Performance of Antenatal Diagnostic Criteria of Twin-Anemia-Polycythemia Sequence
Abstract
1. Introduction
2. Experimental Section
Statistical Analysis
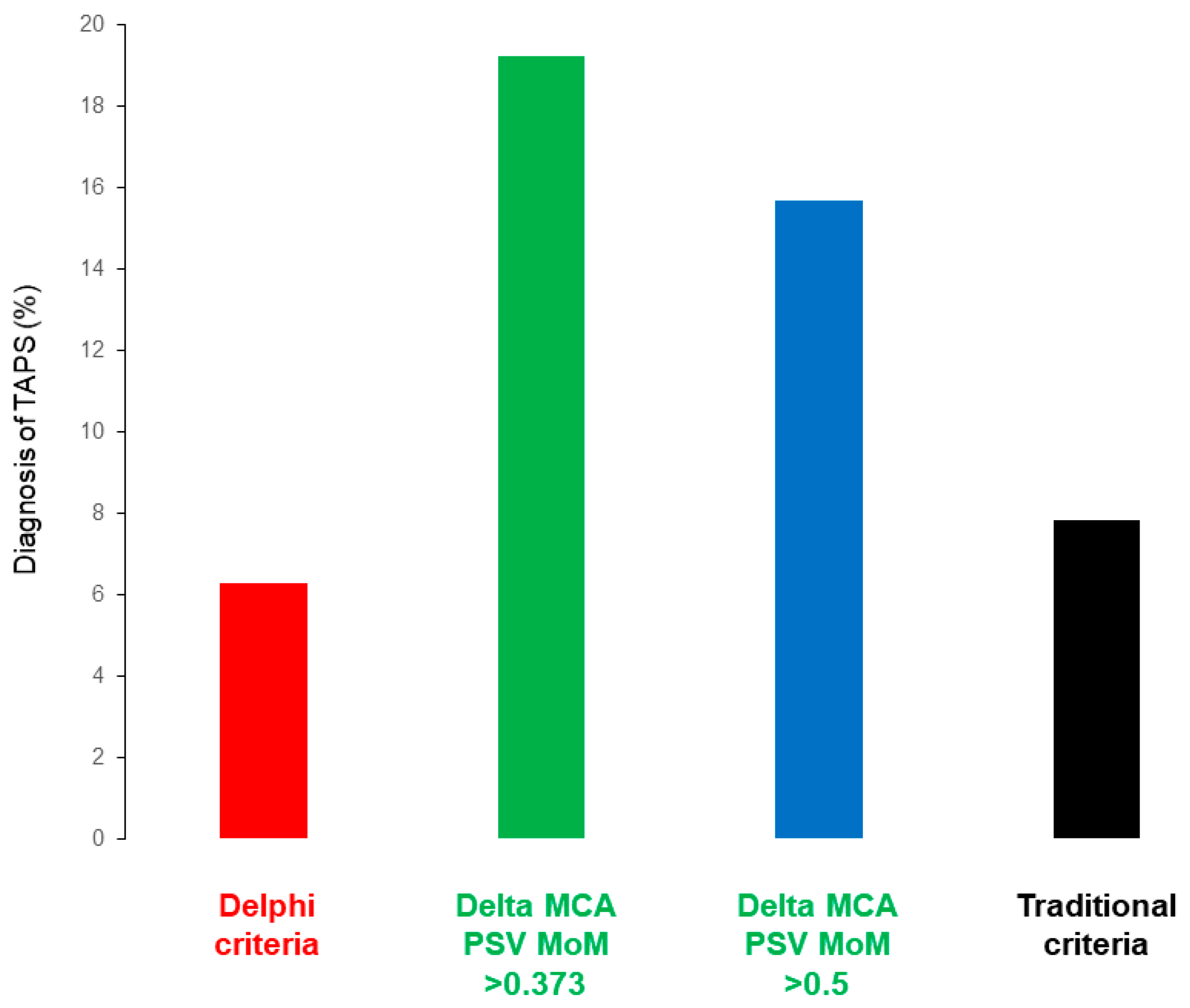
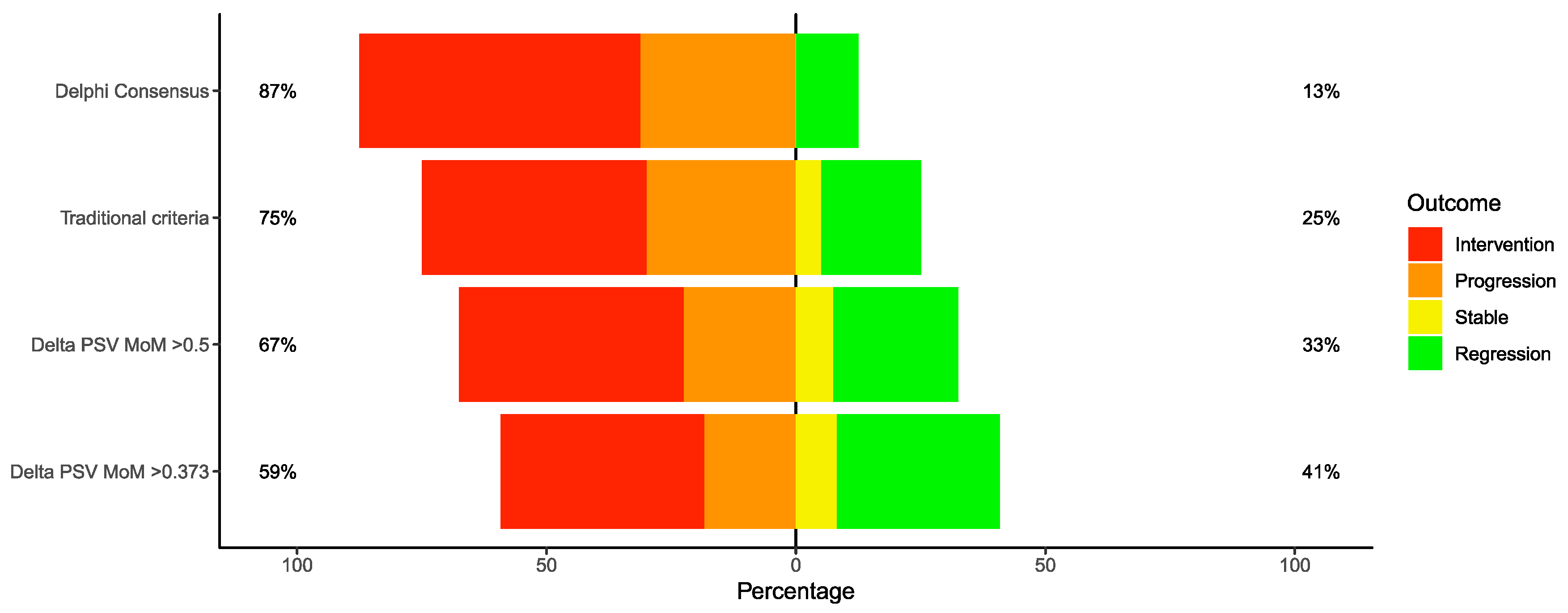
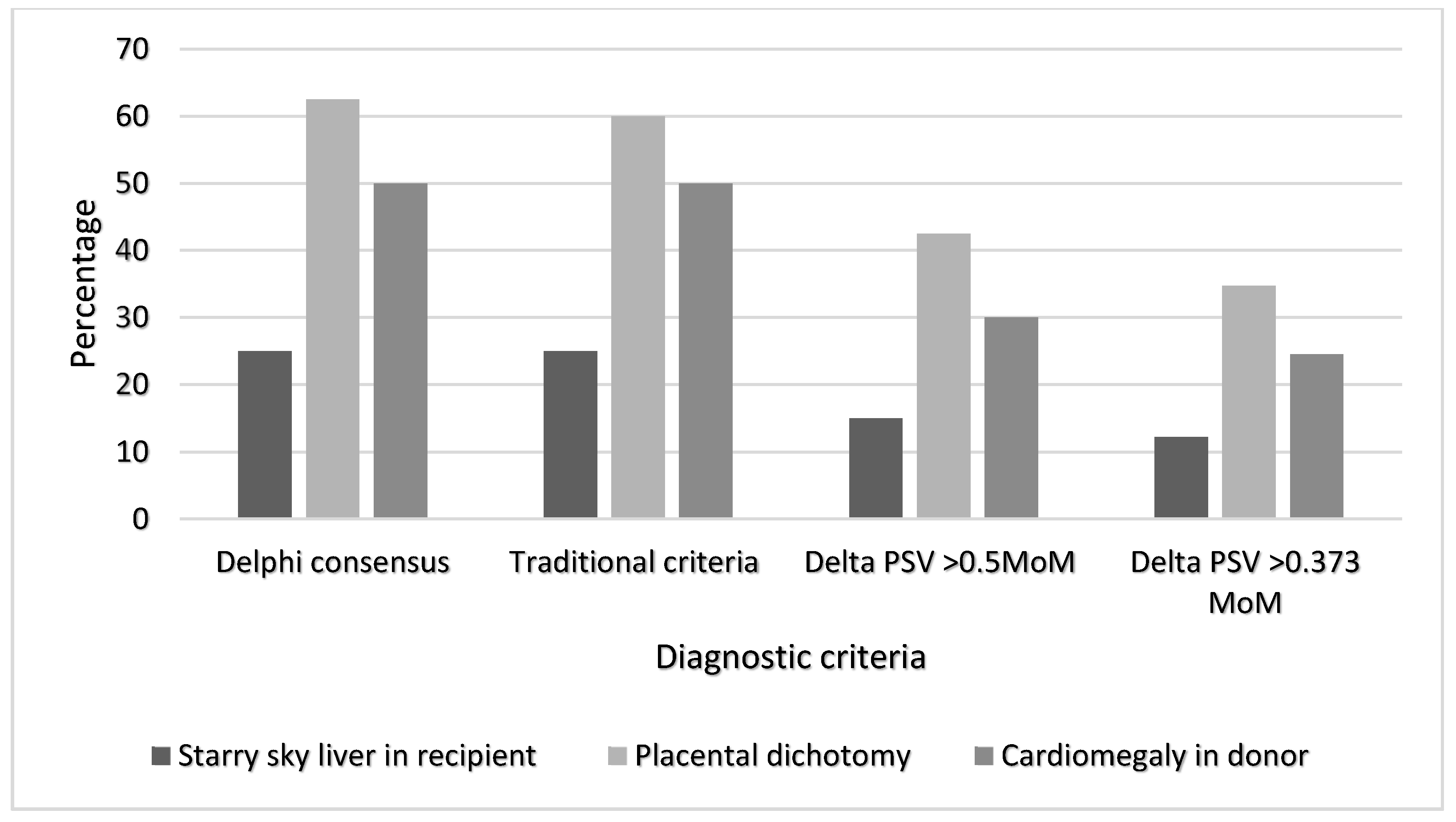
3. Results
Our Study Findings
4. Discussion
4.1. Summary of the Study Findings
4.2. Interpretation of Study Findings and Comparison with Existing Literature
4.3. Clinical and Research Implications
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Slaghekke, F.; Kist, W.J.; Oepkes, D.; Pasman, S.A.; Middeldorp, J.M.; Klumper, F.J.; Walther, F.J.; Vandenbussche, F.P.H.A.; Lopriore, E. Twin Anemia-Polycythemia Sequence: Diagnostic criteria, classification, perinatal management and outcome. Fetal Diagn. Ther. 2010, 27, 181–190. [Google Scholar] [CrossRef]
- Lewi, L.; Jani, J.; Blickstein, I.; Huber, A.; Gucciardo, L.; Van Mieghem, T.; Doné, E.; Boes, A.S.; Hecker, K.; Gratacós, E.; et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: A prospective cohort study. Am. J. Obstet. Gynecol. 2008, 199, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Lopriore, E.; Slaghekke, F.; Middeldorp, J.M.; Klumper, F.J.; Oepkes, D.; Vandenbussche, F.P. Residual anastomoses in twin-to-twin transfusion syndrome treated with selective fetoscopic laser surgery: Localization, size, and consequences. Am. J. Obstet. Gynecol. 2009, 201, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Robyr, R.; Lewi, L.; Salomon, L.J.; Yamamoto, M.; Bernard, J.P.; Deprest, J.; Ville, Y. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2006, 194, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Habli, M.; Bombrys, A.; Lewis, D.; Lim, F.; Polzin, W.; Maxwell, R.; Crombleholme, T.M. Incidence of complications in twin-twin transfusion syndrome after selective fetoscopic laser photocoagulation: A single-center experience. Am. J. Obstet. Gynecol. 2009, 201, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Slaghekke, F.; Lopriore, E.; Lewi, L.; Middeldorp, J.M.; van Zwet, E.W.; Weingertner, A.S.; Klumper, F.J.; DeKoninck, P.; Devlieger, R.; Kilby, M.D.; et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: An open-label randomised controlled trial. Lancet 2014, 383, 2144–2151. [Google Scholar] [CrossRef]
- Slaghekke, F.; van Klink, J.M.; Koopman, H.M.; Middeldorp, J.M.; Oepkes, D.; Lopriore, E. Neurodevelopmental outcome in twin anemia-polycythemia sequence after laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2014, 44, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, L.S.A.; Lopriore, E.; Slaghekke, F.; Oepkes, D.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.; Tan, R.; Rijken, M.; Van Klink, J.M.M. High risk of long-term impairment in donor twins with spontaneous twin anemia polycythemia sequence. Ultrasound Obstet. Gynecol. 2020, 55, 39–46. [Google Scholar] [CrossRef]
- Khalil, A.; Rodgers, M.; Baschat, A.; Bhide, A.; Gratacos, E.; Hecher, K.; Kilby, M.D.; Lewi, L.; Nicolaides, K.H.; Oepkes, D.; et al. ISUOG Practice Guidelines: The role of ultrasound in twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 47, 247–263. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence. Twin and Triplet Pregnancy. NICE Clinical Guideline 137; National Institute for Health and Clinical Excellence: London, UK, 2019.
- Royal College of Obstetricians and Gynaecologists. Monochorionic Twin Pregnancy, Management. Green-Top Guideline No.51; RCOG: London, UK, 2016. [Google Scholar]
- Simpson, L.L. Twin-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2013, 208, 3–18. [Google Scholar] [CrossRef]
- Fishel-Bartal, M.; Weisz, B.; Mazaki-Tovi, S.; Ashwal, E.; Chayen, B.; Lipitz, S.; Yinon, Y. Can middle cerebral artery peak systolic velocity predict polycythemia in monochorionic diamniotic twins? Evidence from a prospective cohort study. Ultrasound Obstet. Gynecol. 2016, 48, 470–475. [Google Scholar] [CrossRef]
- Tollenaar, L.S.A.; Lopriore, E.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.J.; Oepkes, D.; Slaghekke, F. Improved prediction of twin anemia-polycythemia sequence by delta middle cerebral artery peak systolic velocity: New antenatal classification system. Ultrasound Obstet. Gynecol. 2019, 53, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Tavares De Sousa, M.; Fonseca, A.; Hecher, K. Role of fetal intertwin difference in middle cerebral artery peak systolic velocity in predicting neonatal twin anemia-polycythemia sequence. Ultrasound Obstet. Gynecol. 2019, 53, 794–797. [Google Scholar] [CrossRef]
- Khalil, A.; Gordijn, S.; Ganzevoort, W.; Thilaganathan, B.; Johnson, A.; Baschat, A.; Hecher, K.; Reed, K.; Lewi, L.; Deprest, J.; et al. Consensus diagnostic criteria and monitoring of twin anemia polycythemia sequence: A Delphi procedure. Ultrasound Obstet. Gynecol. 2019. [Google Scholar] [CrossRef]
- Quintero, R.A.; Morales, W.J.; Allen, M.H.; Bornick, P.W.; Johnson, P.K.; Kuger, M. Staging of twin-twin transfusion syndrome. J. Perinatol. 1999, 19, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Gratacós, E.; Lewi, L.; Munoz, B.; Acosta-Rojas, R.; Hernandez-Andrade, E.; Martinez, J.M.; Carreras, E.; Deprest, J. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet. Gynecol. 2007, 30, 28–34. [Google Scholar] [CrossRef]
- Mari, G.; Deter, R.L.; Carpenter, R.L.; Rahman, F.; Zimmerman, R.; Moise, K.J., Jr.; Dorman, K.F.; Ludomirsky, A.; Gonzalez, R.; Gomez, R.; et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N. Engl. J. Med. 2000, 342, 9–14. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Jayot, A.; Friszer, S.; Castaigne, V.; Cynober, E.; Pernot, F.; Carbonne, B. Accuracy of middle cerebral artery Doppler assessment between 34 and 37 weeks in fetuses with red cell alloimmunization. Fetal Diagn. Ther. 2017, 42, 225–231. [Google Scholar] [CrossRef]
- Ananth, C.V.; Vintzileos, A.M.; Shen-Schwarz, S.; Smulian, J.C.; Lai, Y.L. Standards of birth weight in twin gestations stratified by placental chorionicity. Obstet. Gynecol. 1998, 91, 917–924. [Google Scholar]
- Stagnati, V.; Pagani, G.; Fichera, A.; Prefumo, F. Intertwin discrepancy in middle cerebral artery peak systolic velocity and third-trimester fetal growth restriction in monochorionic-diamniotic twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 48, 66–71. [Google Scholar] [CrossRef]
- Tollenaar, L.S.A.; Lopriore, E.; Middeldorp, J.M.; Klumper, F.J.C.M.; Haak, M.C.; Oepkes, D.; Slaghekke, F. Prevalence of placental dichotomy, fetal cardiomegaly and starry-sky liver in twin anemia polycythemia sequence. Ultrasound Obstet. Gynecol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mari, G.; Hanif, F.; Kruger, M.; Cosmi, E.; Santolaya-Forgas, J.; Treadwell, M.C. Middle cerebral artery peak systolic velocity: A new Doppler parameter in the assessment of growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2007, 29, 310–316. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, M.E.; Shapiro, S.; Young, L.; Luks, F.I. Placental characteristics of selective birth weight discordance in diamniotic-monochorionic twin gestations. Placenta 2010, 31, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Slaghekke, F.; Pasma, S.; Veujoz, M.; Middeldorp, J.M.; Lewi, L.; Devlieger, R.; Favre, R.; Lopriore, E.; Oepkes, D. Middle cerebral artery peak systolic velocity to predict fetal hemoglobin levels in twin anemia-polycythemia sequence. Ultrasound Obstet. Gynecol. 2015, 46, 432–436. [Google Scholar] [CrossRef] [PubMed]

| Delphi Consensus (n = 16) | Traditional Criteria (n = 20) | Delta PSV MoM > 0.5 (n = 40) | Delta PSV MoM > 0.373 (n = 49) | |
|---|---|---|---|---|
| Maternal age in years, median (IQR) | 35.0 (26.5–36.2) | 34.5 (24.7–36.5) | 32.5 (26.0–36.0) | 33.0 (27.0–36.0) |
| Body mass index in Kg/m2, median (IQR) | 24.9 (22.7–25.5) | 24.3 (22.9–25.5) | 23.4 (21.7–25.4) | 23.7 (21.8–27.1) |
| Gestational age at diagnosis in weeks, median (IQR) | 23.1 (19.6–24.1) | 23.4 (19.9–24.4) | 23.4 (19.4–25.6) | 23.6 (19.7–27.1) |
| TAPS onset | ||||
| Spontaneous | 6 (37.5) | 8 (40.0) | 17 (42.5) | 23 (46.9) |
| Post-laser | 6 (37.5) | 8 (40.0) | 14 (35.0) | 15 (30.6) |
| Coincided with TTTS # | 4 (25.0) | 4 (20.0) | 9 (22.5) | 11 (22.5) |
| Management * | ||||
| Expectant | 2 (12.5) | 5 (25.0) | 15 (37.5) | 22 (44.9) |
| Laser | 10 (62.5) | 10 (50.0) | 19 (47.5) | 21 (42.9) |
| Intrauterine transfusion | 3 (18.7) | 2 (10.0) | 4 (10.0) | 4 (8.2) |
| Selective termination | 2 (12.5) | 4 (20.0) | 3 (7.5) | 4 (8.2) |
| Immediate delivery | 1 (6.2) | 1 (5.0) | 2 (5.0) | 2 (4.4) |
| Gestational age at delivery in weeks, median (IQR) | 32.4 (28.4–33.9) | 32.0 (30.0–34.0) | 32.7 (29.3–34.0) | 32.4 (30.4–34.0) |
| Both twins live born | 12 (75.0) | 16 (88.8) | 31 (77.5) | 40 (81.6) |
| Double IUD | 1 (6.2) | 1 (5.0) | 1 (2.5) | 1 (2.0) |
| Missing data | 0 (0.0) | 2 (10.0) | 2 (5.0) | 2 (4.1) |
| Birth weight—larger twin | 1610 (1321–1893) | 1768 (1354–1952) | 1720 (1372–2022) | 1702 (1430–2016) |
| Birth weight centile—larger twin | 30.1 (15.1–56.2) | 36.1 (23.1–46.9) | 36.3 (25.6–48.2) | 38.8 (24.9–50.2) |
| Birth weight—smaller twin | 1155 (982–1567) | 1240 (995–1596) | 1230 (970–1622) | 1214 (958–1543) |
| Birth weight centile—smaller twin | 5.9 (0.0–16.8) | 5.7 (0.0–16.8) | 1.8 (0.0–16.7) | 1.5 (0.0–16.3) |
| Missing birthweight data | 4 (25.0) | 4 (20.0) | 9 (22.5) | 9 (18.4) |
| Weight discordance | 17.4 (7.5–28.9) | 19.4 (7.5–36.3) | 22.6 (10.1–32.1) | 25.3 (11.2–36.3) |
| NNU admission, any baby | 11 (73.3) | 14 (70.0) | 28 (64.1) | 36 (75.0) |
| Serious neonatal morbidity or mortality, any baby ** | 6 (40.0) | 7 (35.0) | 17 (43.6) | 21 (43.7) |
| Spontaneous (n = 23) | Post-Laser (n = 15) | Coincided with TTTS # (n = 11) | |
|---|---|---|---|
| Maternal age in years, median (IQR) | 32.0 (26.0–36.0) | 32.0 (29.5–34.5) | 33.0 (30.5–36.0) |
| Body mass index in Kg/m2, median (IQR) | 23.9 (22.0–28.7) | 22.4 (20.8–24.3) | 25.3 (22.8–26.4) |
| Gestational age at diagnosis in weeks, median (IQR) | 25.0 (22.6–29.7) | 23.3 (19.6–26.8) | 20.4 (18.0–21.9) |
| Natural history | |||
| Progression | 6 (26.1) | 3 (20.0) | 0 (0.0) |
| Regression | 9 (39.1) | 6 (40.0) | 1 (9.1) |
| Stable | 2 (8.7) | 2 (13.3) | 0 (0.0) |
| NA (immediate treatment) | 6 (26.1) | 4 (26.7) | 10 (90.9) |
| Management * | |||
| Expectant | 13 (56.5) | 8 (53.3) | 1 (9.1) |
| Laser | 7 (30.4) | 4 (26.7) | 10 (90.9) |
| Intrauterine transfusion | 1 (4.3) | 3 (20.0) | 0 (0.0) |
| Selective termination | 2 (8.7) | 1 (6.7) | 0 (0.0) |
| Immediate delivery | 1 (4.3) | 1 (6.7) | 0 (0.0) |
| Disease course following intervention | |||
| Improved | 8 (80.0) | 3 (42.9) | 10 (100.0) |
| Deteriorated | 1 (10.0) | 2 (28.6) | 0 (0.0) |
| Stable | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Delivery post intervention | 1 (10.0) | 2 (28.6) | 0 (0.0) |
| Gestational age at delivery in weeks, median (IQR) | 32.8 (30.6–34.1) | 32.4 (30.2–34.0) | 31.7 (29.9–32.4) |
| Accuracy (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | TP | TN | FP | FN | |
|---|---|---|---|---|---|---|---|
| Traditional criteria | 44.4 (21.5–69.2) | 14.3 (0.36–57.9) | 63.6 (30.8–89.1) | 1 | 7 | 4 | 6 |
| Delta PSV > 0.5 MoM | 50.0 (26.0–74.0) | 85.7 (42.1–99.6) | 27.3 (6.0–61.0) | 6 | 3 | 8 | 1 |
| Delphi consensus | 55.6 (30.8–78.5) | 28.6 (3.7–71.0) | 72.7 (39.0–94.0) | 2 | 8 | 3 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Kalafat, E.; Bhide, A.; Thilaganathan, B.; Khalil, A. Performance of Antenatal Diagnostic Criteria of Twin-Anemia-Polycythemia Sequence. J. Clin. Med. 2020, 9, 2754. https://doi.org/10.3390/jcm9092754
Liu B, Kalafat E, Bhide A, Thilaganathan B, Khalil A. Performance of Antenatal Diagnostic Criteria of Twin-Anemia-Polycythemia Sequence. Journal of Clinical Medicine. 2020; 9(9):2754. https://doi.org/10.3390/jcm9092754
Chicago/Turabian StyleLiu, Becky, Erkan Kalafat, Amar Bhide, Basky Thilaganathan, and Asma Khalil. 2020. "Performance of Antenatal Diagnostic Criteria of Twin-Anemia-Polycythemia Sequence" Journal of Clinical Medicine 9, no. 9: 2754. https://doi.org/10.3390/jcm9092754
APA StyleLiu, B., Kalafat, E., Bhide, A., Thilaganathan, B., & Khalil, A. (2020). Performance of Antenatal Diagnostic Criteria of Twin-Anemia-Polycythemia Sequence. Journal of Clinical Medicine, 9(9), 2754. https://doi.org/10.3390/jcm9092754






