Influence of Conditioned Media on the Re-Differentiation Capacity of Human Chondrocytes in 3D Spheroid Cultures
Abstract
1. Introduction
2. Experimental Section
2.1. Cartilage Harvest
2.2. Generation of Conditioned Medium
2.3. Cell Viability Analysis
2.4. Determination of Cell Count in Spheroid Cultures
2.5. Analysis of Cartilage Conditioned Media
2.6. Isolation and Cultivation of Chondrocytes in 3D Spheroid Cultures
2.7. Histological Analysis
2.8. Immunhistochemical Staining
2.9. Gene Expression Analysis by Semiquantitative RT-PCR
2.10. Statistical Analysis
3. Results
3.1. High Hydrostatic Pressure (HHP) Treatment Leads to Cell Death
3.2. Components of Cartilage ECM, Growth Factors and Binding Proteins are Released into Media
3.3. Influence of Conditioned Media in Spheroid Culture
3.3.1. Influence of Conditioned Media on Cell Number in Spheroid Culture
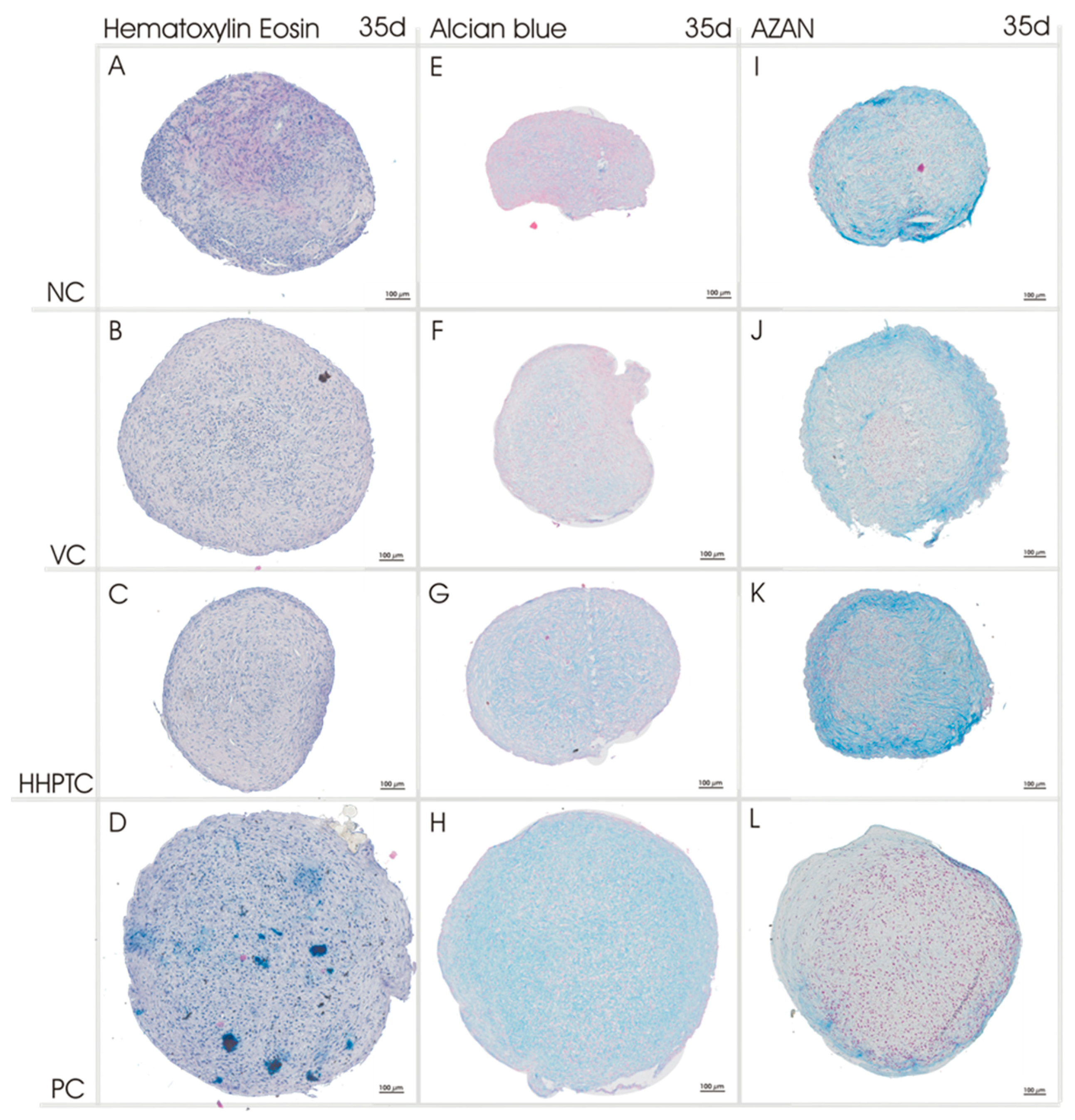
3.3.2. Histological Analyses of Chondrogenic Re-Differentiation in Spheroid Culture
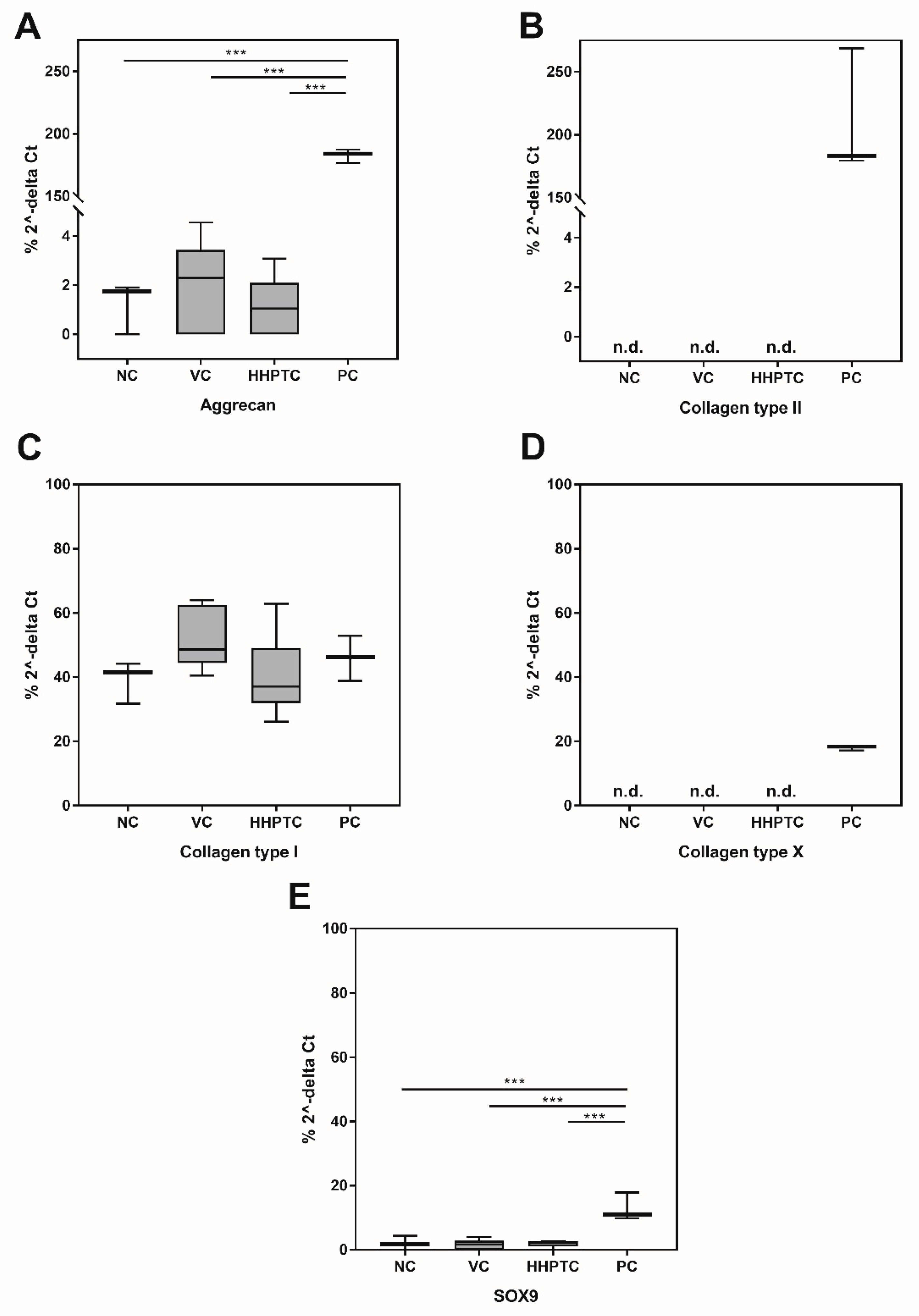
3.3.3. Influence of Conditioned Media on Gene Expression of Chondrogenic Markers
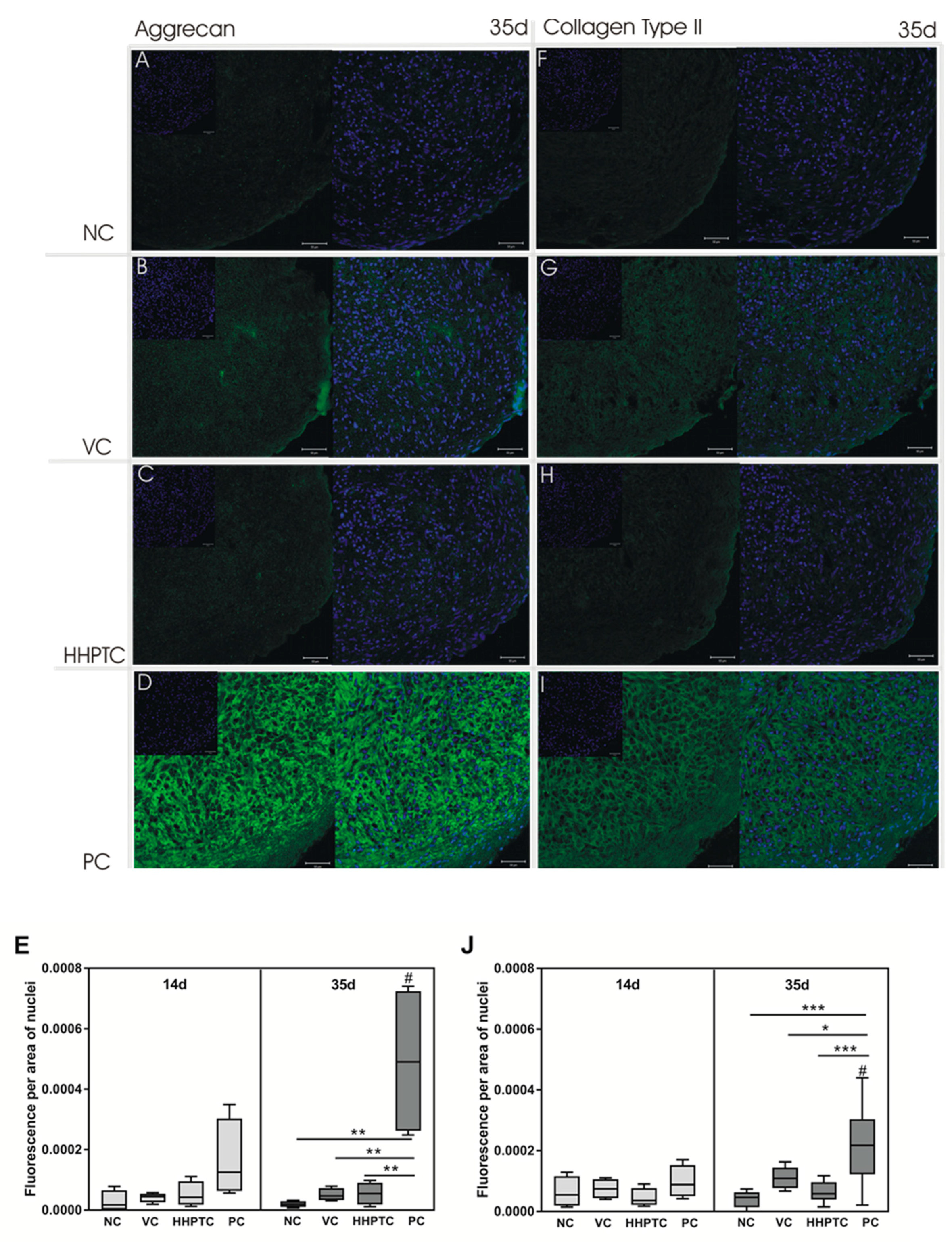
3.3.4. Immunohistochemical Analyses of Collagen Type II and Aggrecan
3.3.5. Influence of Conditioned Media on TGF-β1 Release from Chondrocytic Spheroid Cultures
4. Discussion
4.1. Analyses of VC and HHPTC Conditioned Media
4.2. Induction of Hyaline Matrix Deposition by Conditioned Media
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schneevoigt, J.; Fabian, C.; Leovsky, C.; Seeger, J.; Bahramsoltani, M. In vitro Expression of the Extracellular Matrix Components Aggrecan, Collagen Types I and II by Articular Cartilage-Derived Chondrocytes. Anat. Histol. Embryol. 2017, 46, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Das, A.K.; Chullikana, A.; Majumdar, A.S. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res. Ther. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gikas, P.D.; Bayliss, L.; Bentley, G.; Briggs, T.W. An overview of autologous chondrocyte implantation. J. Bone Jt. Surg. Br. 2009, 91, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Godkin, O.; Schulze-Tanzil, G. The Challenge in Using Mesenchymal Stromal Cells for Recellularization of Decellularized Cartilage. Stem Cell Rev. Rep. 2017, 13, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Ploegert, S.; Heberer, M.; Martin, I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003, 48, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.M.; Athanasiou, K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Activation and dedifferentiation of chondrocytes: Implications in cartilage injury and repair. Ann. Anat. 2009, 191, 325–338. [Google Scholar] [CrossRef]
- Dell’Accio, F.; De Bari, C.; Luyten, F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001, 44, 1608–1619. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigie, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Kaps, C.; Frauenschuh, S.; Endres, M.; Ringe, J.; Haisch, A.; Lauber, J.; Buer, J.; Krenn, V.; Haupl, T.; Burmester, G.R.; et al. Gene expression profiling of human articular cartilage grafts generated by tissue engineering. Biomaterials 2006, 27, 3617–3630. [Google Scholar] [CrossRef]
- Barlic, A.; Drobnic, M.; Malicev, E.; Kregar-Velikonja, N. Quantitative analysis of gene expression in human articular chondrocytes assigned for autologous implantation. J. Orthop. Res. 2008, 26, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fitzgerald, J.B.; Xu, J.; Willers, C.; Wood, D.; Grodzinsky, A.J.; Zheng, M.H. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 2008, 26, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.A.; Shahdadfar, A.; Brinchmann, J.E. Human primary articular chondrocytes, chondroblasts-like cells, and dedifferentiated chondrocytes: Differences in gene, microRNA, and protein expression and phenotype. Tissue Eng. Part C Methods 2011, 17, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Leijten, J.C.; Wu, L.; Kip, M.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthr. Cartil. 2013, 21, 599–603. [Google Scholar] [CrossRef]
- Jonitz, A.; Lochner, K.; Peters, K.; Salamon, A.; Pasold, J.; Mueller-Hilke, B.; Hansmann, D.; Bader, R. Differentiation Capacity of Human Chondrocytes Embedded in Alginate Matrix. Connect. Tissue Res. 2011, 52, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Jonitz, A.; Lochner, K.; Tischer, T.; Hansmann, D.; Bader, R. TGF-β1 and IGF-1 influence the re-differentiation capacity of human chondrocytes in 3D spheroid cultures in relation to different oxygen concentrations. Int. J. Mol. Med. 2012, 30, 666–672. [Google Scholar] [CrossRef]
- Pasold, J.; Zander, K.; Heskamp, B.; Grüttner, C.; Lüthen, F.; Tischer, T.; Jonitz-Heincke, A.; Bader, R. Positive impact of IGF-1-coupled nanoparticles on the differentiation potential of human chondrocytes cultured on collagen scaffolds. Int. J. Nanomed. 2015, 10, 1131–1143. [Google Scholar] [CrossRef]
- Jonitz-Heincke, A.; Klinder, A.; Boy, D.; Salamon, A.; Hansmann, D.; Pasold, J.; Buettner, A.; Bader, R. In vitro Analysis of the Differentiation Capacity of Postmortally Isolated Human Chondrocytes Influenced by Different Growth Factors and Oxygen Levels. Cartilage 2017, 10, 111–119. [Google Scholar] [CrossRef]
- Witt, A.; Salamon, A.; Boy, D.; Hansmann, D.; Büttner, A.; Wree, A.; Bader, R.; Jonitz-Heincke, A. Gene expression analysis of growth factor receptors in human chondrocytes in monolayer and 3D spheroid cultures. Int. J. Mol. Med. 2017, 40, 10–20. [Google Scholar] [CrossRef]
- Lin, Z.; Willers, C.; Xu, J.; Zheng, M.H. The chondrocyte: Biology and clinical application. Tissue Eng. 2006, 12, 1971–1984. [Google Scholar] [CrossRef]
- Schneiderman, R.; Snir, E.; Popper, O.; Hiss, J.; Stein, H.; Maroudas, A. Insulin-like growth factor-I and its complexes in normal human articular cartilage: Studies of partition and diffusion. Arch. Biochem. Biophys. 1995, 324, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, L.; Yang, W.; Cao, Y.; Shi, Y.; Li, X.; Zhang, Q. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthr. Cartil. 2017, 25, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Albro, M.B.; Nims, R.J.; Durney, K.M.; Cigan, A.D.; Shim, J.J.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. Heterogeneous engineered cartilage growth results from gradients of media-supplemented active TGF-β and is ameliorated by the alternative supplementation of latent TGF-β. Biomaterials 2016, 77, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Morales, T.I.; Joyce, M.E.; Sobel, M.E.; Danielpour, D.; Roberts, A.B. Transforming growth factor-beta in calf articular cartilage organ cultures: Synthesis and distribution. Arch. Biochem. Biophys. 1991, 288, 397–405. [Google Scholar] [CrossRef]
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef] [PubMed]
- Hahn, O.; Kieb, M.; Jonitz-Heincke, A.; Bader, R.; Peters, K.; Tischer, T. Dose-Dependent Effects of Platelet-Rich Plasma Powder on Chondrocytes In Vitro. Am. J. Sports Med. 2020, 48, 1727–1734. [Google Scholar] [CrossRef]
- Zhao, X.; Hwang, N.S.; Bichara, D.A.; Saris, D.B.; Malda, J.; Vacanti, J.P.; Pomerantseva, I.; Sundback, C.A.; Langer, R.; Anderson, D.G.; et al. Chondrogenesis by bone marrow-derived mesenchymal stem cells grown in chondrocyte-conditioned medium for auricular reconstruction. J. Tissue Eng. Regen. Med. 2017, 11, 2763–2773. [Google Scholar] [CrossRef]
- da Silva, M.L.A.; Costa-Pinto, A.R.; Martins, A.; Correlo, V.; Sol, P.; Bhattacharya, M.; Faria, S.; Reis, R.L.; Neves, N.M. Conditioned medium as a strategy for human stem cells chondrogenic differentiation. J. Tissue Eng. Regen. Med. 2015, 9, 714–723. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhou, G.; Xiao, R.; Cao, Y. Conditioned Medium from Chondrocyte/Scaffold Constructs Induced Chondrogenic Differentiation of Bone Marrow Stromal Cells. Anat. Rec. 2012, 295, 1109–1116. [Google Scholar] [CrossRef]
- Platas, J.; Guillén, M.I.; del Caz, M.P.; Gomar, F.; Castejón, M.; Mirabet, V.; Alcaraz, M.J. Paracrine effects of human adipose-derived mesenchymal stem cells in inflammatory stress-induced senescence features of osteoarthritic chondrocytes. Aging (Albany NY) 2016, 8, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Platas, J.; Guillén, M.I.; del Caz, M.D.; Gomar, F.; Mirabet, V.; Alcaraz, M.J. Conditioned Media from Adipose-Tissue-Derived Mesenchymal Stem Cells Downregulate Degradative Mediators Induced by Interleukin-1β in Osteoarthritic Chondrocytes. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Sutherland, A.J.; Converse, G.L.; Hopkins, R.A.; Detamore, M.S. The Bioactivity of Cartilage Extracellular Matrix in Articular Cartilage Regeneration. Adv. Healthc. Mater. 2015, 4, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hiemer, B.; Genz, B.; Jonitz-Heincke, A.; Pasold, J.; Wree, A.; Dommerich, S.; Bader, R. Devitalisation of human cartilage by high hydrostatic pressure treatment: Subsequent cultivation of chondrocytes and mesenchymal stem cells on the devitalised tissue. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [PubMed]
- Schnabel, M.; Marlovits, S.; Eckhoff, G.; Fichtel, I.; Gotzen, L.; Vécsei, V.; Schlegel, J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthr. Cartil. 2002, 10, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Risteli, J.; Melkko, J.; Niemi, S.; Risteli, L. Use of a marker of collagen formation in osteoporosis studies. Calcif. Tissue Int. 1991, 49, S24–S25. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar]
- van der Kraan, P.M.; Buma, P.; van Kuppevelt, T.; van den Berg, W.B. Interaction of chondrocytes, extracellular matrix and growth factors: Relevance for articular cartilage tissue engineering. Osteoarthr. Cartil. 2002, 10, 631–637. [Google Scholar] [CrossRef]
- Martin, J.A.; Miller, B.A.; Scherb, M.B.; Lembke, L.A.; Buckwalter, J.A. Co-localization of insulin-like growth factor binding protein 3 and fibronectin in human articular cartilage. Osteoarthr. Cartil. 2002, 10, 556–563. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; de Souza, P.; Castrejon, H.V.; John, T.; Merker, H.J.; Scheid, A.; Shakibaei, M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002, 308, 371–379. [Google Scholar] [PubMed]
- Ruggiero, F.; Petit, B.; Ronziere, M.C.; Farjanel, J.; Hartmann, D.J.; Herbage, D. Composition and organization of the collagen network produced by fetal bovine chondrocytes cultured at high density. J. Histochem. Cytochem. 1993, 41, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.C.; Saunders, K.M.; Burton-Wurster, N.; Macleod, J.N. Phenotypic stability of articular chondrocytes in vitro: The effects of culture models, bone morphogenetic protein 2, and serum supplementation. J. Bone Miner. Res. 2000, 15, 166–174. [Google Scholar] [CrossRef]
- Zhang, L.; Gardiner, B.S.; Smith, D.W.; Pivonka, P.; Grodzinsky, A. The effect of cyclic deformation and solute binding on solute transport in cartilage. Arch. Biochem. Biophys. 2007, 457, 47–56. [Google Scholar] [CrossRef]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef]
- Albro, M.B.; Nims, R.J.; Cigan, A.D.; Yeroushalmi, K.J.; Alliston, T.; Hung, C.T.; Ateshian, G.A. Accumulation of exogenous activated TGF-β in the superficial zone of articular cartilage. Biophys. J. 2013, 104, 1794–1804. [Google Scholar] [CrossRef]
- Indrawattana, N.; Chen, G.; Tadokoro, M.; Shann, L.H.; Ohgushi, H.; Tateishi, T.; Tanaka, J.; Bunyaratvej, A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem. Biophys. Res. Commun. 2004, 320, 914–919. [Google Scholar] [CrossRef]
- Gavénis, K.; Schmidt-Rohlfing, B.; Mueller-Rath, R.; Andereya, S.; Schneider, U. In vitro Comparison of Six Different Matrix Systems for the Cultivation of Human Chondrocytes. Cell. Dev. Biol. Anim. 2006, 42, 159–167. [Google Scholar] [CrossRef]
- Loeser, R.F. Integrins and chondrocyte–matrix interactions in articular cartilage. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef]
- Rutgers, M.; Saris, D.B.; Vonk, L.A.; van Rijen, M.H.; Akrum, V.; Langeveld, D.; van Boxtel, A.; Dhert, W.J.; Creemers, L.B. Effect of Collagen Type I or Type II on Chondrogenesis by Cultured Human Articular Chondrocytes. Tissue Eng. Part A 2013, 19, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Matsiko, A.; Dickson, G.R.; O’Brien, F.J.; Gleeson, J.P. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014, 10, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Lauing, K.L.; Cortes, M.; Domowicz, M.S.; Henry, J.G.; Baria, A.T.; Schwartz, N.B. Aggrecan is required for growth plate cytoarchitecture and differentiation. Dev. Biol. 2014, 396, 224–236. [Google Scholar] [CrossRef] [PubMed]
- French, M.M.; Rose, S.; Canseco, J.; Athanasiou, K.A. Chondrogenic differentiation of adult dermal fibroblasts. Ann. Biomed. Eng. 2004, 32, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hu, J.C.; Athanasiou, K.A. Isolation and chondroinduction of a dermis-isolated, aggrecan-sensitive subpopulation with high chondrogenic potential. Arthritis Rheum. 2007, 56, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ingavle, G.C.; Frei, A.W.; Gehrke, S.H.; Detamore, M.S. Incorporation of aggrecan in interpenetrating network hydrogels to improve cellular performance for cartilage tissue engineering. Tissue Eng. Part A 2013, 19, 1349–1359. [Google Scholar] [CrossRef]
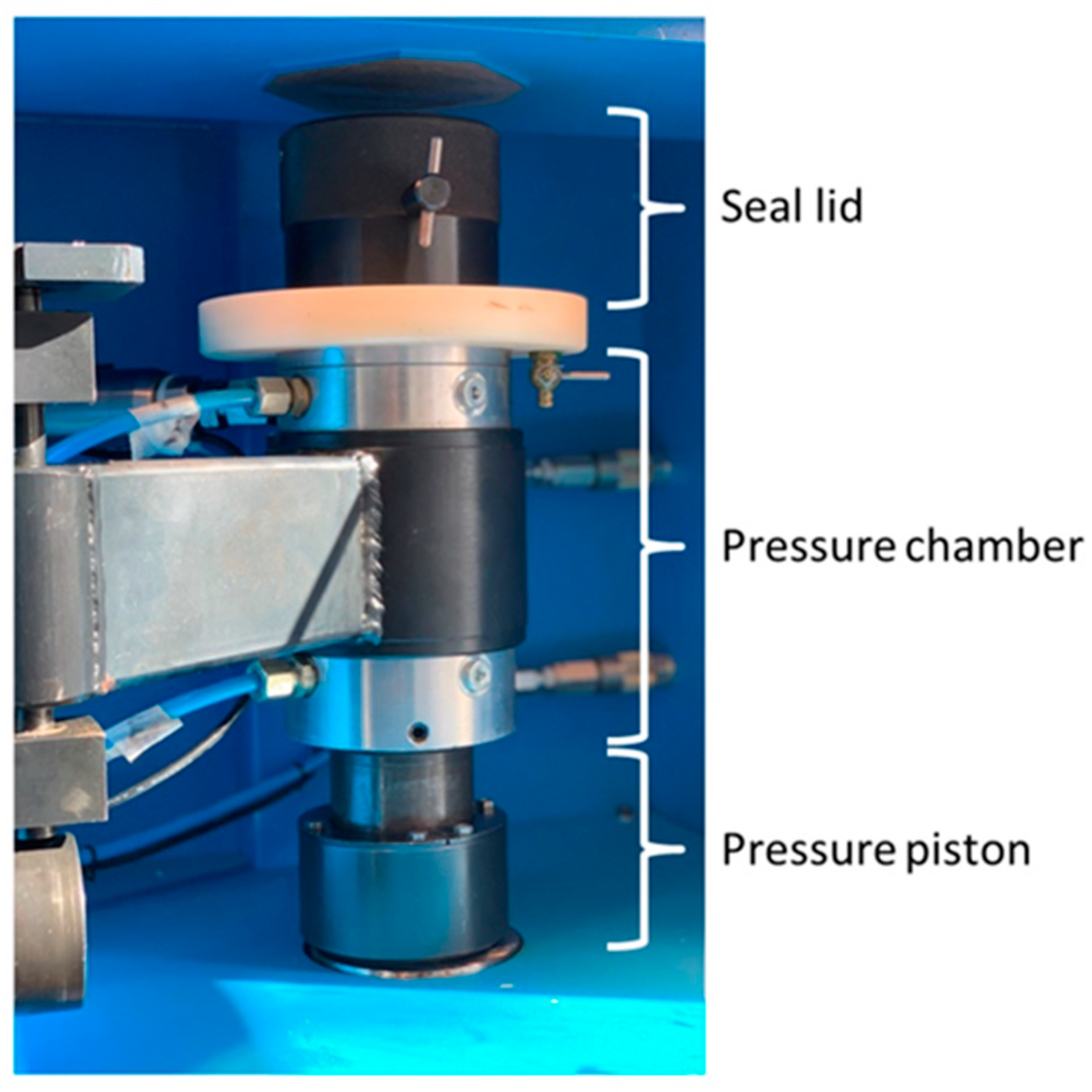
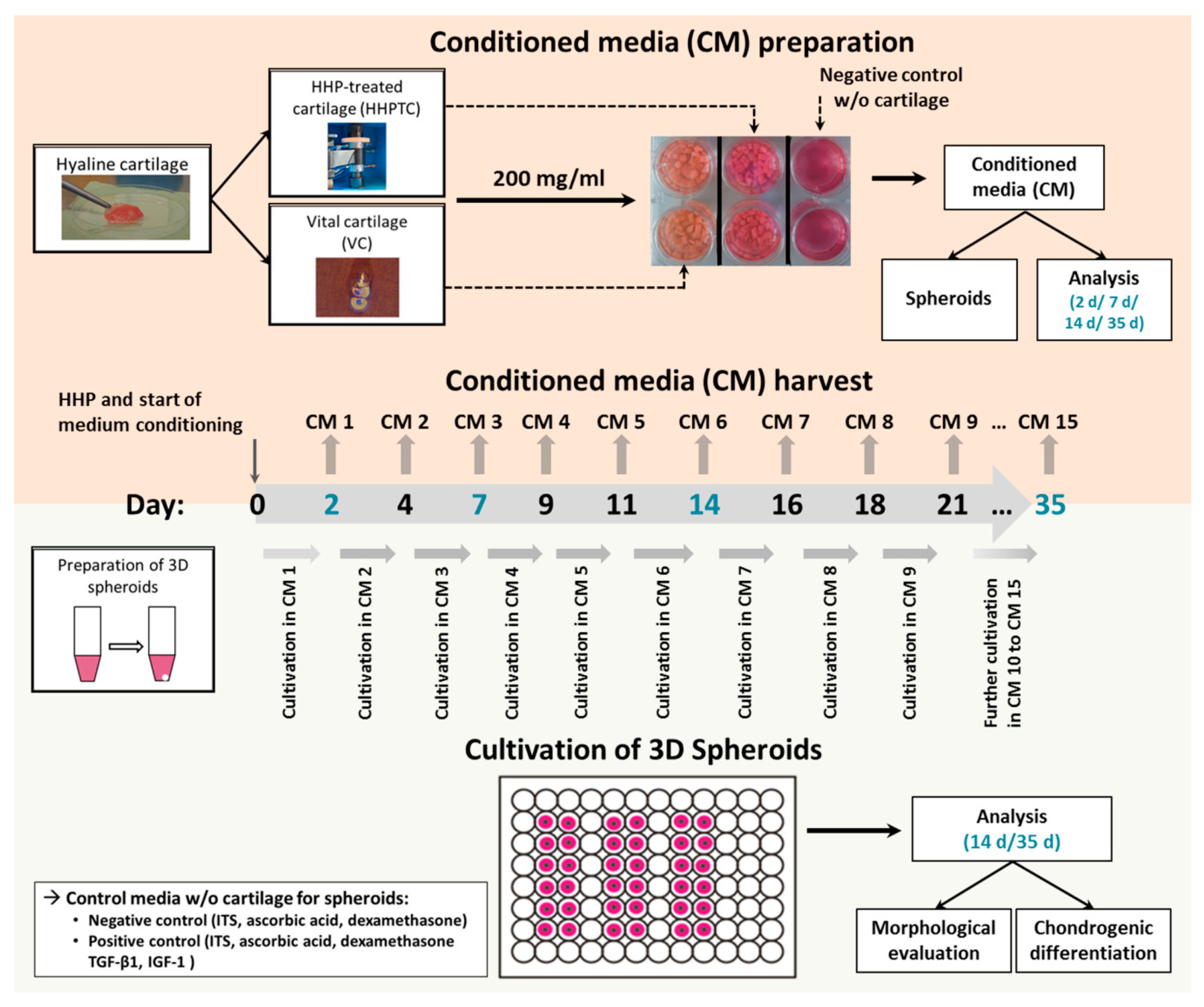
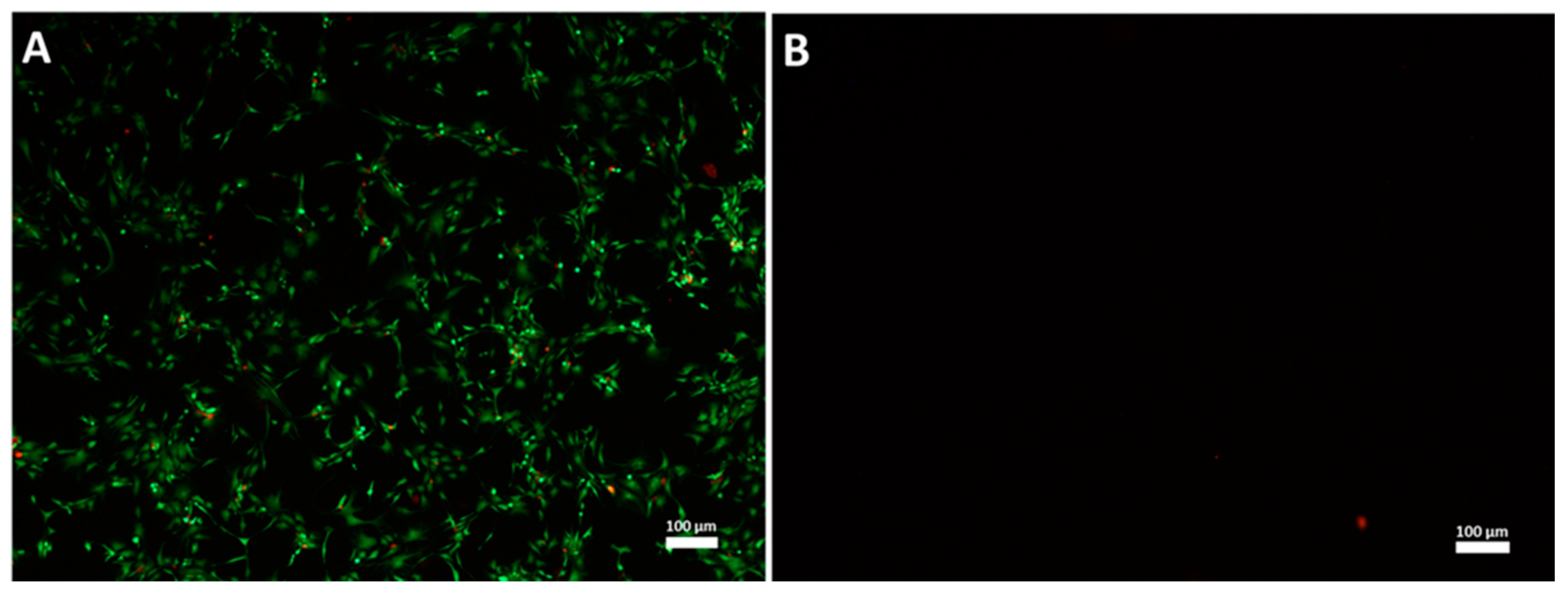

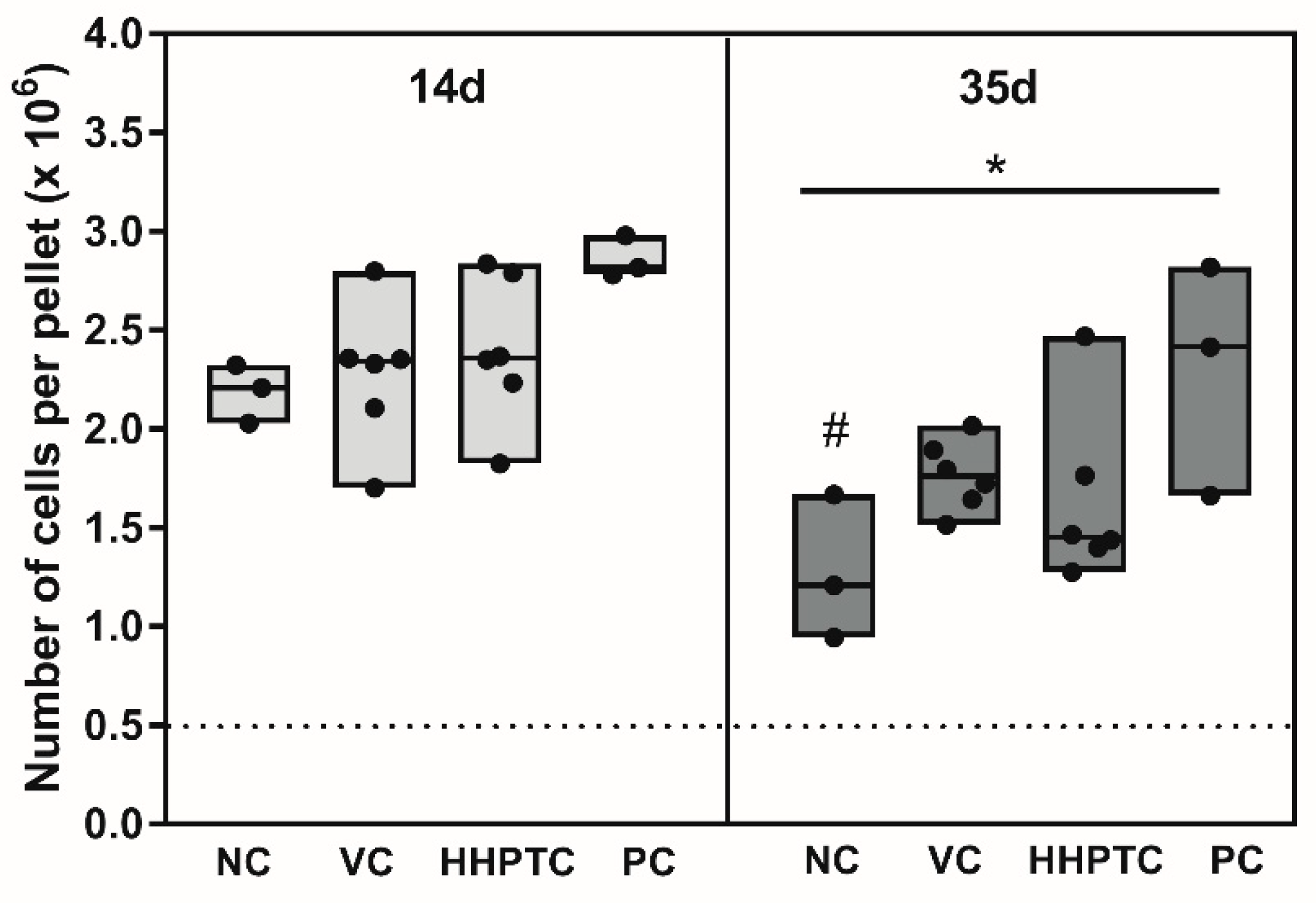

| Components | Chondrocyte Cultivation Medium/NC | PC | VC-CM | HHPTC-CM |
|---|---|---|---|---|
| VC-CM (in µL) | - | - | 500 | - |
| HHPTC-CM (in µL) | - | - | - | 500 |
| DMEM (in µL), supplemented with 1% pen/strep, 1% fungicide | 1000 | 1000 | 500 | 500 |
| ITS™ | 1:100 | 1:100 | 1:100 | 1:100 |
| Ascorbic acid | 50 µg/mL | 50 µg/mL | 50 µg/mL | 50 µg/mL |
| Dexamethasone | 100 nM | 100 nM | 100 nM | 100 nM |
| IGF-1 | - | 50 ng/mL | - | - |
| TGF-β1 | - | 50 ng/mL | - | - |
| Transcript | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-Actin | CTTCCTGGGCATGGAGTC | AGCACTGTGTTGGCGTACAG |
| Aggrecan | ACAAGGTCTCACTGCCCAAC | AATGGAACACGATGCCTTTC |
| Collagen 1 (Col1A1) | ACGAAGACATCCCACCAATC | AGATCACGTCATCGCACAAC |
| Collagen 2 (Col2A1) | AATGGTGGCTTCCATTCAG | GTGATGTTCTGGGAGCCTTC |
| Collagen 10 (Col10A1) | GAACTCCCAGCACGCAGAATC | AGTGGGCCTTTTATGCCTGT |
| SRY-box 9 (Sox9) | AGTACCCGCACCTGCACAAC | CGCTTCTCGCTCTCGTTCAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinder, A.; Kussauer, S.; Hiemer, B.; Wree, A.; Bader, R.; Jonitz-Heincke, A. Influence of Conditioned Media on the Re-Differentiation Capacity of Human Chondrocytes in 3D Spheroid Cultures. J. Clin. Med. 2020, 9, 2798. https://doi.org/10.3390/jcm9092798
Klinder A, Kussauer S, Hiemer B, Wree A, Bader R, Jonitz-Heincke A. Influence of Conditioned Media on the Re-Differentiation Capacity of Human Chondrocytes in 3D Spheroid Cultures. Journal of Clinical Medicine. 2020; 9(9):2798. https://doi.org/10.3390/jcm9092798
Chicago/Turabian StyleKlinder, Annett, Sophie Kussauer, Bettina Hiemer, Andreas Wree, Rainer Bader, and Anika Jonitz-Heincke. 2020. "Influence of Conditioned Media on the Re-Differentiation Capacity of Human Chondrocytes in 3D Spheroid Cultures" Journal of Clinical Medicine 9, no. 9: 2798. https://doi.org/10.3390/jcm9092798
APA StyleKlinder, A., Kussauer, S., Hiemer, B., Wree, A., Bader, R., & Jonitz-Heincke, A. (2020). Influence of Conditioned Media on the Re-Differentiation Capacity of Human Chondrocytes in 3D Spheroid Cultures. Journal of Clinical Medicine, 9(9), 2798. https://doi.org/10.3390/jcm9092798




