Clinical Significance of Timing of Intubation in Critically Ill Patients with COVID-19: A Multi-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection and Definitions
2.3. Classification by Intubation Timing and Status
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Laboratory Indices, Severity of Illness, and Clinical Course
3.3. Clinical Outcomes
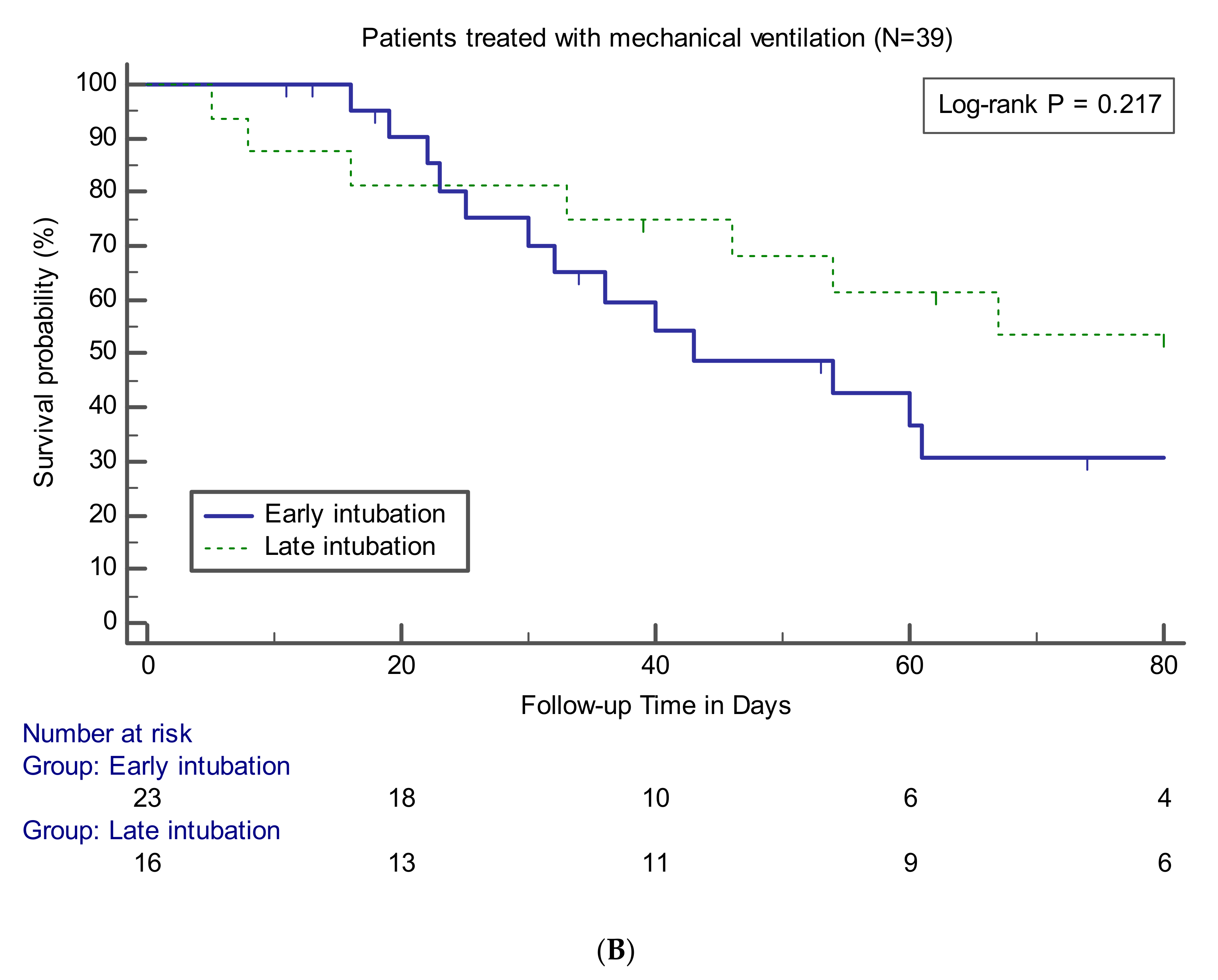
3.4. Effects of Early Intubation on Mortality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. General’s Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 31 July 2020).
- World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report-192, 30 July 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200730-covid-19-sitrep-192.pdf?sfvrsn=5e52901f_4 (accessed on 31 July 2020).
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19—Preliminary report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 65, 854–887. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.R.; Fitzpatrick, M.C.; Sah, P.; Shoukat, A.; Pandey, A.; El-Sayed, A.M.; Singer, B.H.; Moghadas, S.M.; Galvani, A.P. Projecting the demand for ventilators at the peak of the COVID-19 outbreak in the USA. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Kangelaris, K.N.; Ware, L.B.; Wang, C.Y.; Janz, D.R.; Hanjing, Z.; Matthay, M.A.; Calfee, C.S. Timing of Intubation and Clinical outcomes in Adults with ARDS. Crit. Care Med. 2016, 44, 120–129. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Brealey, D.; Howell, D.; Singer, M. Use of non-invasive ventilation for patients with COVID-19: A cause for concern? Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Fowler, R.A.; Guest, C.B.; Lapinsky, S.E.; Sibbald, W.J.; Louie, M.; Tang, P.; Simor, A.E.; Stewart, T.E. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am. J. Respir. Crit. Care Med. 2004, 169, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-ncov) Infection Is Suspected: Interim Guidance. 2020, p. 21. Available online: https://apps.who.int/iris/handle/10665/330893 (accessed on 2 July 2020).
- Force, A.D.T.; Ranieri, V.; Rubenfeld, G.; Thompson, B.; Ferguson, N.; Caldwell, E. Acute respiratory distress syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Quah, P.; Li, A.; Phua, J. Mortality rates of patients with COVID-19 in the intensive care unit: A systematic review of the emerging literature. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.A.; Bonten, M.J.; Kollef, M.H.; Hall, J.B. Risk factors for ventilator-associated pneumonia: From epidemiology to patient management. Clin. Infect. Dis. 2004, 38, 1141–1149. [Google Scholar]
- Sztrymf, B.; Messika, J.; Bertrand, F.; Hurel, D.; Leon, R.; Dreyfuss, D.; Ricard, J.-D. Beneficial effects of humidified high flow nasal oxygen in critical care patients: A prospective pilot study. Intensive Care Med. 2011, 37, 1780. [Google Scholar] [CrossRef]
- Sztrymf, B.; Messika, J.; Mayot, T.; Lenglet, H.; Dreyfuss, D.; Ricard, J.-D. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: A prospective observational study. J. Crit. Care 2012, 27, 324.e9–324.e13. [Google Scholar] [CrossRef]
- Parke, R.L.; McGuinness, S.P.; Eccleston, M.L. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir. Care 2011, 56, 265–270. [Google Scholar] [CrossRef]
- Messika, J.; Ahmed, K.B.; Gaudry, S.; Miguel-Montanes, R.; Rafat, C.; Sztrymf, B.; Dreyfuss, D.; Ricard, J.-D. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: A 1-year observational study. Respir. Care 2015, 60, 162–169. [Google Scholar] [CrossRef]
- Leung, C.; Joynt, G.; Gomersall, C.; Wong, W.; Lee, A.; Ling, L.; Chan, P.; Lui, P.; Tsoi, P.; Ling, C. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: A randomized controlled crossover trial. J. Hosp. Infect. 2019, 101, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Roca, O.; Riera, J.; Torres, F.; Masclans, J.R. High-flow oxygen therapy in acute respiratory failure. Respir. Care 2010, 55, 408–413. [Google Scholar] [PubMed]
- Cheung, J.C.-H.; Ho, L.T.; Cheng, J.V.; Cham, E.Y.K.; Lam, K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir. Med. 2020, 8, e19. [Google Scholar] [CrossRef]
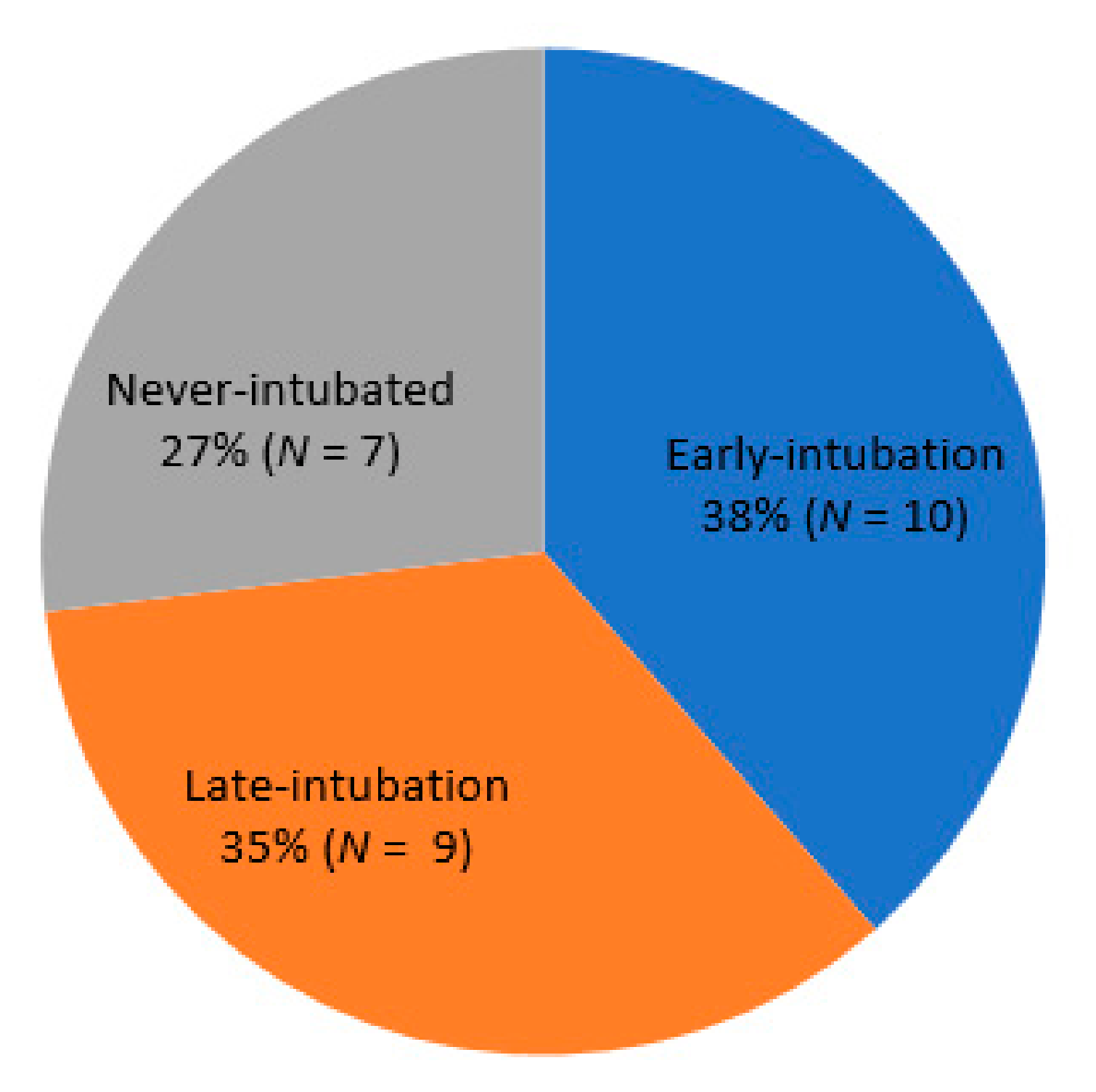

| Variables | Early Intubation (n = 23) | Initially Nonintubated (n = 24) | p Value a | Late Intubation (n = 16) | p Value b |
|---|---|---|---|---|---|
| Age | 72 (64–76) | 69 (60–78) | 0.655 | 66 (59–77) | 0.475 |
| Male | 14 (60.9) | 14 (58.3) | 0.859 | 10 (62.5) | 0.918 |
| Body mass index, kg/m2 | 22.8 (21.0–26.7) | 25.6 (22.5–27.1) | 0.167 | 25.1 (22.7–27.3) | 0.241 |
| Comorbidities | |||||
| Any comorbidities | 16 (69.6) | 19 (79.2) | 0.450 | 13 (81.2) | 0.480 |
| Hypertension | 10 (43.5) | 11 (45.8) | 0.871 | 8 (50) | 0.688 |
| Diabetes | 10 (43.5) | 8 (33.3) | 0.474 | 7 (43.8) | 0.987 |
| Chronic kidney disease | 1 (4.3) | 2 (8.3) | >0.999 | 2 (12.5) | 0.557 |
| Dementia | 2 (8.7) | 3 (12.5) | >0.999 | 1 (6.2) | >0.999 |
| Cerebrovascular disease | 0 (0) | 2 (8.3) | 0.489 | 1 (6.2) | 0.410 |
| Malignancy | 2 (8.7) | 4 (16.7) | 0.666 | 4 (25) | 0.205 |
| Cardiovascular disease | 4 (17.4) | 4 (16.7) | >0.999 | 2 (12.5) | >0.999 |
| Chronic lung disease | 3 (13.0) | 1 (4.2) | 0.348 | 1 (6.2) | 0.631 |
| Chronic liver disease | 1 (4.3) | 2 (8.3) | >0.999 | 0 (0) | >0.999 |
| Duration of symptoms before admission, days | 7 (5–11) | 5 (4–12) | 0.280 | 5 (3–10) | 0.143 |
| Presenting symptoms | |||||
| Fever | 18 (78.3) | 16 (66.7) | 0.374 | 9 (56.2) | 0.174 |
| Dyspnea | 19 (82.6) | 17 (70.8) | 0.341 | 12 (75) | 0.694 |
| Cough | 12 (52.2) | 14 (58.3) | 0.671 | 8 (50) | 0.894 |
| Sputum | 10 (43.5) | 10 (41.7) | 0.900 | 6 (37.5) | 0.709 |
| Myalgia | 5 (21.7) | 5 (20.8) | >0.999 | 4 (25) | >0.999 |
| Fatigue | 3 (13.0) | 7 (29.2) | 0.286 | 6 (37.5) | 0.123 |
| Diarrhea | 2 (8.7) | 5 (20.8) | 0.416 | 2 (12.5) | >0.999 |
| Vital signs at the time of ICU admission | |||||
| Mean arterial pressure, mmHg | 93 (90–107) | 93 (86–102) | 0.539 | 93 (86–97) | 0.388 |
| Heart rate, beats/min | 85 (76–124) | 88 (80–100) | 0.915 | 92 (74–100) | 0.808 |
| Respiratory rate, breaths/min | 28 (22–34) | 21 (20–26) | 0.007 | 21 (20–29) | 0.057 |
| Body temperature, ℃ | 37.3 (36.4–37.8) | 36.8 (36.5–37.4) | 0.781 | 36.9 (36.6–37.4) | 0.863 |
| Variables | Early Intubation (n = 23) | Initially Nonintubated (n = 24) | p Value a | Late Intubation (n = 16) | p Value b |
|---|---|---|---|---|---|
| White blood cells, 103/L | 6.89 (4.73–9.82) | 7.16 (5.21–9.55) | 0.907 | 7.16 (5.9–9.96) | 0.679 |
| Hemoglobin, g/dL | 13.0 (11.2–14.4) | 13.2 (11.5–14.4) | 0.935 | 13.1 (10.9–14.1) | 0.828 |
| Hematocrit, % | 37.6 (33.1–42) | 39.3 (33.2–41.2) | 0.849 | 38.7 (31.8–40.6) | 0.706 |
| Platelets, 103/L | 197 (146–296) | 211 (156–295) | 0.702 | 220 (156–295) | 0.396 |
| C–reactive protein, mg/dL | 9.98 (5.66–15.59) | 10.27 (6.46–13.83) | 0.983 | 11 (7.39–17.43) | 0.668 |
| Procalcitonin, mmol/L | 0.27 (0.12–0.74) | 0.11 (0.1–0.21) | 0.044 | 0.13 (0.1–0.21) | 0.082 |
| Lactate, mmol/L | 1.8 (1.3–2.5) | 1.6 (1.3–2.3) | 0.557 | 1.8 (1.3–2.4) | 0.947 |
| Albumin, g/dL | 3.3 (3–3.5) | 3.4 (3.3–3.5) | 0.298 | 3.4 (3.3–3.7) | 0.329 |
| AST, U/L | 48 (35–81) | 50 (35–91) | 0.717 | 50 (33–65) | 0.875 |
| ALT, U/L | 27 (19–34) | 20 (13–57) | 0.302 | 22 (13–53) | 0.346 |
| Total bilirubin, mg/dL | 0.62 (0.4–0.85) | 0.54 (0.32–0.83) | 0.537 | 0.6 (0.3–0.83) | 0.607 |
| BUN, mg/dL | 15.7 (12.5–24.7) | 15.6 (8.9–22.6) | 0.609 | 16.8 (11.8–35.3) | 0.842 |
| Creatinine, mg/dL | 0.9 (0.7–1.2) | 0.9 (0.67–1.45) | 0.741 | 0.95 (0.7–1.9) | 0.484 |
| Sodium, mmol/L | 134 (132–139) | 137 (133–138) | 0.407 | 137 (132–139) | 0.330 |
| Potassium, mmol/L | 3.9 (3.3–4.3) | 4 (3.3–4.7) | 0.327 | 3.9 (3.3–4.6) | 0.427 |
| Glucose, mg/dL | 156 (117–197) | 139 (111–168) | 0.312 | 161 (122–172) | 0.966 |
| LDH, U/L | 486 (410–559) | 442 (370–632) | 0.606 | 468 (344–698) | 0.818 |
| D-dimer, ug/mL | 2.0 (0.77–3.87) | 2.5 (1.23–6.4) | 0.447 | 2.03 (1.07–3.74) | 0.642 |
| Prothrombin time, INR | 1.13 (1.02–1.27) | 1.08 (1.02–1.26) | 0.910 | 1.08 (1.02–1.27) | 0.908 |
| NT–proBNP, pg/mL | 826 (243–1376) | 570 (316–1395) | 0.648 | 540 (372–2026) | 0.917 |
| Troponin I, ng/mL | 0.03 (0.02–0.18) | 0.02 (0.01–0.02) | 0.073 | 0.02 (0.01–0.02) | 0.114 |
| CK–MB, U/L | 1.5 (1–4.3) | 1.1 (0.8–1.8) | 0.025 | 1 (0.8–1.4) | 0.019 |
| Variables | Early Intubation (n = 23) | Initially Nonintubated (n = 24) | p Value a | Late Intubation (n = 16) | p Value b |
|---|---|---|---|---|---|
| Severity of illness on ICU admission | |||||
| Septic shock | 4 (17.4) | 2 (8.3) | 0.416 | 1 (6.2) | 0.631 |
| Acute kidney injury | 7 (30.4) | 5 (20.8) | 0.450 | 4 (25) | >0.999 |
| Acute cardiac injury | 8 (34.8) | 3 (12.5) | 0.071 | 2 (12.5) | 0.152 |
| SOFA score | 3 (2–7) | 2 (2–4) | 0.134 | 3 (2–4) | 0.336 |
| APACHE II score | 15 (10–17) | 11 (8–14) | 0.042 | 14 (8–15) | 0.252 |
| ABGA at the time of diagnosis of ARDS | |||||
| pH | 7.34 (7.31–7.44) | 7.45 (7.42–7.5) | 0.001 | 7.43 (7.4–7.49) | 0.013 |
| PaCO2, mmHg | 37.6 (33.3–50.2) | 32.2 (26.8–36.5) | 0.001 | 32.3 (23.8–37.1) | 0.002 |
| PaO2, mmHg | 77.3 (55.3–85) | 67.8 (55–82.3) | 0.389 | 67.8 (55.7–79.7) | 0.339 |
| HCO3, mmol/L | 22.6 (21.1–25.4) | 22.4 (18.8–25.5) | 0.672 | 20.8 (17.4–26.3) | 0.259 |
| PF ratio | 86 (69–123) | 144 (70–206) | 0.028 | 120 (62–188) | 0.204 |
| ICU management | |||||
| HFNC | 13 (56.5) | 24 (100) | <0.001 | 16 (100) | 0.002 |
| NM blockade | 15 (65.2) | 9 (37.5) | 0.057 | 9 (56.2) | 0.571 |
| CRRT | 5 (21.7) | 5 (20.8) | >0.999 | 5 (31.2) | 0.711 |
| Tracheostomy | 9 (39.1) | 7 (29.2) | 0.471 | 7 (43.8) | 0.773 |
| ECMO | 3 (13.0) | 4 (16.7) | >0.999 | 4 (25) | 0.415 |
| Medical treatment | |||||
| Antiviral agents | |||||
| Lopinavir-ritonavir | 20 (87.0) | 16 (66.7) | 0.101 | 11 (68.8) | 0.235 |
| Darunavir–cobicistat | 3 (13.0) | 7 (29.2) | 0.286 | 5 (31.2) | 0.235 |
| Antibiotics | 23 (100) | 24 (100) | 16 (100) | ||
| Hydroxychloroquine | 20 (87.0) | 22 (91.7) | 0.666 | 14 (87.5) | >0.999 |
| Glucocorticoid | 18 (78.3) | 19 (79.2) | >0.999 | 15 (93.8) | 0.370 |
| Medical event during ICU care | |||||
| Septic shock | 20 (87.0) | 15 (62.5) | 0.055 | 14 (87.5) | >0.999 |
| Acute kidney injury | 10 (43.5) | 7 (29.2) | 0.307 | 7 (43.8) | 0.987 |
| Acute cardiac injury | 10 (43.5) | 5 (20.8) | 0.096 | 4 (25) | 0.237 |
| VAP or HAP | 7 (30.4) | 1 (4.2) | 0.023 | 1 (6.2) | 0.109 |
| CRBSI | 4 (17.4) | 3 (12.5) | 0.701 | 3 (18.8) | >0.999 |
| Bleeding | 3 (13.0) | 3 (12.5) | >0.999 | 3 (18.8) | 0.674 |
| CPCR | 1 (4.3) | 3 (12.5) | 0.609 | 2 (12.5) | 0.557 |
| Variables | Early Intubation (n = 23) | Initially Nonintubated (n = 24) | p Value a | Late Intubation (n = 16) | p Value b |
|---|---|---|---|---|---|
| In-hospital mortality | 13 (56.5) | 8 (33.3) | 0.110 | 7 (43.8) | 0.433 |
| Main cause of death | |||||
| COVID-19 related ARDS | 7/13 (53.8) | 4/8 (50) | >0.999 | 4/7 (57.1) | >0.999 |
| VAP | 3/13 (23.1) | 1/8 (12.5) | >0.999 | 1/7 (14.3) | >0.999 |
| CRBSI | 2/13 (15.4) | 0/8 (0) | 0.505 | 0/7 (0) | 0.521 |
| Acute kidney injury | 0/13 (0) | 1/8 (12.5) | 0.381 | 1/7 (14.3) | 0.350 |
| Myocardial infarction | 1/13 (7.7) | 1/8 (12.5) | 0.999 | 1/7 (14.3) | >0.999 |
| Unknown c | 0/13 (0) | 1/8 (12.5) | 0.381 | 0/12 (0) | |
| Ventilator-free days | 9 (0–18) | 28 (9–45) | 0.008 | 25 (7–45) | 0.033 |
| ICU days d | 13 (7–33) | 14 (7–48) | 0.691 | 47 (13–74) | 0.101 |
| Days of MV d | 10 (4–24) | 6 (0–25) | 0.325 | 20 (9–57) | 0.177 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.H.; Choi, K.-J.; Choi, S.H.; Lee, S.Y.; Kim, K.C.; Kim, E.J.; Lee, J. Clinical Significance of Timing of Intubation in Critically Ill Patients with COVID-19: A Multi-Center Retrospective Study. J. Clin. Med. 2020, 9, 2847. https://doi.org/10.3390/jcm9092847
Lee YH, Choi K-J, Choi SH, Lee SY, Kim KC, Kim EJ, Lee J. Clinical Significance of Timing of Intubation in Critically Ill Patients with COVID-19: A Multi-Center Retrospective Study. Journal of Clinical Medicine. 2020; 9(9):2847. https://doi.org/10.3390/jcm9092847
Chicago/Turabian StyleLee, Yong Hoon, Keum-Ju Choi, Sun Ha Choi, Shin Yup Lee, Kyung Chan Kim, Eun Jin Kim, and Jaehee Lee. 2020. "Clinical Significance of Timing of Intubation in Critically Ill Patients with COVID-19: A Multi-Center Retrospective Study" Journal of Clinical Medicine 9, no. 9: 2847. https://doi.org/10.3390/jcm9092847
APA StyleLee, Y. H., Choi, K.-J., Choi, S. H., Lee, S. Y., Kim, K. C., Kim, E. J., & Lee, J. (2020). Clinical Significance of Timing of Intubation in Critically Ill Patients with COVID-19: A Multi-Center Retrospective Study. Journal of Clinical Medicine, 9(9), 2847. https://doi.org/10.3390/jcm9092847





