The Relationship between Hematological Markers of Systemic Inflammation (Neutrophil-To-Lymphocyte, Platelet-To-Lymphocyte, Lymphocyte-To-Monocyte Ratios) and Ultrasound Disease Activity Parameters in Patients with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Findings
2.3. Ultrasound Imaging of Joints
- Semi-quantitative grey scale (GS) for grading synovial hypertrophy (0–3) in each joint:
- -
- Grade 0: normal joint with no synovial hypertrophy;
- -
- Grade 1: synovial hypertrophy up to the level of the horizontal line connecting the bone surfaces of an examined joint;
- -
- Grade 2: synovial hypertrophy extending beyond the joint line but with the upper surface flat to the underlying bones;
- -
- Grade 3: synovial hypertrophy extending beyond the joint line but with the upper surface convex to the underlying bones.
- Power Doppler ultrasound (PDUS) semi-quantitative scale (0–3) in each joint:
- -
- Grade 0: no Doppler activity;
- -
- Grade 1: up to three single Doppler spots, or up to one confluent spot and two single spots, or up to two confluent spots;
- -
- Grade 2: greater than grade 1 but <50% Doppler signals in the total GS background;
- -
- Grade 3: greater than grade 2 and >50% Doppler signals of the GS background [20].
2.4. Statistical Analysis
3. Results
3.1. Demographic and Disease-Related Variables in RA Patients
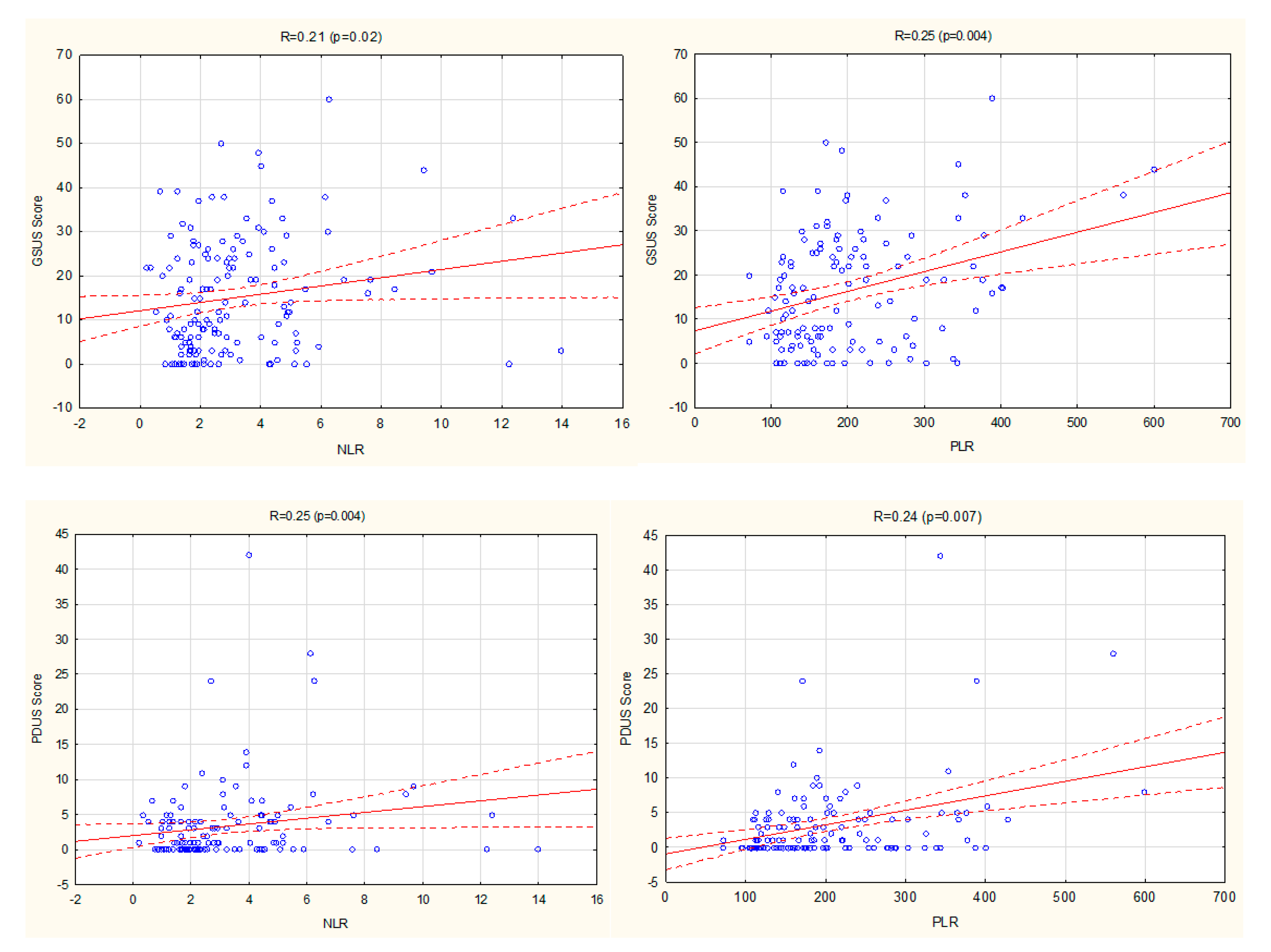
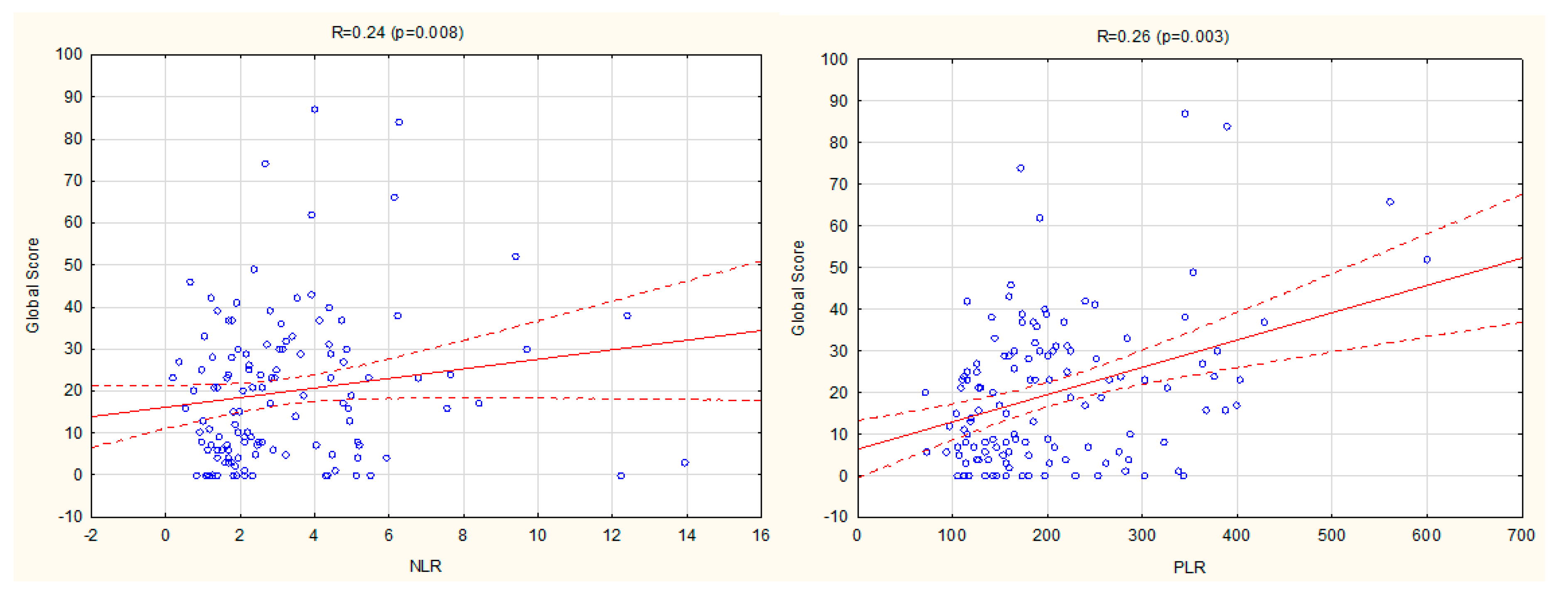
3.2. Relationship between NLR, PLR, LMR, and Ultrasound Disease Activity Markers
3.3. The Relationship between Ultrasound and Other Disease Activity Markers
3.4. The Relationship between NLR, PLR, LMR, and Clinical Disease Activity Markers
3.5. The Relationship between NLR, PLR, LMR, and Laboratory Disease Activity Markers
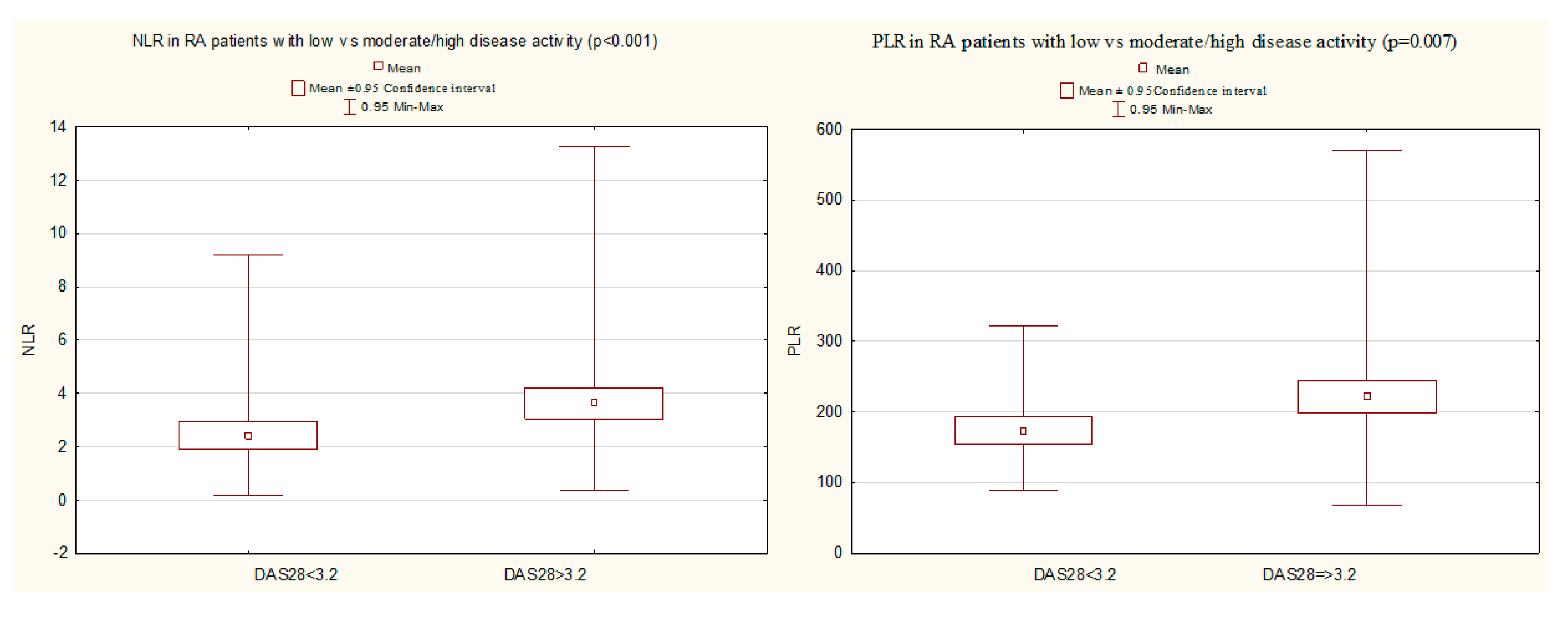
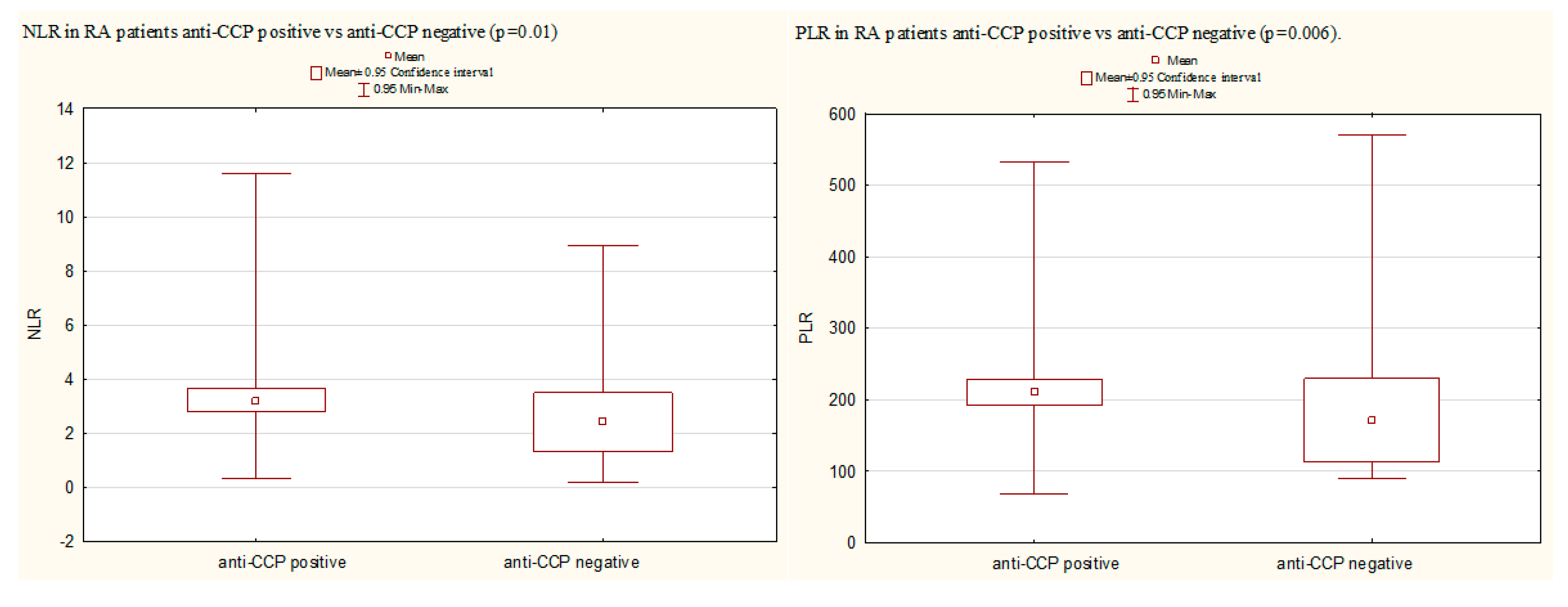
3.6. A Comparison of NLR, PLR, and LMR in Certain Groups of RA Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uslu, A.U.; Küçük, A.; Şahin, A.; Ugan, Y.; Yılmaz, R.; Güngör, T.; Bağcacı, S.; Küçükşen, S. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int. J. Rheum. Dis. 2015, 18, 731–735. [Google Scholar] [CrossRef]
- Chandrashekara, S.; Mukhtar Ahmad, M.; Renuka, P.; Anupama, K.R.; Renuka, K. Characterization of neutrophil-to-lymphocyte ratio as a measure of inflammation in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 1457–1467. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef]
- Fawzy, R.M.; Said, E.A.; Mansour, A.I. Association of neutrophil to lymphocyte ratio with disease activity indices and musculoskeletal ultrasound findings in recent onset rheumatoid arthritis patients. Egypt. Rheumatol. 2017, 39, 203–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.J.; Du, Y.P.; Feng, C.X.; Wang, L.Q.; Chen, M.B. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine 2018, 97, e0144. [Google Scholar] [CrossRef]
- Isaac, V.; Wu, C.Y.; Huang, C.T.; Baune, B.T.; Tseng, C.L.; McLachlan, C.S. Elevated neutrophil to lymphocyte ratio predicts mortality in medical inpatients with multiple chronic conditions. Medicine 2016, 95, e3832. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, J.; Lu, Z.; Chen, M.; Fang, X.; Wang, G. High neutrophil-to-lymphocyte ratio is associated with increased carotid artery intima-media thickness in type 2 diabetes. J. Diabetes Investig. 2017, 8, 101–107. [Google Scholar] [CrossRef]
- Cho, J.H.; Cho, H.J.; Lee, H.Y.; Ki, Y.J.; Jeon, E.S.; Hwang, K.K.; Chae, S.C.; Baek, S.H.; Kang, S.M.; Choi, D.J.; et al. Neutrophil-Lymphocyte Ratio in Patients with Acute Heart Failure Predicts In-Hospital and Long-Term Mortality. J. Clin. Med. 2020, 9, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paliogiannis, P.; Satta, R.; Deligia, G.; Farina, G.; Bassu, S.; Mangoni, A.A. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: A systematic review and meta-analysis. Clin. Exp. Med. 2019, 19, 37–45. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Lin, F.; Ren, Y.; Liu, D.; Zhong, R.; Liang, Y. Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil- lymphocyte ratios among various systemic autoimmune rheumatic diseases. APMIS 2017, 125, 863–871. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, S.M.; Song, J.J.; Park, Y.B.; Lee, S.W. Platelet to lymphocyte ratio is associated with the current activity of ANCA-associated vasculitis at diagnosis: A retrospective monocentric study. Rheumatol. Int. 2018, 38, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, S.; Shi, J.; Zhu, X.; Ying, H.; Zhang, Y.; Chen, S.; Shen, B.; Li, J. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin. Rheumatol. 2017, 36, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Mae, Y.; Takata, T.; Ida, A.; Ogawa, M.; Taniguchi, S.; Yamamoto, M.; Iyama, T.; Fukuda, S.; Isomoto, H. Prognostic Value of Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio for Renal Outcomes in Patients with Rapidly Progressive Glomerulonephritis. J. Clin. Med. 2020, 9, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erre, G.L.; Paliogiannis, P.; Castagna, F.; Mangoni, A.A.; Carru, C.; Passiu, G.; Zinellu, A. Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur. J. Clin. Investig. 2019, 49, e13037. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.N.; Kim, Y.K.; Kim, G.T.; Ahn, E.; So, M.W.; Sohn, D.H.; Lee, S.G. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-α agents in patients with rheumatoid arthritis: A retrospective chart review analysis. Rheumatol. Int. 2019, 39, 859–868. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerek, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts: Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Pincus, T.; Sokka, T.; Kautiainen, H. Further development of a physical function scale on a MDHAQ [corrected] for standard care of patients with rheumatic diseases. J. Rheumatol. 2005, 32, 1432–1439. [Google Scholar]
- van der Heijde, D. How to read radiographs according to the Sharp/van der Heijde method. J. Rheumatol. 2000, 27, 261–263. [Google Scholar]
- D’Agostino, M.-A.; Terslev, L.; Aegerter, P.; Backhaus, M.; Balint, P.; Bruyn, G.A.; Filippucci, E.; Grassi, W.; Iagnocco, A.; Jousse-Joulin, S.; et al. Scoring ultrasound synovitis in rheumatoid arthritis: A EULAR-OMERACT ultrasound taskforce-Part 1: Definition and development of a standardised, consensus-based scoring system. Rheum. Musculoskelet. Dis. Open 2017, 3, e000428. [Google Scholar] [CrossRef]
- Backhaus, M.; Burmester, G.R.; Gerber, T.; Grassi, W.; Machold, K.P.; Swen, W.A.; Wakefield, R.; Manger, B. Guidelines for musculoskeletal ultrasound in rheumatology. Ann. Rheum. Dis. 2001, 60, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Iagnocco, A.; Finucci, A.; Ceccarelli, F.; Perricone, C.; Iorgoveanu, V.; Valesini, G. Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patient with rheumatoid arthritis. Rheumatology 2015, 54, 1890–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, H.B.; Michelsen, B.; Sexton, J.; Haugen, I.K.; Provan, S.A.; Haavardsholm, E.A.; Uhlig, T.; Kvien, T.K. Swollen, but not tender joints, are independently associated with ultrasound synovitis: Results from a longitudinal observational study of patients with established rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
| Data | Results (Mean (SD) (Range) or Number (%)) |
|---|---|
| Age, years | 53.8 (11.9) (20–82) |
| Gender, female/male | 100 (79.4)/26 (20.6) |
| RA related variables: | |
| Disease duration, years | 14.0 (10.9) (1–45) |
| Positive RF-IgM | 110 (87.3) |
| Positive anti-CCP | 104 (82.5) |
| Extra-articular manifestations | 70 (55.6) |
| Erosions (hands/feet) | 107 (84.9) |
| M-HAQ | 1.4 (0.7) (0–3.5) |
| Current conventional DMARD | 124 (98.4) |
| Current biological treatment | 62 (49.2) |
| Current glucocorticoid use | 83 (65.9) |
| Laboratory results: | |
| Hemoglobin, g/dL | 12.8 (1.4) (9.4–16.1) |
| White blood cells | 6.9 (2.4) (3.2–14.6) |
| Platelets, 109/L | 289.2 (78.2) (124–505) |
| Neutrophils, 109/L | 4.6 (2.4) (1.1–12.9) |
| Lymphocytes, 109/L | 1.6 (0.6) (0.6–4.2) |
| Monocytes, 109/L | 0.4 (1.8) (0.1–1.5) |
| NLR | 3.34 (2.4) (0.5–14.0) |
| PLR | 204.5 (95.9) (71.2–600) |
| LMR | 4.4 (2.5) (1.2–15.8) |
| CRP, mg/L | 15.0 (18.7) (0.6–85.0) |
| ESR, mm/h | 35.3 (28.3) (2–120) |
| Clinical parameters of RA activity: | |
| TJC | 5.5 (5.5) (0–21) |
| SJC | 3.7 (4.4) (0–19) |
| PGA (VAS), mm | 37.6 (26.0) (0–98) |
| Morning stiffness, minutes | 56.1 (63.1) (0–300) |
| DAS28 | 4.19 (1.8) (0.7–7.8) |
| Low disease activity (DAS28 < 3.2) | 45 (35.7) |
| GSUS score (hypertrophy) | 16.5 (13.2) (0–60) |
| PDUS score | 3.3 (5.8) (0–42) |
| Global score | 19.8 (17.6) (0–87) |
| Data/p Value/R | CRP | ESR | DAS28 | TJC | SJC | PGA | Morning Stiffness | M-HAQ | WBC Count | Hb |
|---|---|---|---|---|---|---|---|---|---|---|
| NLR | <0.001 | <0.001 | <0.001 | 0.004 | 0.001 | 0.003 | 0.006 | 0.03 | <0.001 | NS |
| 0.5 | 0.37 | 0.37 | 0.26 | 0.3 | 0.26 | 0.27 | 0.2 | 0.54 | ||
| PLR | <0.001 | <0.001 | <0.001 | 0.003 | 0.002 | 0.02 | 0.04 | NS | NS | 0.005 |
| 0.31 | 0.35 | 0.35 | 0.26 | 0.28 | 0.22 | 0.21 | −0.25 | |||
| LMR | 0.02 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| −0.21 | ||||||||||
| GSUS score | 0.02 | 0.006 | <0.001 | 0.007 | <0.001 | <0.001 | 0.05 | NS | NS | NS |
| 0.21 | 0.25 | 0.36 | 0.24 | 0.47 | 0.34 | 0.2 | ||||
| PDUS score | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.02 | NS | NS |
| 0.31 | 0.27 | 0.42 | 0.36 | 0.6 | 0.39 | 0.38 | 0.22 | |||
| Global score | 0.007 | 0.003 | <0.001 | 0.002 | <0.001 | <0.001 | 0.01 | 0.04 | NS | NS |
| 0.24 | 0.27 | 0.4 | 0.28 | 0.52 | 0.36 | 0.25 | 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Targońska-Stępniak, B.; Zwolak, R.; Piotrowski, M.; Grzechnik, K.; Majdan, M. The Relationship between Hematological Markers of Systemic Inflammation (Neutrophil-To-Lymphocyte, Platelet-To-Lymphocyte, Lymphocyte-To-Monocyte Ratios) and Ultrasound Disease Activity Parameters in Patients with Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 2760. https://doi.org/10.3390/jcm9092760
Targońska-Stępniak B, Zwolak R, Piotrowski M, Grzechnik K, Majdan M. The Relationship between Hematological Markers of Systemic Inflammation (Neutrophil-To-Lymphocyte, Platelet-To-Lymphocyte, Lymphocyte-To-Monocyte Ratios) and Ultrasound Disease Activity Parameters in Patients with Rheumatoid Arthritis. Journal of Clinical Medicine. 2020; 9(9):2760. https://doi.org/10.3390/jcm9092760
Chicago/Turabian StyleTargońska-Stępniak, Bożena, Robert Zwolak, Mariusz Piotrowski, Krzysztof Grzechnik, and Maria Majdan. 2020. "The Relationship between Hematological Markers of Systemic Inflammation (Neutrophil-To-Lymphocyte, Platelet-To-Lymphocyte, Lymphocyte-To-Monocyte Ratios) and Ultrasound Disease Activity Parameters in Patients with Rheumatoid Arthritis" Journal of Clinical Medicine 9, no. 9: 2760. https://doi.org/10.3390/jcm9092760
APA StyleTargońska-Stępniak, B., Zwolak, R., Piotrowski, M., Grzechnik, K., & Majdan, M. (2020). The Relationship between Hematological Markers of Systemic Inflammation (Neutrophil-To-Lymphocyte, Platelet-To-Lymphocyte, Lymphocyte-To-Monocyte Ratios) and Ultrasound Disease Activity Parameters in Patients with Rheumatoid Arthritis. Journal of Clinical Medicine, 9(9), 2760. https://doi.org/10.3390/jcm9092760






