Effect of Calcium Chloride Hydrothermal Treatment of Titanium on Protein, Cellular, and Bacterial Adhesion Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium Plates
2.2. Protein Adsorption Assay
2.3. Cell Culture
2.4. Cell Staining
2.5. Attached Cell Count
2.6. Bacterial Culture
2.7. Statistical Procedure
3. Results
3.1. The Surface Characteristics of Titanium Plates
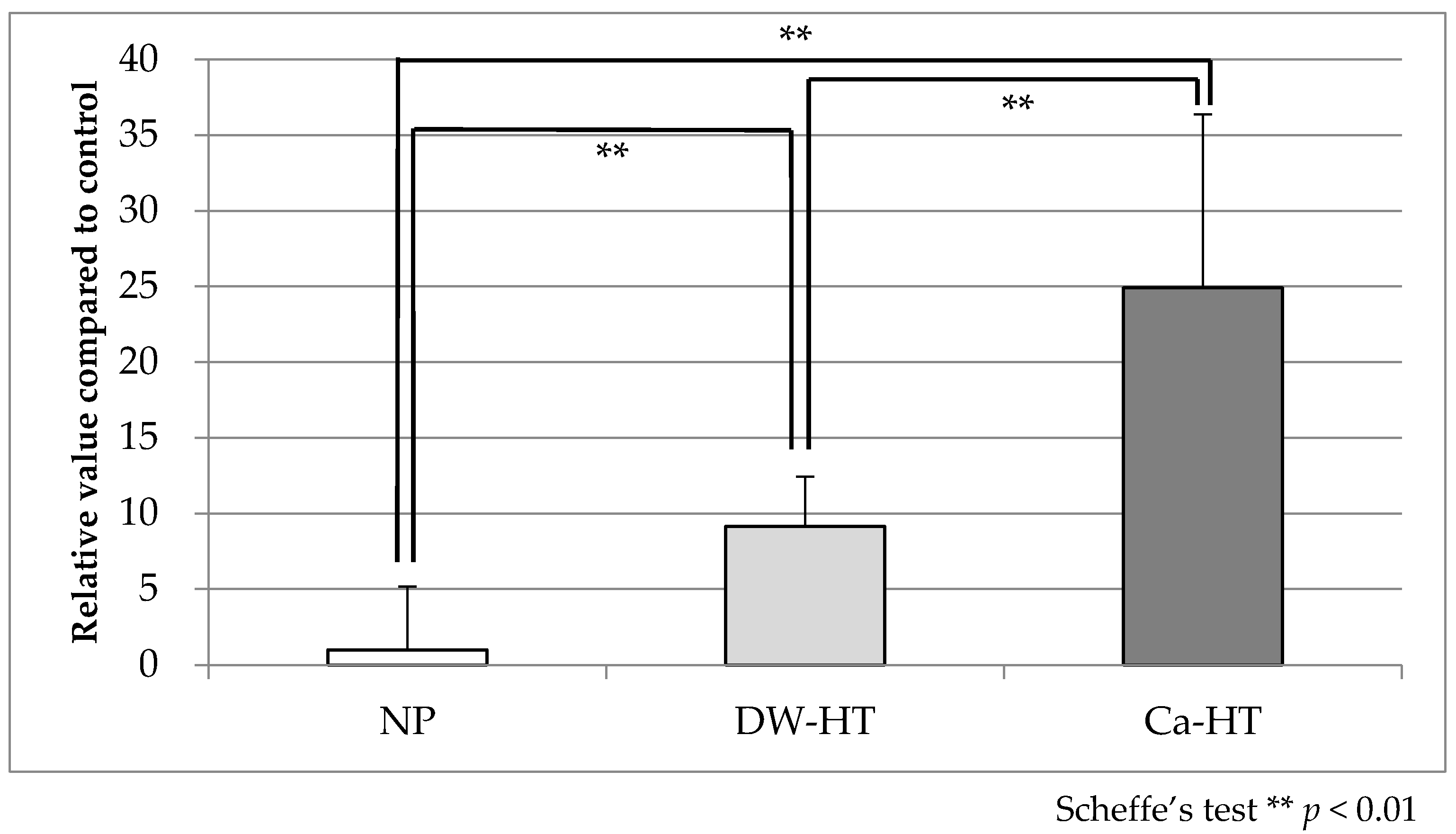
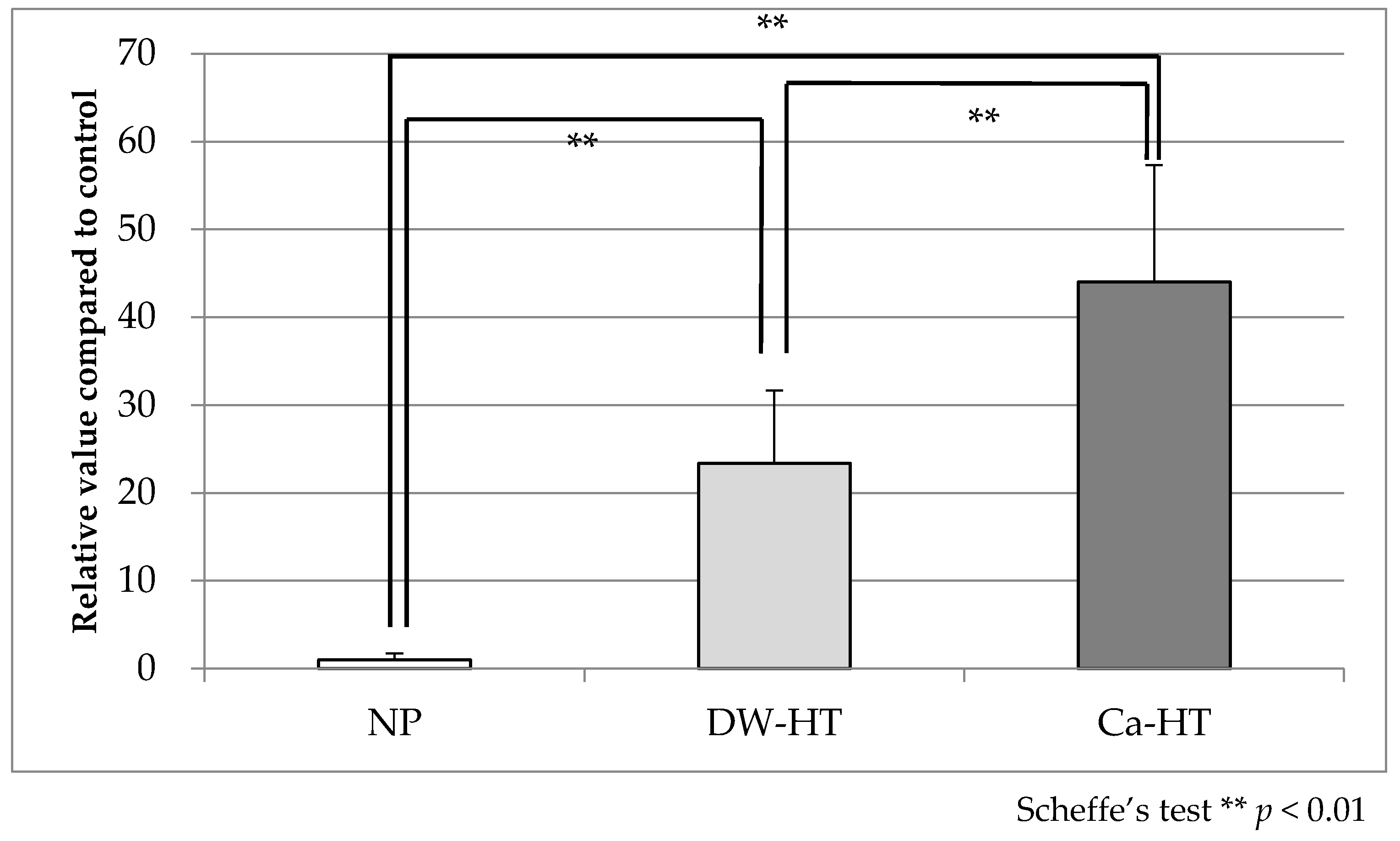
3.2. Protein Adsorption Assay
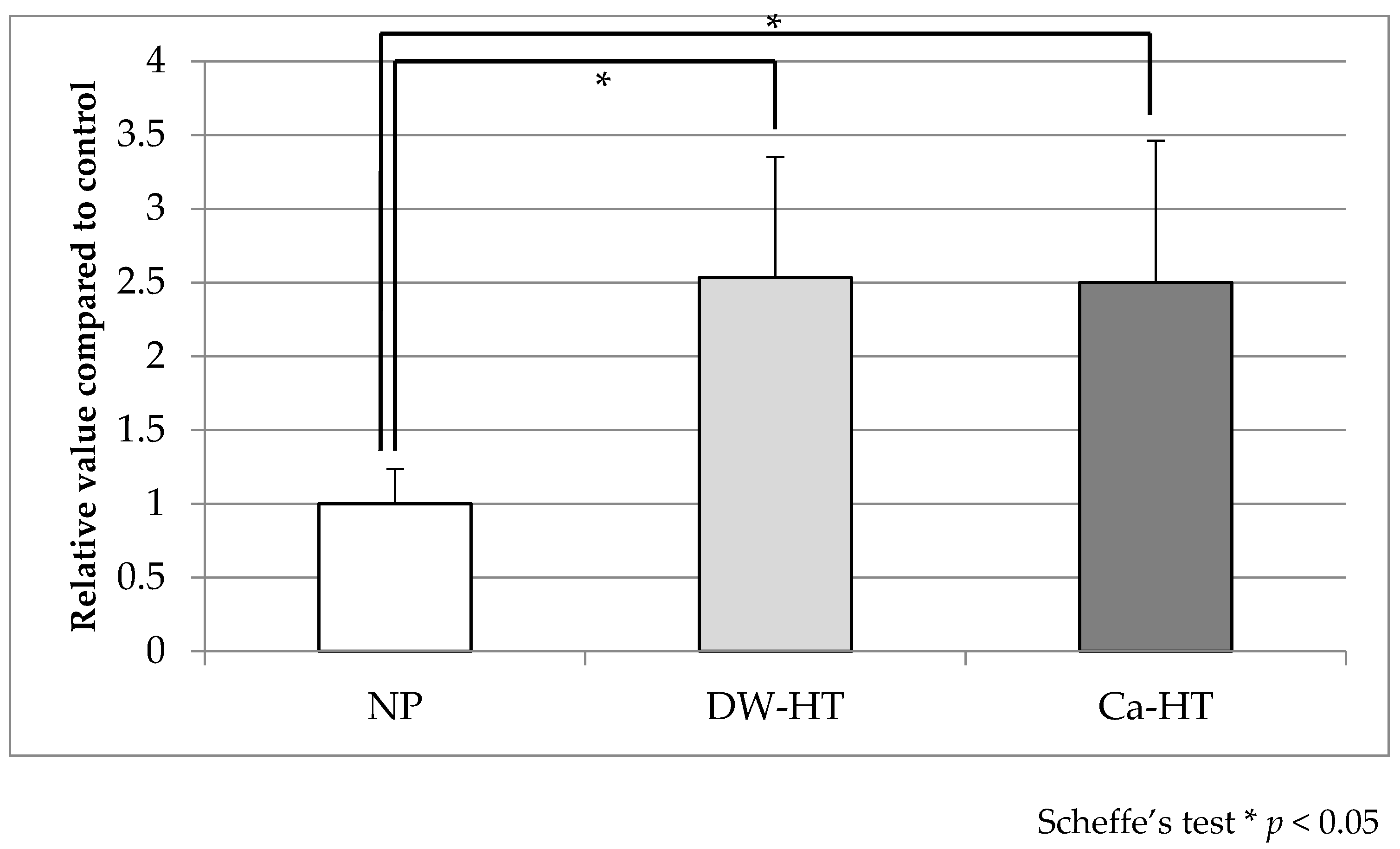
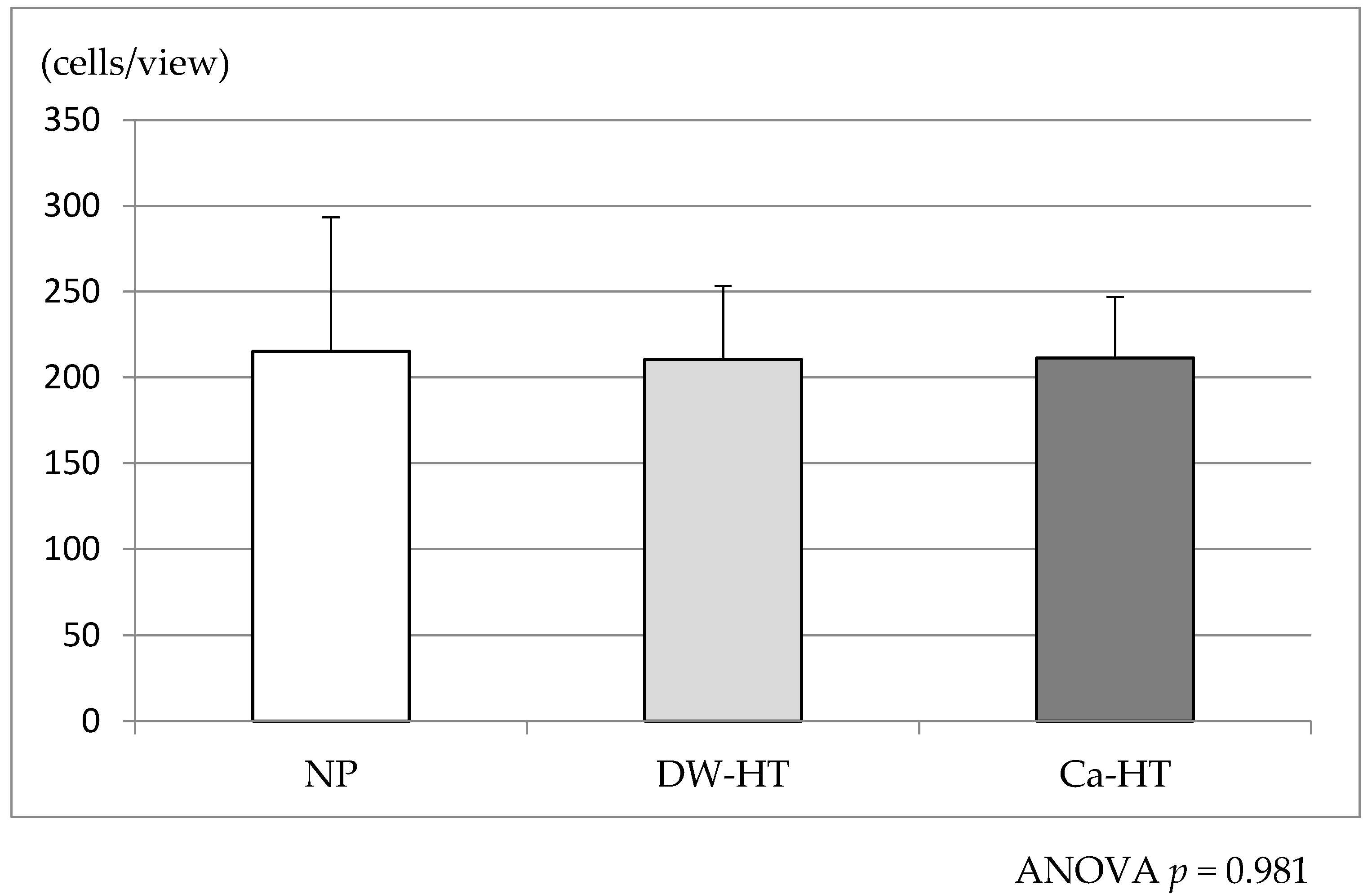
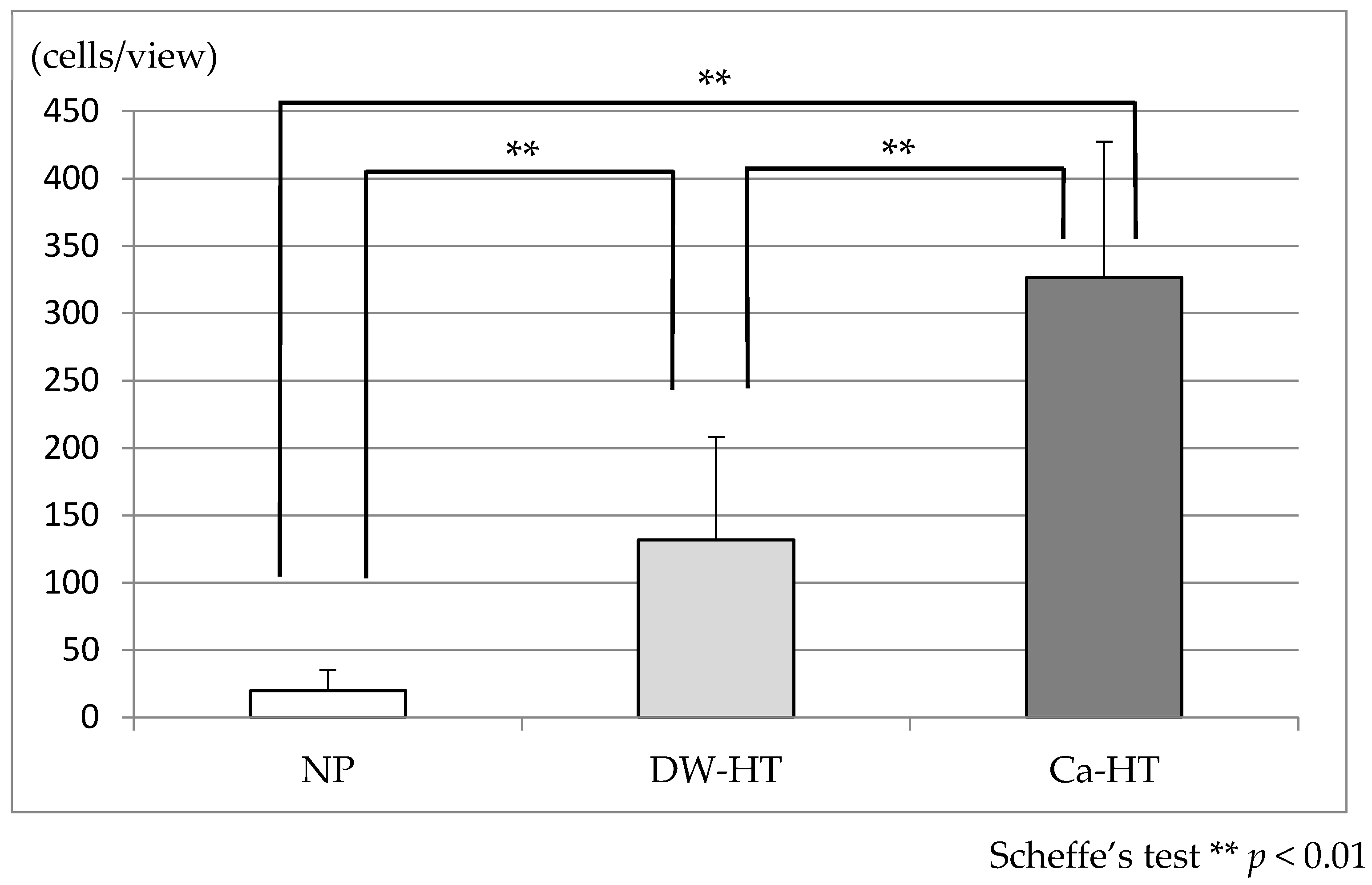
3.3. Initial Cell Adhesion
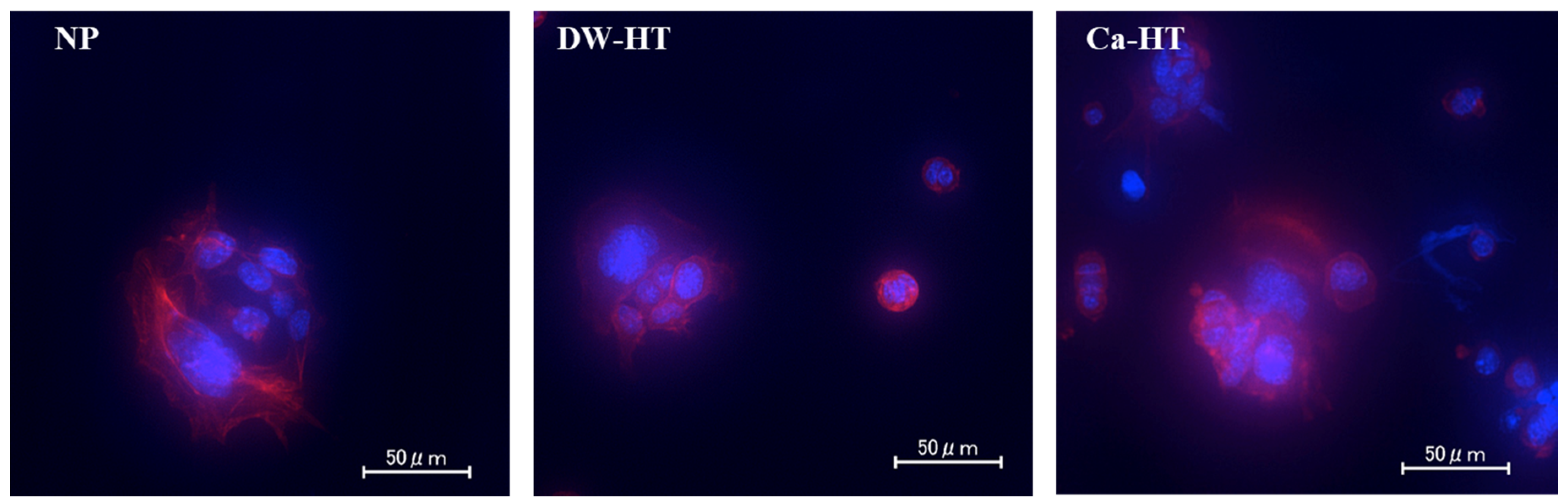
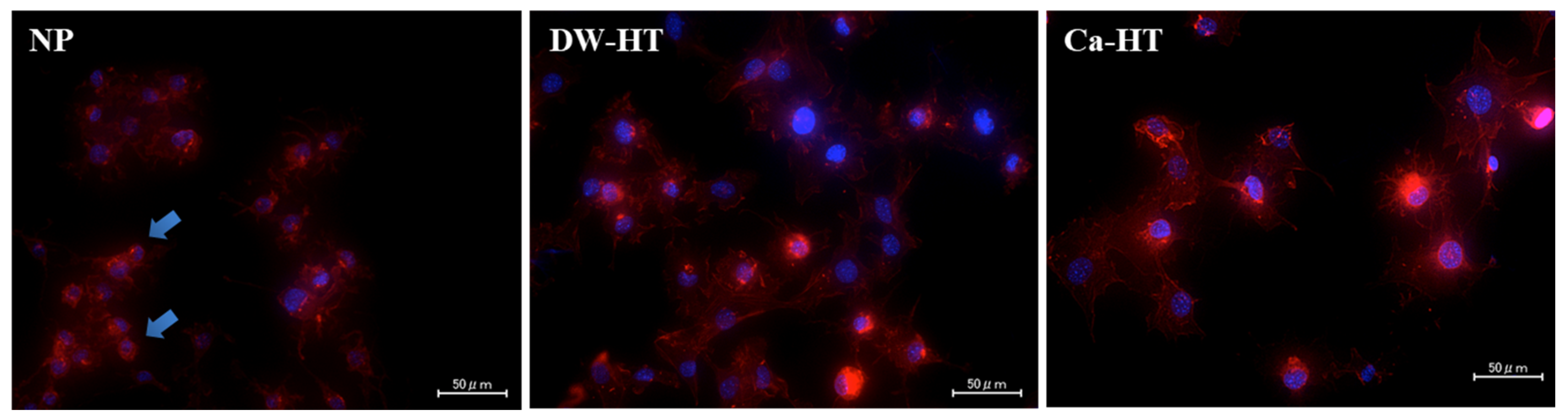
3.4. Cell Morphology
3.5. Bacterial Adhesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adell, R.; Eriksson, B.; Lekholm, U.; Branemark, P.I.; Jemt, T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implant. 1990, 5, 347–359. [Google Scholar]
- Lang, N.P.; Berglundh, T.; Heitz-Mayfield, L.J.; Pjetursson, B.E.; Salvi, G.E.; Sanz, M. Consensus statements and recommended clinical procedures regarding implant survival and complications. Int. J. Oral Maxillofac. Implants 2004, 19, 150–154. [Google Scholar]
- Berglundh, T.; Gotfredsen, K.; Zitzmann, N.U.; Lang, N.P.; Lindhe, J. Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: An experimental study in dogs. Clin. Oral Implant. Res. 2007, 18, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Strom, C. Peri-implantitis in partially edentulous patients: Association with inadequate plaque control. Clin. Oral Implant. Res. 2009, 20, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J.; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus report of the sixth european workshop on periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 282–285. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, C.J.; Bernal, G.; Rungcharassaeng, K.; Kan, J.Y. Clinical complications with implants and implant prostheses. J. Prosthet. Dent. 2003, 90, 121–132. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Yamaza, T.; Tsukiyama, Y.; Koyano, K. Promotive effect of insulin-like growth factor-1 for epithelial sealing to titanium implants. J. Biomed. Mater. Res. A 2013, 101, 2896–2904. [Google Scholar] [CrossRef]
- Ikeda, H.; Shiraiwa, M.; Yamaza, T.; Yoshinari, M.; Kido, M.A.; Ayukawa, Y.; Inoue, T.; Koyano, K.; Tanaka, T. Difference in penetration of horseradish peroxidase tracer as a foreign substance into the peri-implant or junctional epithelium of rat gingivae. Clin. Oral Implant. Res. 2002, 13, 243–251. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ayukawa, Y.; Legeros, R.Z.; Matsuya, S.; Koyano, K.; Ishikawa, K. Tissue-response to calcium-bonded titanium surface. J. Biomed. Mater. Res. A 2010, 95, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases 2012, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Atsuta, I.; Ayukawa, Y.; Narimatsu, I.; Zhou, T.; Hu, J.; Koyano, K. Effects of different divalent cation hydrothermal treatments of titanium implant surfaces for epithelial tissue sealing. Materials 2020, 13, 2038. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ayukawa, Y.; Furuhashi, A.; Kamo, M.; Ikeda, J.; Atsuta, I.; Haraguchi, T.; Koyano, K. Effect of hydrothermal treatment with distilled water on titanium alloy for epithelial cellular attachment. Materials 2019, 12, 2748. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Oshiro, W.; Atsuta, I.; Furuhashi, A.; Kondo, R.; Jinno, Y.; Koyano, K. Long term retention of gingival sealing around titanium implants with CaCl2 hydrothermal treatment: A rodent study. J. Clin. Med. 2019, 8, 1560. [Google Scholar] [CrossRef]
- Narimatsu, I.; Atsuta, I.; Ayukawa, Y.; Oshiro, W.; Yasunami, N.; Furuhashi, A.; Koyano, K. Epithelial and connective tissue sealing around titanium implants with various typical surface finishes. ACS Biomater. Sci. Eng. 2019, 5, 4976–4984. [Google Scholar] [CrossRef]
- Oshiro, W.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Yamazoe, J.; Kondo, R.; Sakaguchi, M.; Matsuura, Y.; Tsukiyama, Y.; Koyano, K. Effects of CaCl2 hydrothermal treatment of titanium implant surfaces on early epithelial sealing. Coll. Surf. B Biointerfaces 2015, 131, 141–147. [Google Scholar] [CrossRef]
- Albelda, S.M.; Buck, C.A. Integrins and other cell adhesion molecules. FASEB J. 1990, 4, 2868–2880. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Ohara-Nemoto, Y.; Yanai, N.; Obinata, M.; Hayashi, S.; Satoh, M. Establishment of gingival epithelial cell lines from transgenic mice harboring temperature sensitive simian virus 40 large T-antigen gene. J. Oral Pathol. Med. 2001, 30, 296–304. [Google Scholar] [CrossRef]
- Milanezi de Almeida, J.; Bosco, A.F.; Bonfante, S.; Theodoro, L.H.; Nagata, M.J.H.; Garcia, V.G. Nicotine-induced damage affects gingival fibroblasts in the gingival tissue of rats. J. Periodontol. 2011, 82, 1206–1211. [Google Scholar] [CrossRef]
- Wang, S.; Indrawati, L.; Wooters, M.; Caro-Aguilar, I.; Field, J.; Kaufhold, R.; Payne, A.; Caulfield, M.J.; Smith, J.G.; Heinrichs, J.H. A novel automated method for enumeration of Chlamydia trachomatis inclusion forming units. J. Immunol. Methods 2007, 324, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Ahn, S.J.; Wen, Z.T.; Brady, L.J.; Burne, R.A. Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva. Infect. Immun. 2008, 76, 4259–4268. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Nakano, Y.; Kumagai, T.; Yasui, M.; Kamio, N.; Shibata, Y.; Shiota, S.; Yamashita, Y. The ecological proportion of indigenous bacterial populations in saliva is correlated with oral health status. ISME J. 2009, 3, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Ayukawa, Y.; Takeshita, T.; Furuhashi, A.; Yamashita, Y.; Koyano, K. Bacterial adhesion affinities of various implant abutment materials. Clin. Oral Implant. Res. 2013, 24, 1310–1315. [Google Scholar] [CrossRef]
- Takeshita, T.; Nakano, Y.; Yamashita, Y. Improved accuracy in terminal restriction fragment length polymorphism phylogenetic analysis using a novel internal size standard definition. Oral Microbiol. Immunol. 2007, 22, 419–428. [Google Scholar] [CrossRef]
- Horbett, T.A.; Schway, M.B. Correlations between mouse 3T3 cell spreading and serum fibronectin adsorption on glass and hydroxyethylmethacrylate-ethylmethacrylate copolymers. J. Biomed. Mater. Res. 1988, 22, 763–793. [Google Scholar] [CrossRef]
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin—A glycoprotein from basement membranes. J. Biol. Chem. 1979, 254, 9933–9937. [Google Scholar]
- Malmstrom, J.; Agheli, H.; Kingshott, P.; Sutherland, D.S. Viscoelastic modeling of highly hydrated laminin layers at homogeneous and nanostructured surfaces: Quantification of protein layer properties using QCM-D and SPR. Langmuir 2007, 23, 9760–9768. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Cheng, Y.S. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J. Biol. Chem. 1993, 268, 17286–17299. [Google Scholar]
- Somerman, M.J.; Prince, C.W.; Butler, W.T.; Foster, R.A.; Moehring, J.M.; Sauk, J.J. Cell attachment activity of the 44 kilodalton bone phosphoprotein is not restricted to bone cells. Matrix 1989, 9, 49–54. [Google Scholar] [CrossRef]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegard, D. Osteopontin—A possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef] [PubMed]
- Oldberg, A.; Franzen, A.; Heinegard, D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc. Natl. Acad. Sci. USA 1986, 83, 8819–8823. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Takeshita, F.; Inoue, T.; Yoshinari, M.; Shimono, M.; Suetsugu, T.; Tanaka, T. An immunoelectron microscopic localization of noncollagenous bone proteins (osteocalcin and osteopontin) at the bone-titanium interface of rat tibiae. J. Biomed. Mater. Res. 1998, 41, 111–119. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Shibata, Y.; Hosaka, M.; Kawai, H.; Miyazaki, T. Glow discharge plasma treatment of titanium plates enhances adhesion of osteoblast-like cells to the plates through the integrin-mediated mechanism. Int. J. Oral Maxillofac. Implant. 2002, 17, 771–777. [Google Scholar]
- Takemoto, S.; Yamamoto, T.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Takashima, S. Platelet adhesion on titanium oxide gels: Effect of surface oxidation. Biomaterials 2004, 25, 3485–3492. [Google Scholar] [CrossRef]
- Kopf, B.S.; Schipanski, A.; Rottmar, M.; Berner, S.; Maniura-Weber, K. Enhanced differentiation of human osteoblasts on Ti surfaces pre-treated with human whole blood. Acta Biomater. 2015, 19, 180–190. [Google Scholar] [CrossRef]
- Boskey, A.L.; Maresca, M.; Ullrich, W.; Doty, S.B.; Butler, W.T.; Prince, C.W. Osteopontin-hydroxyapatite interactions in vitro: Inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Min. 1993, 22, 147–159. [Google Scholar] [CrossRef]
- Klaning, E.; Christensen, B.; Sorensen, E.S.; Vorup-Jensen, T.; Jensen, J.K. Osteopontin binds multiple calcium ions with high affinity and independently of phosphorylation status. Bone 2014, 66, 90–95. [Google Scholar] [CrossRef]
- Vroman, L. The life of an artificial device in contact with blood: Initial events and their effect on its final state. Bull. N. Y. Acad. Med. 1988, 64, 352–357. [Google Scholar] [PubMed]
- Elwing, H.; Askendal, A.; Lundstrom, I. Competition between adsorbed fibrinogen and high-molecular-weight kininogen on solid surfaces incubated in human plasma (the Vroman effect): Influence of solid surface wettability. J. Biomed. Mater. Res. 1987, 21, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Elwing, H.; Askendal, A.; Lundstrom, I. Desorption of fibrinogen and γ-globulin from solid surfaces induced by a nonionic detergent. J. Coll. Interface Sci. 1989, 128, 296–300. [Google Scholar] [CrossRef]
- An, N.; Rausch-fan, X.; Wieland, M.; Matejka, M.; Andrukhov, O.; Schedle, A. Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent. Mater. 2012, 28, 1207–1214. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Cosyn, J.; Van Aelst, L.; Collaert, B.; Persson, G.R.; De Bruyn, H. The peri-implant sulcus compared with internal implant and suprastructure components: A microbiological analysis. Clin. Implant. Dent. Relat. Res. 2011, 13, 286–295. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haraguchi, T.; Ayukawa, Y.; Shibata, Y.; Takeshita, T.; Atsuta, I.; Ogino, Y.; Yasunami, N.; Yamashita, Y.; Koyano, K. Effect of Calcium Chloride Hydrothermal Treatment of Titanium on Protein, Cellular, and Bacterial Adhesion Properties. J. Clin. Med. 2020, 9, 2627. https://doi.org/10.3390/jcm9082627
Haraguchi T, Ayukawa Y, Shibata Y, Takeshita T, Atsuta I, Ogino Y, Yasunami N, Yamashita Y, Koyano K. Effect of Calcium Chloride Hydrothermal Treatment of Titanium on Protein, Cellular, and Bacterial Adhesion Properties. Journal of Clinical Medicine. 2020; 9(8):2627. https://doi.org/10.3390/jcm9082627
Chicago/Turabian StyleHaraguchi, Takuya, Yasunori Ayukawa, Yukie Shibata, Toru Takeshita, Ikiru Atsuta, Yoichiro Ogino, Noriyuki Yasunami, Yoshihisa Yamashita, and Kiyoshi Koyano. 2020. "Effect of Calcium Chloride Hydrothermal Treatment of Titanium on Protein, Cellular, and Bacterial Adhesion Properties" Journal of Clinical Medicine 9, no. 8: 2627. https://doi.org/10.3390/jcm9082627
APA StyleHaraguchi, T., Ayukawa, Y., Shibata, Y., Takeshita, T., Atsuta, I., Ogino, Y., Yasunami, N., Yamashita, Y., & Koyano, K. (2020). Effect of Calcium Chloride Hydrothermal Treatment of Titanium on Protein, Cellular, and Bacterial Adhesion Properties. Journal of Clinical Medicine, 9(8), 2627. https://doi.org/10.3390/jcm9082627





