Whole-Body Cryotherapy Is an Effective Method of Reducing Abdominal Obesity in Menopausal Women with Metabolic Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Study Design
- Waist circumference > 88 cm;
- TG concentration ≥ 150 mg/dL;
- HDL concentration < 50 mg/dL;
- fasting glucose ≥ 100 mg/dL; and
- systolic blood pressure (SBP) ≥ 130 mmHg o diastolic blood pressure (DBP) ≥ 85 mmHg or anti hypersensitive therapy.
2.2. Participants
2.3. Whole-Body Cryotherapy
2.4. Somaticmeasurements and Body Compositionevaluation
2.5. Biochemical Analysis
2.6. Assessment of Physicalactivity
2.7. Assessment of Nutritional Behaviour
2.8. Statistical Analysis
3. Results
3.1. Changes in Body Composition, Circumferences and Skin Fold Thicknesses as an Effect of Applying Whole-Body Cryotherapy Procedures
3.1.1. Body Mass and Composition
3.1.2. Circumferences
3.1.3. Skinfolds
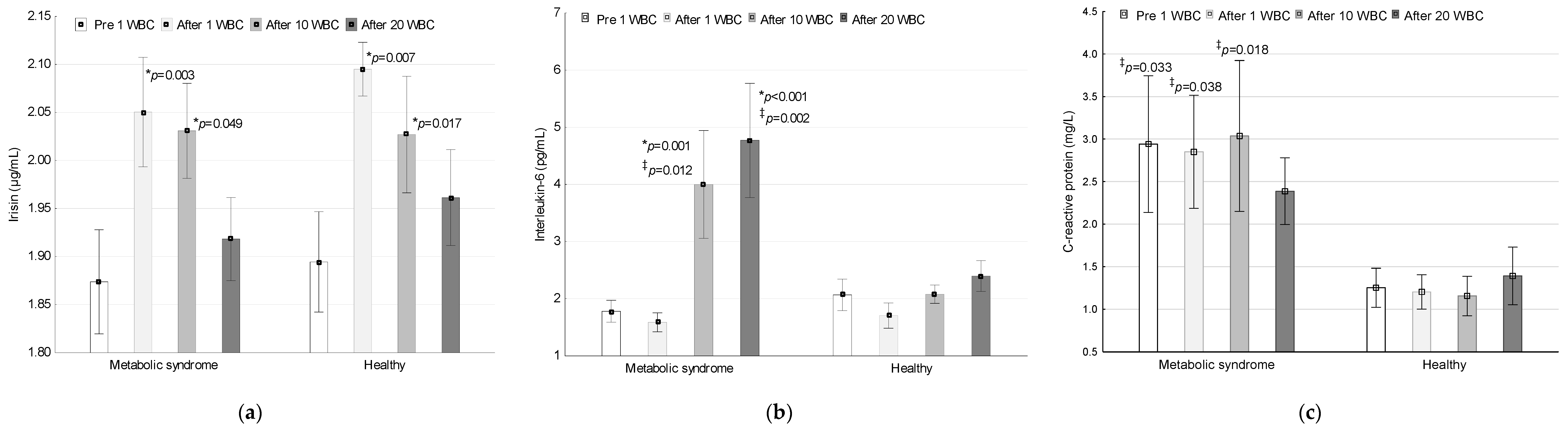
3.2. Irisin, IL-6 and CRP Concentrations during the Application of Whole-Body Cryotherapy
3.2.1. Irisin
3.2.2. Interleukin-6
3.2.3. C-Reactive Protein
3.3. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 July 2020).
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 192 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [Green Version]
- Chooi, Y.U.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janiszewska, R.; Orawiec, R.; Nowak, S. Assessment of body composition, total fatness and fatty tissue distribution in women during process of aging. Probl. Hig. Epidemiol. 2015, 96, 517–522. [Google Scholar]
- Hernandez-Camacho, J.D.; Hernandez-Camacho, M. Clinical update on metabolic syndrome. Rev. Esp. Nutr. Hum. Diet. 2017, 21, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Sonata, J.; Modeneza, D.; Vilatra, R.; Maciel, E.L.; Boccaletto, E.M.; da Silva, C.C. Body composition and quality of life of the elderly offered by the „University Third Age” in Brazil. Arch. Gerontol. Geriatr. 2011, 52, 31–35. [Google Scholar] [CrossRef]
- Ghandehari, H.; Le, V.; Kamal-Bahl, S.; Bassin, S.L.; Wong, N.D. Abdominal obesity and the spectrum of global cardiometabolic risks in US adults. Int. J. Obes. 2009, 33, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Stepaniak, U.; Micek, A.; Waskiewicz, A.; Bielecki, W.; Drygas, W.; Janion, M.; Kozakiewicz, K.; Niklas, A.; Puch-Walczak, A.; Pajak, A. Prevalence of general and abdominal obesity and overweight among adults in Poland. Results of the WOBASZ II study (2013–2014) and comparison with the WOBASZ study (2003–2005). Pol. Arch. Med. Wewn. 2016, 126, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Mbata, O.; Abo El Magd, N.F.; El- Remessy, A.B. Obesity, metabolic syndrome and diabetic retinopathy: Beyond hiperglicemia. World J. Diabetes 2017, 8, 317–329. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Derm. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, N. Metabolic syndrome: Practice essentials, background, pathophysiology. J. Heart Stroke 2018, 3, 1044. [Google Scholar]
- Kalinowski, P.; Mianowana, M. Metabolic syndrome part II: Epidemiology of metabolic syndrome in Poland and in the World. J. Educ. Health Sport 2016, 6, 466–480. [Google Scholar]
- Vishram, J.K.; Borglykke, A.; Andreasen, A.H.; Jeppesen, J.; Ibsen, H.; Jorgensen, T.; Palmieri, L.; Giampao, S.; Donfrancesco, C.; Kee, F.; et al. Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in Europeans, The MORGAM Prospective Cohort Project. PLoS ONE 2014, 9, e107294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drygas, W.; Bielecki, W.; Pajak, A.; Piotrowski, W. Multi-centre national health evaluation of the populationin Poland. In Epidemiology and Prevention of Cardiovascular Diseases, 1st ed.; Kopec, G., Jankowski, P., Pajak, A., Drygas, W., Eds.; Practical Medicine Publishing House: Krakow, Poland, 2015; pp. 43–56. [Google Scholar]
- Riediger, N.D.; Clara, I. Prevalence of metabolic syndrome in the Canadian adult population. CMAJ 2011, 183, 1127–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olszanecka-Glinianowicz, M.; Zahorska-Markiewicz, B. Obesity as inflammatory disease. Postepy Hig. Med. Dosw. 2008, 62, 249–257. [Google Scholar]
- Pihl, E.; Zilmer, K.; Kullisaar, T.; Kairane, C.; Magi, A.; Zilmer, M. Atherogenic inflammatory and oxidative stress markers in relation to overweight values in male former athletes. Int. J. Obes. 2006, 30, 141–146. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic. Dis. 2017, 14, 160287. [Google Scholar] [CrossRef] [Green Version]
- Raza, Q.; Doak, C.M.; Khan, A.; Nicolaou, M.; Seidell, J.C. Obesity and cardiovascular disease risk factors among the indigenous and immigrant Pakistani population: A systematic review. Obes. Facts 2013, 6, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Dudzińska, W.; Bryczkowska, I.; Dołęgowska, B. Body composition, lipid profile, adipokine concentration, and antioxidant capacity changes during interventions to treat overweight with exercise programme and whole-body cryostimulation. Oxid. Med. Cell. Longev. 2015, 2015, 803197. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, A.; Olek, R.A.; Grzywacz, T.; Antosiewicz, J.; Kujach, S.; Łuszczyk, M.; Smaruj, M.; Sledziewska, E.; Laskowski, R. Whole-body cryostimulation as an effective method of reducing low-grade inflammation in obese men. J. Physiol Sci. 2013, 63, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Dulian, K.; Laskowski, R.; Grzywacz, T.; Kujach, S.; Flis, D.J.; Smaruj, M.; Ziemann, E. The whole body cryostimulation modifies irisin concentration and reduces inflammation in middle aged, obese men. Cryobiology 2015, 71, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Wiecek, M.; Szymura, J.; Sproull, J.; Szygula, Z. Decreased blood asprosin in hyperglycemic menopausal women as a result of whole-body cryotherapy regardless of metabolic syndrome. J. Clin. Med. 2019, 8, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilch, W.; Wyrostek, J.; Major, P.; Zuziak, R.; Piotrowska, A.; Czerwinska-Ledwig, O.; Grzybkowska, A.; Zasada, M.; Ziemann, E.; Zychowska, M. The effect of whole-body cryostimulation on body composition and leukocyte expression of HSPA1A, HSPB1, and CRP in obese men. Cryobiology 2020, 94, 100–106. [Google Scholar] [CrossRef]
- Lubkowska, A. Cryotherapy: Physiological considerations and applications to physical therapy. In Physical Therapy Perspectives in the 21st Century—Challenges and Possibilities, 1st ed.; Bettany-Saltikov, J., Ed.; Intech Europe: Rijeka, Croatia, 2012; pp. 155–176. [Google Scholar]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-body cryotherapy in athletes: From therapy to stimulation. An updated review of the literature. Front. Physiol. 2017, 8, 258. [Google Scholar] [CrossRef] [Green Version]
- National Health Fund. Available online: https://www.nfz.gov.pl/zarzadzenia-prezesa/zarzadzenia-prezesa-nfz/zarzadzenie-nr-132019dsoz,6878.html (accessed on 19 August 2020).
- Szymura, J.; Wiecek, M.; Maciejczyk, M.; Gradek, J.; Kantorowicz, M.; Szygula, Z. Unchanged erythrocyte profile after exposure to cryogenic temperatures in elder marathon runners. Front. Physiol. 2018, 9, 659. [Google Scholar] [CrossRef] [Green Version]
- Lubkowska, A.; Szygula, Z.; Chlubek, D.; Banfi, G. The effect of prolonged whole-body cryostimulation treatment with different amounts of sessions on chosen pro- and anti-inflammatory cytokines levels in healthy men. Scand. J. Clin. Lab. Invest. 2011, 71, 419–425. [Google Scholar] [CrossRef]
- Hall, G.; Steensberg, A.; Sacchetti, M.; Fischer, C.; Keller, C.; Schjerling, P.; Hiscock, N.; Moller, K.; Saltin, B.; Febbraio, M.A.; et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J. Clin. Endocrinol. Metab. 2003, 88, 3005–3010. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2007, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PCG-1—Dependent myokine that drives brown-fatlike development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A hope in understanding and managing obesity and metabolic syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, S.; Ulker, N.; Bulmus, O.; Yardimci, A.; Ozcan, M.; Canpolat, S. The modulatory effects of irisin on asprosin, leptin, glucose levels and lipid profile in healthy and obese male and female rats. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]
- Leung, W.; Yu, A.P.; Lai, C.; Siu, P.M. Association of markers of proinflammatory phenotype and beige adipogenesis with metabolic syndrome in Chinese centrally obese adults. J. Diabetes Res. 2018, 2018, 8956509. [Google Scholar] [CrossRef] [Green Version]
- Andrade, P.A.; Souza Silveira, B.K.; Correa Rodrigues, A.; Oliveira da Silva, F.M.; Barbosa Rosa, C.O.; GoncalvesAlfenas, R.C. Effect of exercise on concentrations of irisin in overweight individuals: A systematic review. Sci. Sports 2018, 33, 80–89. [Google Scholar] [CrossRef]
- Sliwicka, E.; Cison, T.; Straburzynska-Lupa, A.; Pilaczynska-Szczesniak, L. Effects of whole-body cryotherapy on 25-hydroxyvitamin D, irisin, myostatin, and interleukin-6 levels in healthy young men of different fitness levels. Sci. Rep. 2020, 10, 6175. [Google Scholar] [CrossRef] [Green Version]
- Jarosz, M.; Wolnicka, K.; Sajor, J.; Wierzejska, R. Recommendations for nutrition and physical activity. In Nutrition Standards for the Polish Population, 1st ed.; Jarosz, M., Ed.; Institute of Food and Nutrition: Warsaw, Poland, 2017; pp. 261–300. [Google Scholar]
- Lubkowska, A.; Szygula, Z. Changes in blood pressure with compensatory heart rate decrease and in the level in aerobic capacity in response to repeated whole-body cryostimulation in normotensive, young and physically active men. Int. J. Occup. Med. Environ. Health 2010, 23, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Biernat, E.; Stupnicki, R.; Gajewski, A.K. International Physical Activity Questionnaire (IPAQ)—Polish version. Phys. Educ. Sport 2007, 51, 47–54. [Google Scholar]
- Lombardo, M.; Perrone, M.A.; Guseva, E.; Aulisa, G.; Padua, E.; Bellia, C.; Della-Morte, D.; Iellamo, F.; Caprio, M.; Bellia, A. Losing weight after menopause with minimal aerobic training and mediterranean diet. Nutrients 2020, 12, 2471. [Google Scholar] [CrossRef] [PubMed]
- Osella, A.R.; Colaianni, G.; Correale, M.; Pesole, P.L.; Bruno, I.; Buongiorno, C.; Deflorio, V.; Leone, C.M.; Colucci, S.C.; Grano, M.; et al. Irisin serum levels in metabolic syndrome patients treated with three different diets: A post-hoc analysis from a randomized controlled clinical trial. Nutrients 2018, 10, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, B.J.; Park, K.H.; Shin, S.; Zaichenko, L.; Davis, C.R.; Crowell, J.A.; Joung, H.; Mantzoros, C.S. Diet quality and diet patterns in relation to circulating cardiometabolic biomarkers. Clin. Nutr. 2016, 35, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Szponar, L.; Wolnicka, K.; Rychli, E. Album of Photographs of Food Products and Dishes; Institute of Food and Nutrition: Warsaw, Poland, 2000; pp. 1–82. [Google Scholar]
- Giglio, B.M.; Schincaglia, R.M.; da Silva, A.S.; Fazani, I.C.S.; Monteiro, P.A.; Mota, J.F.; Cunha, J.P.; Pichard, C.; Pimentel, G.D. Whey protein supplementation compared to collagen increases blood nesfatin concentrations and decreases android fat in overweight women: A randomized double-blind study. Nutrients 2019, 11, 2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef]
- Bronczyk-Puzon, A.; Koszowska, A.; Bieniek, J. Basic anthropometric measurements and derived ratios in dietary counseling: Part one. Piel. Zdr. Publ. 2018, 8, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Yoo, E.G. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J. Pediatr. 2016, 59, 425–431. [Google Scholar] [CrossRef]
- Filliard, J.; Faria, F.C.; Bieuzen, F.; Volondat, M. The effects of the whole-body cryotherapy on the body composition. Br. J. Sports Med. 2016, 50, 38. [Google Scholar] [CrossRef]
- Golozoubova, V.; Hohtola, E.; Matthias, A.; Jacobsson, A.; Cannon, B.; Nedergaard, J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001, 15, 2048–2050. [Google Scholar] [CrossRef]
- Gao, S.; Li, F.; Li, H.; Huang, Y.; Liu, Y.; Chen, Y. Effects and molecular mechanism of GST-Irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PLoS ONE 2016, 11, e0147480. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Q.; Chen, D.; Sun, H.J.; Ding, L.; Wang, J.J.; Chen, Q.; Li, Y.H.; Zhou, Y.B.; Han, Y.; Zhang, F.; et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta 2015, 1852, 1867–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubkowska, A.; Szyguła, Z.; Klimek, A.; Torii, M. Do sessions of cryostimulation have influence on white blood cell count, level of IL6 and total oxidative and antioxidative status in healthy men? Eur. J. Appl. Physiol. 2010, 109, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Ziemann, E.; Olek, R.A.; Kujach, S.; Grzywacz, T.; Antosiewicz, J.; Garstka, T.; Laskowski, R. Five-day whole-body cryostimulation, blood inflammatory markers, and performance in high-ranking professional tennis players. J. Athl. Train. 2012, 47, 664–672. [Google Scholar] [CrossRef] [PubMed]
| Variable | Metabolic Syndrome | Healthy | Total | Min-Max |
|---|---|---|---|---|
| Age (years) | 61.53 ± 3.99 | 60.28 ± 3.63 | 60.92 ± 3.82 | 55.00–67.00 |
| Erythrocytes (106/µL) | 4.62 ± 0.22 | 4.59 ± 0.24 | 4.61 ± 0.23 | 4.20–5.10 |
| Haemoglobin (g/dL) | 13.89 ± 0.55 | 13.94 ± 0.55 | 13.92 ± 0.55 | 13.20–15.30 |
| Haematocrit (%) | 40.82 ± 1.75 | 41.14 ± 1.62 | 40.98 ± 1.67 | 38.60–44.60 |
| ESR (mm/h) | 19.58 ± 10.67 | 16.11 ± 9.65 | 17.89 ± 10.19 | 2.00–43.00 |
| Platelets (103/µL) | 246.53 ± 77.69 | 254.89 ± 56.59 | 250.59 ± 67.44 | 61.00–457.00 |
| Leukocytes (103/µL) | 6.16 ± 1.25 * | 5.29 ± 1.06 | 5.74 ± 1.23 | 3.82–8.53 |
| Neutrophils (103/µL) | 3.04 ± 0.86 | 2.60 ± 0.57 | 2.83 ± 0.76 | 1.60–5.40 |
| Lymphocytes (103/µL) | 2.24 ± 0.56 | 1.92 ± 0.50 | 2.08 ± 0.55 | 1.10–3.40 |
| Monocytes (103/µL) | 0.48 ± 0.11 | 0.50 ± 0.14 | 0.49 ± 0.12 | 0.26–0.84 |
| Eosinophils (103/µL) | 0.18 ± 0.09 | 0.17 ± 0.09 | 0.18 ± 0.09 | 0.06–0.42 |
| Basophils (103/µL) | 0.02 ± 0.03 | 0.04 ± 0.04 | 0.03 ± 0.03 | 0.00–0.10 |
| HbA1C (%) | 5.84 ± 0.28 * | 5.67 ± 0.29 | 5.75 ± 0.29 | 5.20–6.50 |
| Insulin (µIU/mL) | 12.12 ± 5.65 * | 8.27 ± 2.50 | 10.25 ± 4.77 | 4.40–28.20 |
| HOMA-IR | 3.03 ± 1.52 * | 1.92 ± 0.61 | 2.49 ± 1.29 | 0.95–7.19 |
| TCHOL (mg/dL) | 213.50 ± 37.57 | 223.56 ± 35.55 | 218.39 ± 6.45 | 157.90–295.28 |
| LDL (mg/dL) | 127.78 ± 37.13 | 137.81 ± 35.04 | 132.66 ± 35.99 | 57.13–214.12 |
| AIP (log10TG/HDL) | 0.38 ± 0.23 * | 0.22 ± 0.20 | 0.30 ± 0.23 | -0.15–0.93 |
| Homocysteine (µmol/L) | 13.15 ± 1.97 | 12.26 ± 1.71 | 12.72 ± 1.88 | 9.00–17.00 |
| hsCRP (mg/L) | 2.94 ± 3.50 * | 1.25 ± 097 | 2.12 ± 2.70 | 0.27–15.89 |
| Group | WC (cm) | TG (mg/dL) | HDL (mg/dL) | Glucose (mg/dL) | SBP (mmHg) | DBP (mmHg) |
|---|---|---|---|---|---|---|
| Metabolic syndrome | 96.24 ± 9.91 * | 130.65 ± 41.40 | 55.50 ± 12.17 * | 102.39 ± 9.81 * | 127.63 ± 17.27 | 82.63 ± 7.88 |
| Healthy | 84.22 ± 8.71 | 111.47 ± 38.47 | 65.06 ± 14.11 | 92.93 ± 6.94 | 120.17 ± 16.78 | 77.83 ± 7.69 |
| Total | 88.81 ± 8.58 | 121.32 ± 40.63 | 60.15 ± 13.84 | 97.79 ± 9.69 | 124.00 ± 17.22 | 80.30 ± 8.06 |
| Min-Max | 73.60−109.80 | 63.88−215.25 | 41.41−91.72 | 73.60−109.80 | 90−150 | 60−90 |
| Number of people fulfilling given criterion for diagnosis of metabolic syndrome NCEP-ATP III | ||||||
| Metabolic syndrome | 14 (73.7%) | 6 (31.6%) | 7 (36.8%) | 15 (78.9%) | 10 (52.6%) | 7 (36.8%) |
| Healthy | 3 (16.7%) | 3 (16.7%) | 1 (5.6%) | 3 (16.7%) | 6 (33.3%) | 4 (22.2%) |
| Total | 17 (45.9%) | 9 (24.3%) | 8 (21.6%) | 18 (48.6%) | 16 (43.2%) | 11 (29.7%) |
| Number of metabolic syndrome criteria NCEP-ATP III fulfilled by volunteers | ||||||
| Healthy | Metabolic syndrome | |||||
| Number of criteria | None | One | Two | Three | Four | Five |
| Number of people | 4 (10.8%) | 10 (27.0%) | 4 (10.8%) | 11 (29.7%) | 4 (10.8%) | 4 (10.8%) |
| Variable | Group (G) | Treatment (T) | Interaction G×T | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | F | Power Test 1-β | Effect Size η2 | p | F | Power Test 1-β | Effect Size η2 | p | F | Power Test 1-β | Effect Size η2 | |
| Body composition | ||||||||||||
| Body Mass (kg) | 0.001 | 11.98 | 0.92 | 0.25 | 0.001 | 7.31 | 0.93 | 0.17 | 0.780 | 0.25 | 0.09 | 0.01 |
| BMI (kg/m2) | 0.001 | 12.62 | 0.93 | 0.27 | 0.001 | 7.47 | 0.93 | 0.18 | 0.824 | 0.19 | 0.08 | 0.01 |
| Lean Body Mass (kg) | 0.014 | 6.74 | 0.71 | 0.16 | 0.760 | 0.28 | 0.09 | 0.01 | 0.623 | 0.48 | 0.12 | 0.01 |
| Total Body Fat (kg) | <0.001 | 15.49 | 0.97 | 0.31 | 0.001 | 8.42 | 0.96 | 0.19 | 0.954 | 0.05 | 0.06 | <0.01 |
| Total Body Fat (%) | 0.005 | 9.04 | 0.83 | 0.21 | 0.036 | 3.50 | 0.63 | 0.09 | 0.840 | 0.18 | 0.08 | <0.01 |
| Arm Fat (%) | 0.216 | 1.59 | 0.23 | 0.04 | 0.221 | 1.54 | 0.32 | 0.04 | 0.976 | 0.02 | 0.05 | <0.01 |
| Leg Fat (%) | 0.091 | 3.01 | 0.39 | 0.08 | 0.006 | 5.59 | 0.84 | 0.14 | 0.986 | 0.01 | 0.05 | <0.01 |
| Trunk Fat (%) | 0.003 | 10.32 | 0.88 | 0.23 | 0.113 | 2.25 | 0.44 | 0.06 | 0.654 | 0.43 | 0.12 | 0.01 |
| Android Fat (%) | 0.004 | 9.52 | 0.85 | 0.21 | 0.049 | 3.06 | 0.57 | 0.08 | 0.484 | 0.73 | 0.17 | 0.02 |
| Gynoid Fat (%) | 0.098 | 2.88 | 0.38 | 0.08 | 0.277 | 1.31 | 0.27 | 0.04 | 0.353 | 1.06 | 0.23 | 0.03 |
| A/G | 0.020 | 5.94 | 0.66 | 0.15 | 0.314 | 1.18 | 0.25 | 0.03 | 0.792 | 0.23 | 0.09 | 0.01 |
| Circumferences | ||||||||||||
| WC (cm) | 0.002 | 11.94 | 0.91 | 0.31 | <0.001 | 13.54 | 0.99 | 0.33 | 0.516 | 0.67 | 0.16 | 0.02 |
| AC (cm) | 0.004 | 9.95 | 0.86 | 0.27 | 0.001 | 7.52 | 0.93 | 0.22 | 0.471 | 0.76 | 0.17 | 0.03 |
| HC (cm) | 0.004 | 9.93 | 0.86 | 0.27 | <0.001 | 9.67 | 0.98 | 0.26 | 0.372 | 1.01 | 0.22 | 0.04 |
| WHR | 0.124 | 2.52 | 0.35 | 0.09 | 0.080 | 2.71 | 0.51 | 0.09 | 0.363 | 1.03 | 0.22 | 0.04 |
| WHtR | 0.002 | 11.60 | 0.91 | 0.30 | <0.001 | 13.19 | 0.99 | 0.33 | 0.521 | 0.66 | 0.16 | 0.02 |
| Skinfold thickness | ||||||||||||
| Abdominal (mm) | 0.030 | 5.12 | 0.59 | 0,13 | 0.002 | 6.94 | 0.91 | 0.17 | 0.052 | 3.09 | 0.58 | 0.08 |
| Subscapular (mm) | 0.214 | 1.60 | 0.23 | 0.04 | 0.643 | 0.45 | 0.12 | 0.01 | 0.572 | 0.56 | 0.14 | 0.02 |
| Triceps (mm) | 0.003 | 9.96 | 0.87 | 0.22 | <0.001 | 9.84 | 0.98 | 0.22 | 0.357 | 1.04 | 0.23 | 0.03 |
| Metabolic | Syndrome | Healthy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Pre 1 WBC | After 10 WBC | After 20 WBC | Δ 10 WBC | Δ 20 WBC | Pre 1 WBC | After 10 WBC | After 20 WBC | Δ 10 WBC | Δ 20 WBC |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean (95% CI) | Mean (95% CI) | Mean ± SD | Mean ± SD | Mean ± SD | Mean (95% CI) | Mean (95% CI) | |
| Body composition | ||||||||||
| Body Mass (kg) | 77.36 ± 11.95 | 76.97 ± 12.06 | 76.80 ± 12.13 * | −0.38 (−0.89; 0.12) | −0.56 (−1.20; 0.08) | 66.32 ± 6.23 ‡ | 66.06 ± 6.50 ‡ | 65.66 ± 6.32 *,‡ | −0.27 (−0.64; 0.11) | −0.67 (−0.98; −0.35) |
| BMI (kg/m2) | 30.09 ± 4.98 | 29.93 ± 4.93 | 29.86 ± 4.94 * | −0.16 (−0.36; 0.03) | −0.24 (−0.49; 0.02) | 25.50 ± 2.37 ‡ | 25.39 ± 2.45 ‡ | 25.24 ± 2.43 *,‡ | −0.11 (−0.25; 0.04) | −0.25 (−0.37; 0.14) |
| Lean Body Mass (kg) | 44.19 ± 4.75 | 44.14 ± 5.11 | 44.21 ± 4.83 | −0.47 (−0.59; 0.50) | 0.24 (−0.43; 0.48) | 40.76 ± 3.31 ‡ | 40.68 ± 3.28 ‡ | 40.52 ± 2.96 ‡ | −0.08 (−0.43; 0.27) | −0.24 (−0.65; 0.17) |
| Total Body Fat (kg) | 34.42 ± 8.58 | 34.15 ± 8.55 | 33.92 ± 8.81 * | −0.27 (−0.61; 0.08) | −0.50 (−0.93; −0.07) | 25.52 ± 4.08 ‡ | 25.32 ± 4.33 ‡ | 25.01 ± 4.33 *,‡ | −0.20 (−0.53; 0.12) | −0.51 (−0.89; −0.12) |
| Total Body Fat (%) | 42.42 ± 4.32 | 42.21 ± 4.45 | 41.93 ± 4.59 * | −0.21 (−0.69; 0.28) | −0.48 (−0.91; −0.05) | 38.38 ± 3.48 ‡ | 38.22 ± 3.63 ‡ | 38.07 ± 3.54 ‡ | −0.16 (−0.56; 0.25) | −0.31 (−0.84; 0.22) |
| Arm Fat (%) | 42.67 ± 4.37 | 43.18 ± 3.96 | 42.87 ± 4.18 | 0.51 (−0.42; 1.44) | 0.20 (−0.52; 0.92) | 41.06 ± 3.88 | 41.51 ± 4.44 | 41.14 ± 4.02 | 0.44 (−0.55; 1.44) | 0.08 (−0.73; 0.89) |
| Leg Fat (%) | 39.62 ± 4.99 | 39.40 ± 5.08 | 39.07 ± 5.01 * | −0.22 (0.73; 0.29) | −0.55 (−1.01; −0.09) | 36.99 ± 3.97 | 36.82 ± 4.13 | 36.49 ± 4.07 * | −0.17 (−0.52; 0.18) | −0.51 (−1.10; 0.09) |
| Trunk Fat (%) | 46.54 ± 5.08 | 46.21 ± 5.42 | 45.90 ± 5.69 * | −0.33 (−1.01; 0.34) | −0.64 (−1.34; 0.06) | 41.02 ± 4.88 ‡ | 40.72 ± 4.85 ‡ | 40.75 ± 4.89 ‡ | −0.31 (−0.91; 0.30) | −0.27 (−0.98; 0.43) |
| Android Fat (%) | 48.98 ± 5.93 | 48.40 ± 6.34 | 47.90 ± 6.57 * | −0.58 (−1.39; 0.22) | −1.08 (−1.98; −0.19) | 42.74 ± 5.58 ‡ | 42.25 ± 5.82 ‡ | 42.34 ± 5.61 ‡ | −0.49 (−1.36; 0.38) | −0.39 (−1.45; 0.66) |
| Gynoid Fat (%) | 44.24 ± 4.66 | 43.90 ± 4.67 | 43.69 ± 4.90 | −0.34 (−0.91; 0.23) | −0.55 (−1.14; 0.05) | 41.0 ± 3.40 | 41.69 ± 3.58 | 41.55 ± 3.74 | 0.09 (−0.47; 0.66) | −0.05 (−0.68; 0.58) |
| A/G | 1.11 ± 0.10 | 1.10 ± 0.09 | 1.10 ± 0.11 | −0.01 (−0.03; 0.01) | −0.01 (−0.04; 0.02) | 1.03 ± 0.11‡ | 1.01 ± 0.12 ‡ | 1.02 ± 0.11 ‡ | −0.01 (−0.03; 0.00) | −0.01 (−0.03; 0.01) |
| Circumferences | ||||||||||
| WC (cm) | 96.24 ± 9.91 | 94.06 ± 8.25 * | 94.27 ± 9.96 * | −2.17 (−3.78; −0.56) | −1.96 (−3.38; −0.55) | 84.22 ± 8.71 ‡ | 82.94 ± 8.46 *,‡ | 82.66 ± 8.27 *,‡ | −1.28 (−2.30; −0.25) | −1.57 (−2.63; −0.50) |
| AC (cm) | 104.94 ± 10.98 | 103.16 ± 9.76 | 102.46 ± 10.76 * | −1.77 (−4.82; 1.27) | −2.47 (−5.25; −0.30) | 93.91 ± 7.40 ‡ | 93.54 ± 7.6 ‡ | 91.73 ± 7.77 *,‡ | −0.37 (−1.66; 0.93) | −2.17 (−3.69; −0.66) |
| HC (cm) | 112.06 ± 11.71 | 111.21 ± 11.90 | 111.13 ± 12.24 | −0.85 (−2.07; 0.36) | −0.94 (−2.30; 0.42) | 102.13 ± 6.18 ‡ | 101.09 ± 5.43 *,‡ | 100.34 ± 5.4 *,‡ | −1.04 (−1.86; −0.22) | −1.79 (−2.54; −1.05) |
| WHR | 0.86 ± 0.04 | 0.85 ± 0.03 | 0.85 ± 0.04 | −0.01 (−0.02; 0.00) | −0.01 (−0.02; −0.00) | 0.82 ± 0.06 | 0.82 ± 0.06 | 0.82 ± 0.06 | 0.00 (−0.01; 0.00) | 0.00 (−0.01; 0.00) |
| WHtR | 0.59 ± 0.06 | 0.58 ± 0.05* | 0.58 ± 0.06* | −0.01 (−0.02; −0.00) | −0.01 (−0.02; −0.00) | 0.52 ± 0.05 ‡ | 0.51 ± 0.05 *,‡ | 0.51 ± 0.05 *,‡ | −0.01 (−0.01; 0.00) | −0.01 (−0.02; 0.00) |
| Skinfold thickness | ||||||||||
| Abdominal (mm) | 29.44 ± 8.65 | 29.66 ± 8.26 | 28.56 ± 8.17 | 0.22 (−0.63; 1.06) | −0.88 (−1.85; 0.08) | 25.17 ± 7.12 ‡ | 23.18 ± 7.08 *,‡ | 22.61 ± 6.23 *,‡ | −1.98 (−4.05; 0.08) | −2.56 (−4.75; −0.36) |
| Subscapular (mm) | 24.91 ± 7.95 | 24.91 ± 7.48 | 24.96 ± 7.48 | 0.01 (−0.43; 0.44) | 0.06 (−0.66; 0.78) | 21.87 ± 6.76 | 22.24 ± 6.20 | 21.88 ± 6.38 | 0.37 (−0.36; 1.11) | 0.02 (−0.79; 0.83) |
| Triceps (mm) | 24.31 ± 4.95 | 24.30 ± 4.73 | 23.77 ± 4.63 * | −0.01 (−0.45; 0.44) | −0.54 (−1.09; 0.01) | 19.84 ± 4.90‡ | 19.41 ± 4.47 ‡ | 18.83 ± 4.05 *,‡ | −0.43 (−1.03; 0.17) | −1.01 (−1.82; −0.20) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiecek, M.; Szymura, J.; Sproull, J.; Szygula, Z. Whole-Body Cryotherapy Is an Effective Method of Reducing Abdominal Obesity in Menopausal Women with Metabolic Syndrome. J. Clin. Med. 2020, 9, 2797. https://doi.org/10.3390/jcm9092797
Wiecek M, Szymura J, Sproull J, Szygula Z. Whole-Body Cryotherapy Is an Effective Method of Reducing Abdominal Obesity in Menopausal Women with Metabolic Syndrome. Journal of Clinical Medicine. 2020; 9(9):2797. https://doi.org/10.3390/jcm9092797
Chicago/Turabian StyleWiecek, Magdalena, Jadwiga Szymura, Justyna Sproull, and Zbigniew Szygula. 2020. "Whole-Body Cryotherapy Is an Effective Method of Reducing Abdominal Obesity in Menopausal Women with Metabolic Syndrome" Journal of Clinical Medicine 9, no. 9: 2797. https://doi.org/10.3390/jcm9092797
APA StyleWiecek, M., Szymura, J., Sproull, J., & Szygula, Z. (2020). Whole-Body Cryotherapy Is an Effective Method of Reducing Abdominal Obesity in Menopausal Women with Metabolic Syndrome. Journal of Clinical Medicine, 9(9), 2797. https://doi.org/10.3390/jcm9092797





