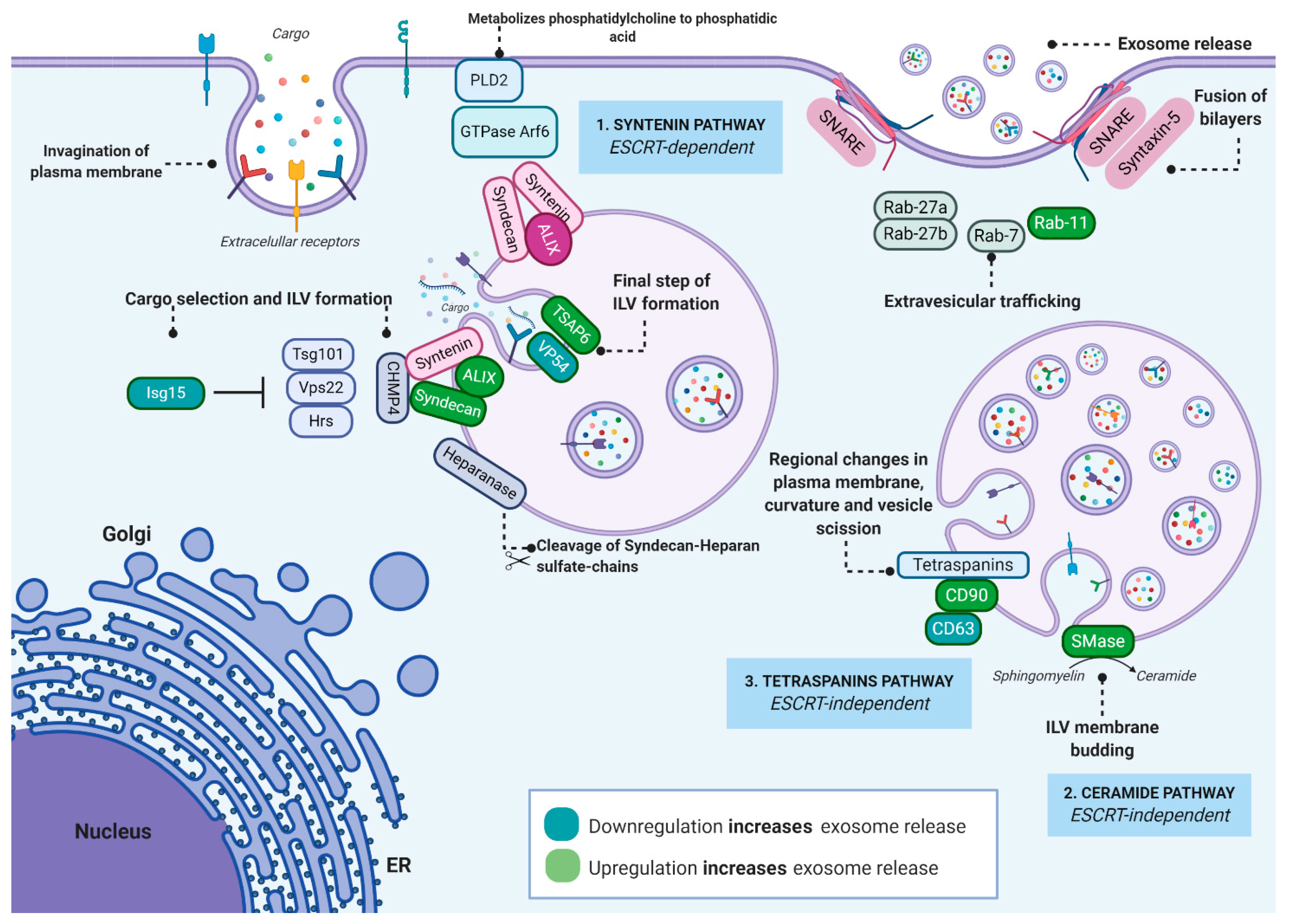
2. Exosome Biogenesis, Regulation and Function
2.1. Exosome Biogenesis
2.2. Regulation and Function
3. Different Types and Functions of Cells that Release Exosomes
3.1. Exosomes Derived from Mesenchymal Stem/Stromal Cells of Different Sources Involved in a Wide Range of Diseases and Metabolic Pathways
3.2. Exosomes Derived from Immune System-Related Cells That Play a Key Role in Several Immunological Processes
4. Exosome Manufacturing Status and Challenges
4.1. Large Scale Production of Exosomes
4.2. High-Quality Uniform Exosomes
4.3. Storage Conditions
4.4. Quality Control
4.5. Treatments for Humans
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Santucci, L.; Bruschi, M.; Del Zotto, G.; Antonini, F.; Ghiggeri, G.M.; Panfoli, I.; Candiano, G. Biological surface properties in extracellular vesicles and their effect on cargo proteins. Sci. Rep. 2019, 9, 13048. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Dini, L.; Tacconi, S.; Carata, E.; Tata, A.M.; Vergallo, C.; Panzarini, E. Microvesicles and exosomes in metabolic diseases and inflammation. Cytokine Growth Factor Rev. 2020, 51, 27–39. [Google Scholar] [CrossRef]
- Lv, Y.; Tan, J.; Miao, Y.; Zhang, Q. The role of microvesicles and its active molecules in regulating cellular biology. J. Cell. Mol. Med. 2019, 23, 7894–7904. [Google Scholar] [CrossRef]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramirez, R.M. Microvesicles: ROS scavengers and ROS producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology (Bethesda) 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Wilhelm, E.N.; Mourot, L.; Rakobowchuk, M. Exercise-Derived Microvesicles: A Review of the Literature. Sports Med. 2018, 48, 2025–2039. [Google Scholar] [CrossRef]
- Aharon, A.; Brenner, B. Microvesicles in Thrombosis and Inflammation. Isr. Med. Assoc. J. 2016, 18, 530–533. [Google Scholar]
- Liu, M.L.; Williams, K.J.; Werth, V.P. Microvesicles in Autoimmune Diseases. Adv. Clin. Chem. 2016, 77, 125–175. [Google Scholar] [CrossRef]
- Larson, M.C.; Hillery, C.A.; Hogg, N. Circulating membrane-derived microvesicles in redox biology. Free Radic. Biol. Med. 2014, 73, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Sabin, K.; Kikyo, N. Microvesicles as mediators of tissue regeneration. Transl. Res. 2014, 163, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, P.; Mulzer, L.M.; Oberstein, T.J.; Munoz, L.E.; Lewczuk, P.; Kornhuber, J.; Herrmann, M.; Maler, J.M. Microvesicles from cerebrospinal fluid of patients with Alzheimer’s disease display reduced concentrations of tau and APP protein. Sci. Rep. 2019, 9, 7089. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Suwanpradid, J.; Kim, I.H.; Staats, H.F.; Haniffa, M.; MacLeod, A.S.; Abraham, S.N. Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science 2018, 362, eaao0666. [Google Scholar] [CrossRef] [PubMed]
- Tower, C.M.; Reyes, M.; Nelson, K.; Leca, N.; Kieran, N.; Muczynski, K.; Jefferson, J.A.; Blosser, C.; Kukla, A.; Maurer, D.; et al. Plasma C4d+ Endothelial Microvesicles Increase in Acute Antibody-Mediated Rejection. Transplantation 2017, 101, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, K.; Llodra, J.; Roth, E.W.; Tsai, J.; Gordo, S.; Wucherpfennig, K.W.; Kam, L.C.; Stokes, D.L.; Dustin, M.L. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 2014, 507, 118–123. [Google Scholar] [CrossRef]
- Oehmcke, S.; Westman, J.; Malmstrom, J.; Morgelin, M.; Olin, A.I.; Kreikemeyer, B.; Herwald, H. A novel role for pro-coagulant microvesicles in the early host defense against streptococcus pyogenes. PLoS Pathog. 2013, 9, e1003529. [Google Scholar] [CrossRef]
- Park, J.O.; Choi, D.Y.; Choi, D.S.; Kim, H.J.; Kang, J.W.; Jung, J.H.; Lee, J.H.; Kim, J.; Freeman, M.R.; Lee, K.Y.; et al. Identification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusions. Proteomics 2013, 13, 2125–2134. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, D.Y.; Yun, S.J.; Choi, S.M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Lazzari, B.; Perrini, C.; Pizzi, F.; Stella, A.; Cremonesi, F.; Capra, E. MicroRNAs of Equine Amniotic Mesenchymal Cell-derived Microvesicles and Their Involvement in Anti-inflammatory Processes. Cell Transplant. 2018, 27, 45–54. [Google Scholar] [CrossRef]
- Tofino-Vian, M.; Guillen, M.I.; Perez Del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Leroyer, A.S.; Rautou, P.E.; Silvestre, J.S.; Castier, Y.; Leseche, G.; Devue, C.; Duriez, M.; Brandes, R.P.; Lutgens, E.; Tedgui, A.; et al. CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J. Am. Coll. Cardiol. 2008, 52, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ye, K.; Yu, J.; Gao, J.; Zhang, L.; Ji, X.; Chen, T.; Wang, H.; Dai, Y.; Tang, B.; et al. Regeneration of sciatic nerves by transplanted microvesicles of human neural stem cells derived from embryonic stem cells. Cell Tissue Bank. 2020, 21, 233–248. [Google Scholar] [CrossRef]
- Noubouossie, D.F.; Henderson, M.W.; Mooberry, M.; Ilich, A.; Ellsworth, P.; Piegore, M.; Skinner, S.C.; Pawlinski, R.; Welsby, I.; Renne, T.; et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood 2020, 135, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Zhou, J.; Zhou, S.S.; Zhang, Y.D.; Ji, T.Y.; Zhang, X.L.; Wang, S.M.; Du, T.; Ding, D.G. Microvesicles derived from human Wharton’s jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR-100. Stem Cell Res. Ther. 2020, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, J.; Chen, T.; Chen, X.; Ji, X.; Ye, K.; Yu, J.; Tang, B.; Wei, Y.; Xu, H.; et al. Microvesicles Derived from Human Embryonic Neural Stem Cells Inhibit the Apoptosis of HL-1 Cardiomyocytes by Promoting Autophagy and Regulating AKT and mTOR via Transporting HSP-70. Stem Cells Int. 2019, 2019, 6452684. [Google Scholar] [CrossRef]
- Kulkarni, R.S.; Bajaj, M.S.; Kale, V.P. Induction and Detection of Autophagy in Aged Hematopoietic Stem Cells by Exposing Them to Microvesicles Secreted by HSC-Supportive Mesenchymal Stromal Cells. Methods Mol. Biol. 2019, 1854, 21–34. [Google Scholar] [CrossRef]
- Berezin, A.E.; Kremzer, A.A.; Samura, T.A.; Berezina, T.A. Altered signature of apoptotic endothelial cell-derived microvesicles predicts chronic heart failure phenotypes. Biomark. Med. 2019, 13, 737–750. [Google Scholar] [CrossRef]
- Schwartz, Y.S.; Dolganova, O.M.; Rudina, M.I.; Pyshnaya, I.A.; Bgatova, N.P. Influence of Apoptotic Bodies and Apoptotic Microvesicles on NO Production in Macrophages. Bull. Exp. Biol. Med. 2018, 165, 453–456. [Google Scholar] [CrossRef]
- Dieker, J.; Hilbrands, L.; Thielen, A.; Dijkman, H.; Berden, J.H.; van der Vlag, J. Enhanced activation of dendritic cells by autologous apoptotic microvesicles in MRL/lpr mice. Arthr. Res. Ther. 2015, 17, 103. [Google Scholar] [CrossRef]
- Razi, M.; Futter, C.E. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol. Biol. Cell 2006, 17, 3469–3483. [Google Scholar] [CrossRef]
- Yu, X.; Harris, S.L.; Levine, A.J. The regulation of exosome secretion: A novel function of the p53 protein. Cancer Res. 2006, 66, 4795–4801. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Raiborg, C.; Bache, K.G.; Gillooly, D.J.; Madshus, I.H.; Stang, E.; Stenmark, H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002, 4, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Thery, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; Belting, M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014, 35, 51–55. [Google Scholar] [CrossRef]
- Jella, K.K.; Rani, S.; O’Driscoll, L.; McClean, B.; Byrne, H.J.; Lyng, F.M. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat. Res. 2014, 181, 138–145. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef]
- Al-Mayah, A.; Bright, S.; Chapman, K.; Irons, S.; Luo, P.; Carter, D.; Goodwin, E.; Kadhim, M. The non-targeted effects of radiation are perpetuated by exosomes. Mutat. Res. 2015, 772, 38–45. [Google Scholar] [CrossRef]
- Lespagnol, A.; Duflaut, D.; Beekman, C.; Blanc, L.; Fiucci, G.; Marine, J.C.; Vidal, M.; Amson, R.; Telerman, A. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008, 15, 1723–1733. [Google Scholar] [CrossRef]
- Charrin, S.; le Naour, F.; Silvie, O.; Milhiet, P.E.; Boucheix, C.; Rubinstein, E. Lateral organization of membrane proteins: Tetraspanins spin their web. Biochem. J. 2009, 420, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Schaper, F.; van Spriel, A.B. Antitumor Immunity Is Controlled by Tetraspanin Proteins. Front. Immunol. 2018, 9, 1185. [Google Scholar] [CrossRef] [PubMed]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome release of beta-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.H.; Odintsova, E.; Haigh, T.A.; Rickinson, A.B.; Taylor, G.S.; Berditchevski, F. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur. J. Immunol. 2011, 41, 2556–2561. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Loewith, R.; Riezman, H.; Winssinger, N. Sphingolipids and membrane targets for therapeutics. Curr. Opin. Chem. Biol. 2019, 50, 19–28. [Google Scholar] [CrossRef]
- Clarke, C.J. Neutral Sphingomyelinases in Cancer: Friend or Foe? Adv. Cancer Res. 2018, 140, 97–119. [Google Scholar] [CrossRef]
- Gatta, A.T.; Carlton, J.G. The ESCRT-machinery: Closing holes and expanding roles. Curr. Opin. Cell Biol. 2019, 59, 121–132. [Google Scholar] [CrossRef]
- Singh, R.K.; Furze, R.C.; Birrell, M.A.; Rankin, S.M.; Hume, A.N.; Seabra, M.C. A role for Rab27 in neutrophil chemotaxis and lung recruitment. BMC Cell Biol. 2014, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Ramstead, A.G.; Bauer, K.M.; Lee, S.H.; Runtsch, M.C.; Wallace, J.; Huffaker, T.B.; Larsen, D.K.; Tolmachova, T.; Seabra, M.C.; et al. Rab27-Dependent Exosome Production Inhibits Chronic Inflammation and Enables Acute Responses to Inflammatory Stimuli. J. Immunol. 2017, 199, 3559–3570. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Kauskot, A.; Kurowska, M.; Goudin, N.; Munoz, I.; Bordet, J.C.; Huang, J.D.; Bryckaert, M.; Fischer, A.; Borgel, D.; et al. Kinesin-1 Is a New Actor Involved in Platelet Secretion and Thrombus Stability. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Al Hawas, R.; Ren, Q.; Ye, S.; Karim, Z.A.; Filipovich, A.H.; Whiteheart, S.W. Munc18b/STXBP2 is required for platelet secretion. Blood 2012, 120, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Jae, N.; McEwan, D.G.; Manavski, Y.; Boon, R.A.; Dimmeler, S. Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett. 2015, 589, 3182–3188. [Google Scholar] [CrossRef]
- Loomis, R.J.; Holmes, D.A.; Elms, A.; Solski, P.A.; Der, C.J.; Su, L. Citron kinase, a RhoA effector, enhances HIV-1 virion production by modulating exocytosis. Traffic 2006, 7, 1643–1653. [Google Scholar] [CrossRef]
- Baker, R.W.; Hughson, F.M. Chaperoning SNARE assembly and disassembly. Nat. Rev. Mol. Cell Biol. 2016, 17, 465–479. [Google Scholar] [CrossRef]
- Fader, C.M.; Sanchez, D.G.; Mestre, M.B.; Colombo, M.I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: Two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta 2009, 1793, 1901–1916. [Google Scholar] [CrossRef]
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 2012, 14, 1036–1045. [Google Scholar] [CrossRef]
- Alenquer, M.; Amorim, M.J. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses 2015, 7, 5066–5083. [Google Scholar] [CrossRef]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavik, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef] [PubMed]
- Glingston, R.S.; Deb, R.; Kumar, S.; Nagotu, S. Organelle dynamics and viral infections: At cross roads. Microbes. Infect. 2019, 21, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ma, P.; Deng, L.; Liu, Z.; Wang, X.; Liu, X.; Long, G. Hepatitis A virus structural protein pX interacts with ALIX and promotes the secretion of virions and foreign proteins through exosome-like vesicles. J. Extracell. Vesicles 2020, 9, 1716513. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Chahar, H.S.; Corsello, T.; Kudlicki, A.S.; Komaravelli, N.; Casola, A. Respiratory Syncytial Virus Infection Changes Cargo Composition of Exosome Released from Airway Epithelial Cells. Sci. Rep. 2018, 8, 387. [Google Scholar] [CrossRef]
- Babatunde, K.A.; Yesodha Subramanian, B.; Ahouidi, A.D.; Martinez Murillo, P.; Walch, M.; Mantel, P.Y. Role of Extracellular Vesicles in Cellular Cross Talk in Malaria. Front. Immunol. 2020, 11, 22. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, S.; Yang, N.; Feng, Y.; Sang, X.; Wang, Y.; Wahlgren, M.; Chen, Q. Characterization of the Catalytic Subunits of the RNA Exosome-like Complex in Plasmodium falciparum. J. Eukaryot. Microbiol. 2018, 65, 843–853. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Miura, T.; Kasai, K.; Fujishima, Y.; Nakata, A.; Yoshida, M. Radiation-Induced Bystander Effect is Mediated by Mitochondrial DNA in Exosome-Like Vesicles. Sci. Rep. 2019, 9, 9103. [Google Scholar] [CrossRef]
- Mo, L.J.; Song, M.; Huang, Q.H.; Guan, H.; Liu, X.D.; Xie, D.F.; Huang, B.; Huang, R.X.; Zhou, P.K. Exosome-packaged miR-1246 contributes to bystander DNA damage by targeting LIG4. Br. J. Cancer 2018, 119, 492–502. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Ding, N.; Hu, W.; Zhang, X.; Wang, B.; Hua, J.; Wei, W.; Zhu, Q. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015, 12, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Goulielmaki, E.; Ioannidou, A.; Tsekrekou, M.; Stratigi, K.; Poutakidou, I.K.; Gkirtzimanaki, K.; Aivaliotis, M.; Evangelou, K.; Topalis, P.; Altmuller, J.; et al. Tissue-infiltrating macrophages mediate an exosome-based metabolic reprogramming upon DNA damage. Nat. Commun. 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, H.; Zhang, W.; Ding, F.; Fan, Z.; Zeng, Z. Exosomes Derived From T Regulatory Cells Suppress CD8+ Cytotoxic T Lymphocyte Proliferation and Prolong Liver Allograft Survival. Med. Sci. Monit. 2019, 25, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, X.; Zhao, T.; Li, W.; Xiang, J. Natural CD8(+)25(+) regulatory T cell-secreted exosomes capable of suppressing cytotoxic T lymphocyte-mediated immunity against B16 melanoma. Biochem. Biophys. Res. Commun. 2013, 438, 152–155. [Google Scholar] [CrossRef]
- Zhang, J.P.; Chen, B.B.; Zheng, H.H.; Yang, H.J.; Song, L. [Discharge and Contents of Exosome from Leukocyte-Reduced Apheresis Platelet Stored for Different Storage Time]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019, 27, 1993–1997. [Google Scholar] [CrossRef]
- Kovuru, N.; Raghuwanshi, S.; Gutti, R.K. Exosome mediated differentiation of megakaryocytes: A study on TLR mediated effects. J. Thromb. Thrombolysis 2019, 48, 171–173. [Google Scholar] [CrossRef]
- Shelke, G.V.; Yin, Y.; Jang, S.C.; Lasser, C.; Wennmalm, S.; Hoffmann, H.J.; Li, L.; Gho, Y.S.; Nilsson, J.A.; Lotvall, J. Endosomal signalling via exosome surface TGFbeta-1. J. Extracell. Vesicles 2019, 8, 1650458. [Google Scholar] [CrossRef]
- Chen, B.; Li, M.Y.; Guo, Y.; Zhao, X.; Liu, Y.Y. Mast cell-derived exosome participates in acupoint-stimulation initiated local network activities. Zhen Ci Yan Jiu 2015, 40, 82–85. [Google Scholar]
- Mathews, P.M.; Levy, E. Exosome Production Is Key to Neuronal Endosomal Pathway Integrity in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1347. [Google Scholar] [CrossRef]
- Bowers, E.C.; Hassanin, A.A.I.; Ramos, K.S. In vitro models of exosome biology and toxicology: New frontiers in biomedical research. Toxicol. In Vitro 2020, 64, 104462. [Google Scholar] [CrossRef]
- Benjamins, J.A.; Nedelkoska, L.; Touil, H.; Stemmer, P.M.; Carruthers, N.J.; Jena, B.P.; Naik, A.R.; Bar-Or, A.; Lisak, R.P. Exosome-enriched fractions from MS B cells induce oligodendrocyte death. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e550. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.J.; Park, H.T.; Shin, Y.K. Exosomes derived from differentiated Schwann cells inhibit Schwann cell migration via microRNAs. Neuroreport 2020, 31, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chopp, M.; Szalad, A.; Lu, X.; Zhang, Y.; Wang, X.; Cepparulo, P.; Lu, M.; Li, C.; Zhang, Z.G. Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes 2020, 69, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hu, M.; He, S.; Li, B.; Liu, C.; Min, J.; Hong, L. Effects of RSC96 Schwann Cell-Derived Exosomes on Proliferation, Senescence, and Apoptosis of Dorsal Root Ganglion Cells In Vitro. Med. Sci. Monit. 2018, 24, 7841–7849. [Google Scholar] [CrossRef]
- Jia, L.; Chopp, M.; Wang, L.; Lu, X.; Szalad, A.; Zhang, Z.G. Exosomes derived from high-glucose-stimulated Schwann cells promote development of diabetic peripheral neuropathy. FASEB J. 2018, 32, 6911–6922. [Google Scholar] [CrossRef]
- Elahi, F.M.; Farwell, D.G.; Nolta, J.A.; Anderson, J.D. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 2020, 38, 15–21. [Google Scholar] [CrossRef]
- Vilaca-Faria, H.; Salgado, A.J.; Teixeira, F.G. Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s Disease? Cells 2019, 8, 118. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Yang, P.C. Induced Pluripotent Stem Cell (iPSC)-Derived Exosomes for Precision Medicine in Heart Failure. Circ. Res. 2018, 122, 661–663. [Google Scholar] [CrossRef]
- Jung, J.H.; Fu, X.; Yang, P.C. Exosomes Generated From iPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases. Circ. Res. 2017, 120, 407–417. [Google Scholar] [CrossRef]
- Lin, X.; Ling, Q.; Lv, Y.; Ye, W.; Huang, J.; Li, X.; Guo, Q.; Wang, J.; Li, Z.; Jin, J. Plasma exosome-derived microRNA-532 as a novel predictor for acute myeloid leukemia. Cancer Biomark. 2020, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Bun, P.; Huveneers, S.; van Niel, G.; Pegtel, D.M.; Verweij, F.J. Real-time imaging of multivesicular body-plasma membrane fusion to quantify exosome release from single cells. Nat. Protoc. 2020, 15, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.T.; Lee, W.T.; Abedini, M.R.; Wang, P.W.; Shieh, D.B.; Dilworth, F.J.; Carmona, E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2020, 39, 1600–1616. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Shah, S.H.; Tamayo, A.; Robbins, P.D.; Golberg, R.B.; Mendez, A.J.; Ricordi, C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci. Rep. 2017, 7, 5998. [Google Scholar] [CrossRef]
- Yazarlou, F.; Mowla, S.J.; Oskooei, V.K.; Motevaseli, E.; Tooli, L.F.; Afsharpad, M.; Nekoohesh, L.; Sanikhani, N.S.; Ghafouri-Fard, S.; Modarressi, M.H. Urine exosome gene expression of cancer-testis antigens for prediction of bladder carcinoma. Cancer Manag. Res. 2018, 10, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Koritzinsky, E.H.; Glispie, D.M.; Yuen, P.S.T. Urine Exosome Isolation and Characterization. Methods Mol. Biol. 2017, 1641, 413–423. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, J.; Donovan, M.J.; O’Neill, V.; Bentink, S.; Noerholm, M.; Belzer, S.; Skog, J.; Kattan, M.W.; Partin, A.; Andriole, G.; et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016, 2, 882–889. [Google Scholar] [CrossRef]
- Yang, J.; Wei, F.; Schafer, C.; Wong, D.T. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS ONE 2014, 9, e110641. [Google Scholar] [CrossRef]
- Dixon, C.L.; Sheller-Miller, S.; Saade, G.R.; Fortunato, S.J.; Lai, A.; Palma, C.; Guanzon, D.; Salomon, C.; Menon, R. Amniotic Fluid Exosome Proteomic Profile Exhibits Unique Pathways of Term and Preterm Labor. Endocrinology 2018, 159, 2229–2240. [Google Scholar] [CrossRef]
- Torregrosa Paredes, P.; Gutzeit, C.; Johansson, S.; Admyre, C.; Stenius, F.; Alm, J.; Scheynius, A.; Gabrielsson, S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy 2014, 69, 463–471. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- de Araujo Farias, V.; Carrillo-Galvez, A.B.; Martin, F.; Anderson, P. TGF-beta and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018, 43, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, N.; Rossignoli, F.; Golinelli, G.; Grisendi, G.; Spano, C.; Candini, O.; Osturu, S.; Catani, F.; Paolucci, P.; Horwitz, E.M.; et al. Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med. 2015, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Al-Nbaheen, M.; Kadalmani, B.; Aldahmash, A.; Ramesh, T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012, 347, 419–427. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Manikandan, M.; Al-Nbaheen, M.; Kadalmani, B.; Aldahmash, A.; Alajez, N.M. In vitro differentiation of human skin-derived multipotent stromal cells into putative endothelial-like cells. BMC Dev. Biol. 2012, 12, 7. [Google Scholar] [CrossRef]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PLoS ONE 2014, 9, e104662. [Google Scholar] [CrossRef]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.M.; Cancemi, P.; Geraci, F. Mesenchymal and Induced Pluripotent Stem Cells-Derived Extracellular Vesicles: The New Frontier for Regenerative Medicine? Cells 2020, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, J.; Wang, J.; Li, Y.; Hu, X.; Luo, S.; Xiang, D. Extracellular vesicles derived from different sources of mesenchymal stem cells: Therapeutic effects and translational potential. Cell Biosci. 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Lelek, J.; Zuba-Surma, E.K. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int. J. Mol. Sci. 2020, 21, 799. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Alessio, N.; Pipino, C.; Mandatori, D.; Di Tomo, P.; Ferone, A.; Marchiso, M.; Melone, M.A.B.; Peluso, G.; Pandolfi, A.; Galderisi, U. Mesenchymal stromal cells from amniotic fluid are less prone to senescence compared to those obtained from bone marrow: An in vitro study. J. Cell. Physiol. 2018, 233, 8996–9006. [Google Scholar] [CrossRef]
- Squillaro, T.; Antonucci, I.; Alessio, N.; Esposito, A.; Cipollaro, M.; Melone, M.A.B.; Peluso, G.; Stuppia, L.; Galderisi, U. Impact of lysosomal storage disorders on biology of mesenchymal stem cells: Evidences from in vitro silencing of glucocerebrosidase (GBA) and alpha-galactosidase A (GLA) enzymes. J. Cell. Physiol. 2017, 232, 3454–3467. [Google Scholar] [CrossRef]
- Khong, D.; Li, M.; Singleton, A.; Chin, L.Y.; Mukundan, S.; Parekkadan, B. Orthogonal potency analysis of mesenchymal stromal cell function during ex vivo expansion. Exp. Cell Res. 2018, 362, 102–110. [Google Scholar] [CrossRef]
- Pachon-Pena, G.; Yu, G.; Tucker, A.; Wu, X.; Vendrell, J.; Bunnell, B.A.; Gimble, J.M. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J. Cell. Physiol. 2011, 226, 843–851. [Google Scholar] [CrossRef]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef] [PubMed]
- Casado-Diaz, A.; Quesada-Gomez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, R.; Chen, Q.; Shao, J.; Yu, J.; Hu, S. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res. Ther. 2017, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Shin, J.Y.; Kim, H.N.; Oh, S.H.; Song, S.K.; Lee, P.H. Mesenchymal stem cells stabilize the blood-brain barrier through regulation of astrocytes. Stem Cell Res. Ther. 2015, 6, 187. [Google Scholar] [CrossRef]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Fraga, J.S.; Graos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N.; Salgado, A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012, 3, 18. [Google Scholar] [CrossRef]
- Huang, J.H.; Yin, X.M.; Xu, Y.; Xu, C.C.; Lin, X.; Ye, F.B.; Cao, Y.; Lin, F.Y. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma. 2017, 34, 3388–3396. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Sousa, N.; Salgado, A.J. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell. Mol. Life Sci. 2013, 70, 3871–3882. [Google Scholar] [CrossRef]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell. Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef]
- Qu, Q.; Pang, Y.; Zhang, C.; Liu, L.; Bi, Y. Exosomes derived from human umbilical cord mesenchymal stem cells inhibit vein graft intimal hyperplasia and accelerate reendothelialization by enhancing endothelial function. Stem Cell Res. Ther. 2020, 11, 133. [Google Scholar] [CrossRef]
- Xiong, Z.H.; Wei, J.; Lu, M.Q.; Jin, M.Y.; Geng, H.L. Protective effect of human umbilical cord mesenchymal stem cell exosomes on preserving the morphology and angiogenesis of placenta in rats with preeclampsia. Biomed. Pharmacother. 2018, 105, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Xu, Q.; Wu, Z.; Chen, Y.; Shu, Y.; Cao, X.; Chen, M.; Zhang, B.; Zhou, Y.; Yao, R.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res. Ther. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, Y.; Chen, L.; Li, D.; Feng, H.; Lu, Z.; Fan, T.; Chen, Z.; Livingston, M.J.; Geng, Q. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J. Cell. Physiol. 2020, 235, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, S.; Hu, H.; Yang, J.; Wang, X.; Ma, Y.; Jiang, J.; Wang, J.; Zhong, L.; Chen, M.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem. Biophys. Res. Commun. 2019, 508, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Tian, J.; Wang, S. Role of T cell-derived exosomes in immunoregulation. Immunol. Res. 2018, 66, 313–322. [Google Scholar] [CrossRef]
- Ventimiglia, L.N.; Alonso, M.A. Biogenesis and Function of T Cell-Derived Exosomes. Front. Cell Dev. Biol. 2016, 4, 84. [Google Scholar] [CrossRef]
- Wahlgren, J.; Karlson Tde, L.; Glader, P.; Telemo, E.; Valadi, H. Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling. PLoS ONE 2012, 7, e49723. [Google Scholar] [CrossRef]
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Fan, X.; Lin, F.; et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019, 10, 4355. [Google Scholar] [CrossRef]
- Tang, X.J.; Sun, X.Y.; Huang, K.M.; Zhang, L.; Yang, Z.S.; Zou, D.D.; Wang, B.; Warnock, G.L.; Dai, L.J.; Luo, J. Therapeutic potential of CAR-T cell-derived exosomes: A cell-free modality for targeted cancer therapy. Oncotarget 2015, 6, 44179–44190. [Google Scholar] [CrossRef] [PubMed]
- Tumne, A.; Prasad, V.S.; Chen, Y.; Stolz, D.B.; Saha, K.; Ratner, D.M.; Ding, M.; Watkins, S.C.; Gupta, P. Noncytotoxic suppression of human immunodeficiency virus type 1 transcription by exosomes secreted from CD8+ T cells. J. Virol. 2009, 83, 4354–4364. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, J.; Xie, F.; Tian, J.; Tang, X.; Guo, H.; Ma, J.; Xu, P.; Mao, L.; Xu, H.; et al. CD4(+) T Cell-Released Extracellular Vesicles Potentiate the Efficacy of the HBsAg Vaccine by Enhancing B Cell Responses. Adv. Sci. (Weinh.) 2019, 6, 1802219. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chibbar, R.; Xiang, J. Novel EXO-T vaccine using polyclonal CD4(+) T cells armed with HER2-specific exosomes for HER2-positive breast cancer. Onco Targ. Ther. 2018, 11, 7089–7093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Y.; Li, W.; Chibbar, R.; Xiong, S.; Xiang, J. CD4(+) T cell-released exosomes inhibit CD8(+) cytotoxic T-lymphocyte responses and antitumor immunity. Cell. Mol. Immunol. 2011, 8, 23–30. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Yu, Z.; Wu, M. New Insights into Regulatory T Cells: Exosome- and Non-Coding RNA-Mediated Regulation of Homeostasis and Resident Treg Cells. Front. Immunol. 2016, 7, 574. [Google Scholar] [CrossRef]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014, 41, 89–103. [Google Scholar] [CrossRef]
- Smyth, L.A.; Ratnasothy, K.; Tsang, J.Y.; Boardman, D.; Warley, A.; Lechler, R.; Lombardi, G. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur. J. Immunol. 2013, 43, 2430–2440. [Google Scholar] [CrossRef]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef]
- Stagg, J.; Divisekera, U.; Duret, H.; Sparwasser, T.; Teng, M.W.; Darcy, P.K.; Smyth, M.J. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011, 71, 2892–2900. [Google Scholar] [CrossRef]
- Yu, A.; Snowhite, I.; Vendrame, F.; Rosenzwajg, M.; Klatzmann, D.; Pugliese, A.; Malek, T.R. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes 2015, 64, 2172–2183. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Villarroya-Beltri, C.; Fernandez-Delgado, I.; Latorre-Pellicer, A.; Acin-Perez, R.; Martin-Cofreces, N.B.; Jaso-Tamame, A.L.; Iborra, S.; Jorge, I.; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018, 9, 2658. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Lin, L.; Wang, J.; Xiao, H.; Li, J.; Peng, X.; Dai, H.; Li, L. Mast Cell-Derived Exosomes Promote Th2 Cell Differentiation via OX40L-OX40 Ligation. J. Immunol. Res. 2016, 2016, 3623898. [Google Scholar] [CrossRef]
- Pace, A.L.D.; Tumino, N.; Besi, F.; Alicata, C.; Conti, L.A.; Munari, E.; Maggi, E.; Vacca, P.; Moretta, L. Characterization of Human NK Cell-Derived Exosomes: Role of DNAM1 Receptor In Exosome-Mediated Cytotoxicity Against Tumor. Cancers (Basel) 2020, 12, 661. [Google Scholar] [CrossRef]
- Neviani, P.; Wise, P.M.; Murtadha, M.; Liu, C.W.; Wu, C.H.; Jong, A.Y.; Seeger, R.C.; Fabbri, M. Natural Killer-Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer Res. 2019, 79, 1151–1164. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef]
- Tian, H.; Li, W. Dendritic cell-derived exosomes for cancer immunotherapy: Hope and challenges. Ann. Transl. Med. 2017, 5, 221. [Google Scholar] [CrossRef]
- Pitt, J.M.; Charrier, M.; Viaud, S.; Andre, F.; Besse, B.; Chaput, N.; Zitvogel, L. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J. Immunol. 2014, 193, 1006–1011. [Google Scholar] [CrossRef]
- Pitt, J.M.; Andre, F.; Amigorena, S.; Soria, J.C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Leone, D.A.; Rees, A.J.; Kain, R. Dendritic cells and routing cargo into exosomes. Immunol. Cell Biol. 2018, 96, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Li, H.; Zhang, M.; Xu, H.; Yue, L.T.; Zhang, X.X.; Wang, S.; Wang, C.C.; Li, Y.B.; Dou, Y.C.; et al. Exosomes derived from atorvastatin-modified bone marrow dendritic cells ameliorate experimental autoimmune myasthenia gravis by up-regulated levels of IDO/Treg and partly dependent on FasL/Fas pathway. J. Neuroinflamm. 2016, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, Z.; Wan, S.; Wu, F.; Chen, W.; Zhang, B.; Lin, D.; Liu, J.; Xie, H.; Sun, X.; et al. Exosomes Derived from Dendritic Cells Treated with Schistosoma japonicum Soluble Egg Antigen Attenuate DSS-Induced Colitis. Front. Pharmacol. 2017, 8, 651. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.; Zhou, Z.; Lin, W.; Chen, S.; Chen, M.; Ye, Y. Exosomes derived from rAAV/AFP-transfected dendritic cells elicit specific T cell-mediated immune responses against hepatocellular carcinoma. Cancer Manag. Res. 2018, 10, 4945–4957. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Peng, Y.; Yu, S.; Liu, J.; Wu, L.; Zhang, L.; Wu, Q.; Chang, X.; Yu, X.; et al. Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget 2018, 9, 2887–2894. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.; Zhu, L.; Hua, W.; Zhang, Y.; Zhang, H.; Zhang, L.; Li, Z.; Xing, P.; Hong, B.; et al. Glia-derived exosomes: Promising therapeutic targets. Life Sci. 2019, 239, 116951. [Google Scholar] [CrossRef]
- Shi, S.; Rao, Q.; Zhang, C.; Zhang, X.; Qin, Y.; Niu, Z. Dendritic Cells Pulsed with Exosomes in Combination with PD-1 Antibody Increase the Efficacy of Sorafenib in Hepatocellular Carcinoma Model. Transl. Oncol. 2018, 11, 250–258. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Z.; Ling, W.; Zhu, D.; Feng, Z.; Kong, L. Exosomes Derived from Dendritic Cells Attenuate Liver Injury by Modulating the Balance of Treg and Th17 Cells After Ischemia Reperfusion. Cell. Physiol. Biochem. 2018, 46, 740–756. [Google Scholar] [CrossRef]
- Pang, X.L.; Wang, Z.G.; Liu, L.; Feng, Y.H.; Wang, J.X.; Xie, H.C.; Yang, X.L.; Li, J.F.; Feng, G.W. Immature dendritic cells derived exosomes promotes immune tolerance by regulating T cell differentiation in renal transplantation. Aging (Albany N. Y.) 2019, 11, 8911–8924. [Google Scholar] [CrossRef]
- Agarwal, A.; Fanelli, G.; Letizia, M.; Tung, S.L.; Boardman, D.; Lechler, R.; Lombardi, G.; Smyth, L.A. Regulatory T cell-derived exosomes: Possible therapeutic and diagnostic tools in transplantation. Front. Immunol. 2014, 5, 555. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Meng, S.; Jiang, H.; Zhu, C.; Wu, W. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J. Surg. Res. 2011, 171, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. (Weinh.) 2019, 6, 1900513. [Google Scholar] [CrossRef]
- Yang, R.; Liao, Y.; Wang, L.; He, P.; Hu, Y.; Yuan, D.; Wu, Z.; Sun, X. Exosomes Derived From M2b Macrophages Attenuate DSS-Induced Colitis. Front. Immunol. 2019, 10, 2346. [Google Scholar] [CrossRef]
- Walker, N.D.; Elias, M.; Guiro, K.; Bhatia, R.; Greco, S.J.; Bryan, M.; Gergues, M.; Sandiford, O.A.; Ponzio, N.M.; Leibovich, S.J.; et al. Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma. Cell Death Dis. 2019, 10, 59. [Google Scholar] [CrossRef]
- Aiastui, A. Should Cell Culture Platforms Move towards EV Therapy Requirements? Front. Immunol. 2015, 6, 8. [Google Scholar] [CrossRef]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Jiang, X.R.; Song, A.; Bergelson, S.; Arroll, T.; Parekh, B.; May, K.; Chung, S.; Strouse, R.; Mire-Sluis, A.; Schenerman, M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat. Rev. Drug Discov. 2011, 10, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.R.; Walsh, C.T.; Almarsson, O. Drugs as materials: Valuing physical form in drug discovery. Nat. Rev. Drug Discov. 2004, 3, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Kosaka, N.; Takeshita, F.; Ochiya, T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 2013, 13, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.J.; Lee, C.; Rojalin, T.; Carney, R.P.; Hazari, S.; Knudson, A.; Lam, K.; Saari, H.; Ibanez, E.L.; Viitala, T.; et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J. Extracell. Vesicles 2015, 4, 28533. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Jong, A.Y.; Wu, C.H.; Li, J.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles 2017, 6, 1294368. [Google Scholar] [CrossRef]
- Bogdanova, A.; Berzins, U.; Nikulshin, S.; Skrastina, D.; Ezerta, A.; Legzdina, D.; Kozlovska, T. Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J. Stem Cells 2014, 9, 135–148. [Google Scholar]
- Lunyak, V.V.; Amaro-Ortiz, A.; Gaur, M. Mesenchymal Stem Cells Secretory Responses: Senescence Messaging Secretome and Immunomodulation Perspective. Front. Genet. 2017, 8, 220. [Google Scholar] [CrossRef]
- Rochette, L.; Mazini, L.; Meloux, A.; Zeller, M.; Cottin, Y.; Vergely, C.; Malka, G. Anti-Aging Effects of GDF11 on Skin. Int. J. Mol. Sci. 2020, 21, 2598. [Google Scholar] [CrossRef]
- Zarei, F.; Abbaszadeh, A. Application of Cell Therapy for Anti-Aging Facial Skin. Curr. Stem Cell Res. Ther. 2019, 14, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Boulestreau, J.; Maumus, M.; Rozier, P.; Jorgensen, C.; Noel, D. Mesenchymal Stem Cell Derived Extracellular Vesicles in Aging. Front. Cell Dev. Biol. 2020, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Aprile, D.; Squillaro, T.; Di Bernardo, G.; Finicelli, M.; Melone, M.A.; Peluso, G.; Galderisi, U. The senescence-associated secretory phenotype (SASP) from mesenchymal stromal cells impairs growth of immortalized prostate cells but has no effect on metastatic prostatic cancer cells. Aging (Albany N. Y.) 2019, 11, 5817–5828. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Snijders, A.P.; Gil, J. Unbiased characterization of the senescence-associated secretome using SILAC-based quantitative proteomics. Methods Mol. Biol. 2013, 965, 175–184. [Google Scholar] [CrossRef]
- Lei, Q.; Liu, T.; Gao, F.; Xie, H.; Sun, L.; Zhao, A.; Ren, W.; Guo, H.; Zhang, L.; Wang, H.; et al. Microvesicles as Potential Biomarkers for the Identification of Senescence in Human Mesenchymal Stem Cells. Theranostics 2017, 7, 2673–2689. [Google Scholar] [CrossRef]
- Alessio, N.; Squillaro, T.; Di Bernardo, G.; Galano, G.; De Rosa, R.; Melone, M.A.; Peluso, G.; Galderisi, U. Increase of circulating IGFBP-4 following genotoxic stress and its implication for senescence. Elife 2020, 9, e54523. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Lotvall, J.; Lasser, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23111. [Google Scholar] [CrossRef]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mager, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.; Hallbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 2015, 11, 879–883. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef]
- Heinemann, M.L.; Ilmer, M.; Silva, L.P.; Hawke, D.H.; Recio, A.; Vorontsova, M.A.; Alt, E.; Vykoukal, J. Benchtop isolation and characterization of functional exosomes by sequential filtration. J. Chromatogr. A 2014, 1371, 125–135. [Google Scholar] [CrossRef]
- Morales-Kastresana, A.; Telford, B.; Musich, T.A.; McKinnon, K.; Clayborne, C.; Braig, Z.; Rosner, A.; Demberg, T.; Watson, D.C.; Karpova, T.S.; et al. Labeling Extracellular Vesicles for Nanoscale Flow Cytometry. Sci. Rep. 2017, 7, 1878. [Google Scholar] [CrossRef]
- Corso, G.; Mager, I.; Lee, Y.; Gorgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.A.; Nordin, J.Z.; Andaloussi, S.E. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017, 7, 11561. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, G.; Dhondt, B.; Van Deun, J.; De Smedt, E.; Berx, G.; Timmerman, E.; Gevaert, K.; Miinalainen, I.; Cocquyt, V.; Braems, G.; et al. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci. Rep. 2017, 7, 2704. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.; Capece, M.; Cocucci, E.; Tessari, A.; Palmieri, D. Cancer-Derived Extracellular Vesicle-Associated MicroRNAs in Intercellular Communication: One Cell’s Trash Is Another Cell’s Treasure. Int. J. Mol. Sci. 2019, 20, 6109. [Google Scholar] [CrossRef] [PubMed]
- Perycz, M.; Urbanska, A.S.; Krawczyk, P.S.; Parobczak, K.; Jaworski, J. Zipcode binding protein 1 regulates the development of dendritic arbors in hippocampal neurons. J. Neurosci. 2011, 31, 5271–5285. [Google Scholar] [CrossRef]
- Vallhov, H.; Gutzeit, C.; Johansson, S.M.; Nagy, N.; Paul, M.; Li, Q.; Friend, S.; George, T.C.; Klein, E.; Scheynius, A.; et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J. Immunol. 2011, 186, 73–82. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Schapira, A.H.; Gardiner, C.; Sargent, I.L.; Wood, M.J.; Cooper, J.M. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 2011, 42, 360–367. [Google Scholar] [CrossRef]
- Wahlgren, J.; De, L.K.T.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic. Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface functionalization of exosomes using click chemistry. Bioconjug. Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Lasser, C.; Gho, Y.S.; Lotvall, J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 2014, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.; Skotland, T.; Sylvanne, T.; Kauhanen, D.; Rog, T.; Orlowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta 2013, 1831, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid. Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Zoller, M. Exosomes in Cancer Disease. Methods Mol. Biol. 2016, 1381, 111–149. [Google Scholar] [CrossRef]
- Mateescu, B.; Kowal, E.J.; van Balkom, B.W.; Bartel, S.; Bhattacharyya, S.N.; Buzas, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef]
- Ullal, A.J.; Pisetsky, D.S.; Reich, C.F., 3rd. Use of SYTO 13, a fluorescent dye binding nucleic acids, for the detection of microparticles in in vitro systems. Cytom. A 2010, 77, 294–301. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.; Hwang, D.W.; Lee, M. Delivery of High Mobility Group Box-1 siRNA Using Brain-Targeting Exosomes for Ischemic Stroke Therapy. J. Biomed. Nanotechnol. 2019, 15, 2401–2412. [Google Scholar] [CrossRef]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef]
- Lu, Y.B.; Chen, C.X.; Huang, J.; Tian, Y.X.; Xie, X.; Yang, P.; Wu, M.; Tang, C.; Zhang, W.P. Nicotinamide phosphoribosyltransferase secreted from microglia via exosome during ischemic injury. J. Neurochem. 2019, 150, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Aucher, A.; Rudnicka, D.; Davis, D.M. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J. Immunol. 2013, 191, 6250–6260. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, G.G.; Zelante, B.B.; Toniolo, P.A.; Migliori, I.K.; Barbuto, J.A. Dendritic Cell-Derived Exosomes may be a Tool for Cancer Immunotherapy by Converting Tumor Cells into Immunogenic Targets. Front. Immunol. 2014, 5, 692. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, S.P.; Elkahloun, A.G.; Wu, W.; Abu-Asab, M.S.; Roberts, D.D. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. 2014, 37, 49–59. [Google Scholar] [CrossRef]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther. 2019, 10, 95. [Google Scholar] [CrossRef]
- Zhu, L.L.; Huang, X.; Yu, W.; Chen, H.; Chen, Y.; Dai, Y.T. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 2018, 50, e12871. [Google Scholar] [CrossRef]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.; El-Baba, M.D.; Saxena, P.; Auslander, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhai, L.; Ge, Q.; Liu, Z.; Tao, R. Vacuolar Protein Sorting 4B (VPS4B) Regulates Apoptosis of Chondrocytes via p38 Mitogen-Activated Protein Kinases (MAPK) in Osteoarthritis. Inflammation 2017, 40, 1924–1932. [Google Scholar] [CrossRef]
- Scheffer, L.L.; Sreetama, S.C.; Sharma, N.; Medikayala, S.; Brown, K.J.; Defour, A.; Jaiswal, J.K. Mechanism of Ca(2)(+)-triggered ESCRT assembly and regulation of cell membrane repair. Nat. Commun. 2014, 5, 5646. [Google Scholar] [CrossRef] [PubMed]
- Dany, M.; Gencer, S.; Nganga, R.; Thomas, R.J.; Oleinik, N.; Baron, K.D.; Szulc, Z.M.; Ruvolo, P.; Kornblau, S.; Andreeff, M.; et al. Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood 2016, 128, 1944–1958. [Google Scholar] [CrossRef]
- Ganesan, V.; Colombini, M. Regulation of ceramide channels by Bcl-2 family proteins. FEBS Lett. 2010, 584, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Faure, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Kramer-Albers, E.M.; Bretz, N.; Tenzer, S.; Winterstein, C.; Mobius, W.; Berger, H.; Nave, K.A.; Schild, H.; Trotter, J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin. Appl. 2007, 1, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef]
- Messenger, S.W.; Woo, S.S.; Sun, Z.; Martin, T.F.J. A Ca(2+)-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J. Cell Biol. 2018, 217, 2877–2890. [Google Scholar] [CrossRef] [PubMed]

| Sponsor, City and State | NCT No | Disease | Exo Source |
|---|---|---|---|
| Wuhan Jinyintan Hospital, Wuhan, China | NCT04276987 | Severe novel coronavirus pneumonia | Mesenchymal stem cells (MSCs) |
| Beni-Suef University, Bani Sweif, Egypt | NCT04270006 | Periodontitis | MSCs |
| Fujian Medical University, Fujian, China | NCT04356300 | Multiple organ dysfunction syndrome | MSCs |
| TC Erciyes University, Talas, Turkey | NCT04389385 | Severe novel coronavirus pneumonia | T Cell |
| Sun Yat-sen University, Guangzhou, China | NCT04213248 | Dry eye in patients with chronic graft-versus-host disease (cGVHD) | MSCs |
| M.D. Anderson Cancer Center, Houston, TX, USA | NCT03608631 | Metastatic pancreas cancer with KrasG12D mutation | MSCs |
| El Sahel Teaching Hospital, Cairo, Egypt | NCT02138331 | Type 1 diabetes (T1DM) | MSCs |
| Ruijin Hospital, Shanghai, China | NCT04313647 | Clinical tolerance in healthy volunteers | MSCs |
| Tianjin Medical University, Tianjin, China | NCT03437759 | MSC-Exo promotes MH healing | MSCs |
| Ruijin Hospital, Shanghai, China | NCT04388982 | Alzheimer’s disease | MSCs |
| Aegle Therapeutics, Arlington, MA, USA | NCT04173650 | Dystrophic epidermolysis bullosa | MSCs |
| Stem Cell and Cancer Institute, Kalbe Farma, Jakarta, Indonesia | NCT04134676 | Chronic ulcer wounds | MSCs |
| Neurological Associates of West Los Angeles, CA, USA | NCT04202783 | Craniofacial neuralgia | MSCs |
| Kumamoto University, Kumamoto, Japan | NCT02565264 | Cutaneous wound healing | Plasma |
| Saeed Oraei Yazdani, Tehran, Iran | NCT03384433 | Acute ischemic stroke | MSCs |
| Neurological Associates of West Los Angeles, CA, USA | NCT04202770 | Depression, anxiety and dementias | MSCs |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aheget, H.; Tristán-Manzano, M.; Mazini, L.; Cortijo-Gutierrez, M.; Galindo-Moreno, P.; Herrera, C.; Martin, F.; Marchal, J.A.; Benabdellah, K. Exosome: A New Player in Translational Nanomedicine. J. Clin. Med. 2020, 9, 2380. https://doi.org/10.3390/jcm9082380
Aheget H, Tristán-Manzano M, Mazini L, Cortijo-Gutierrez M, Galindo-Moreno P, Herrera C, Martin F, Marchal JA, Benabdellah K. Exosome: A New Player in Translational Nanomedicine. Journal of Clinical Medicine. 2020; 9(8):2380. https://doi.org/10.3390/jcm9082380
Chicago/Turabian StyleAheget, Houssam, María Tristán-Manzano, Loubna Mazini, Marina Cortijo-Gutierrez, Pablo Galindo-Moreno, Concha Herrera, Francisco Martin, Juan Antonio Marchal, and Karim Benabdellah. 2020. "Exosome: A New Player in Translational Nanomedicine" Journal of Clinical Medicine 9, no. 8: 2380. https://doi.org/10.3390/jcm9082380
APA StyleAheget, H., Tristán-Manzano, M., Mazini, L., Cortijo-Gutierrez, M., Galindo-Moreno, P., Herrera, C., Martin, F., Marchal, J. A., & Benabdellah, K. (2020). Exosome: A New Player in Translational Nanomedicine. Journal of Clinical Medicine, 9(8), 2380. https://doi.org/10.3390/jcm9082380









