Localization of Integrin Beta-4 Subunit at Soft Tissue–Titanium or Zirconia Interface
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
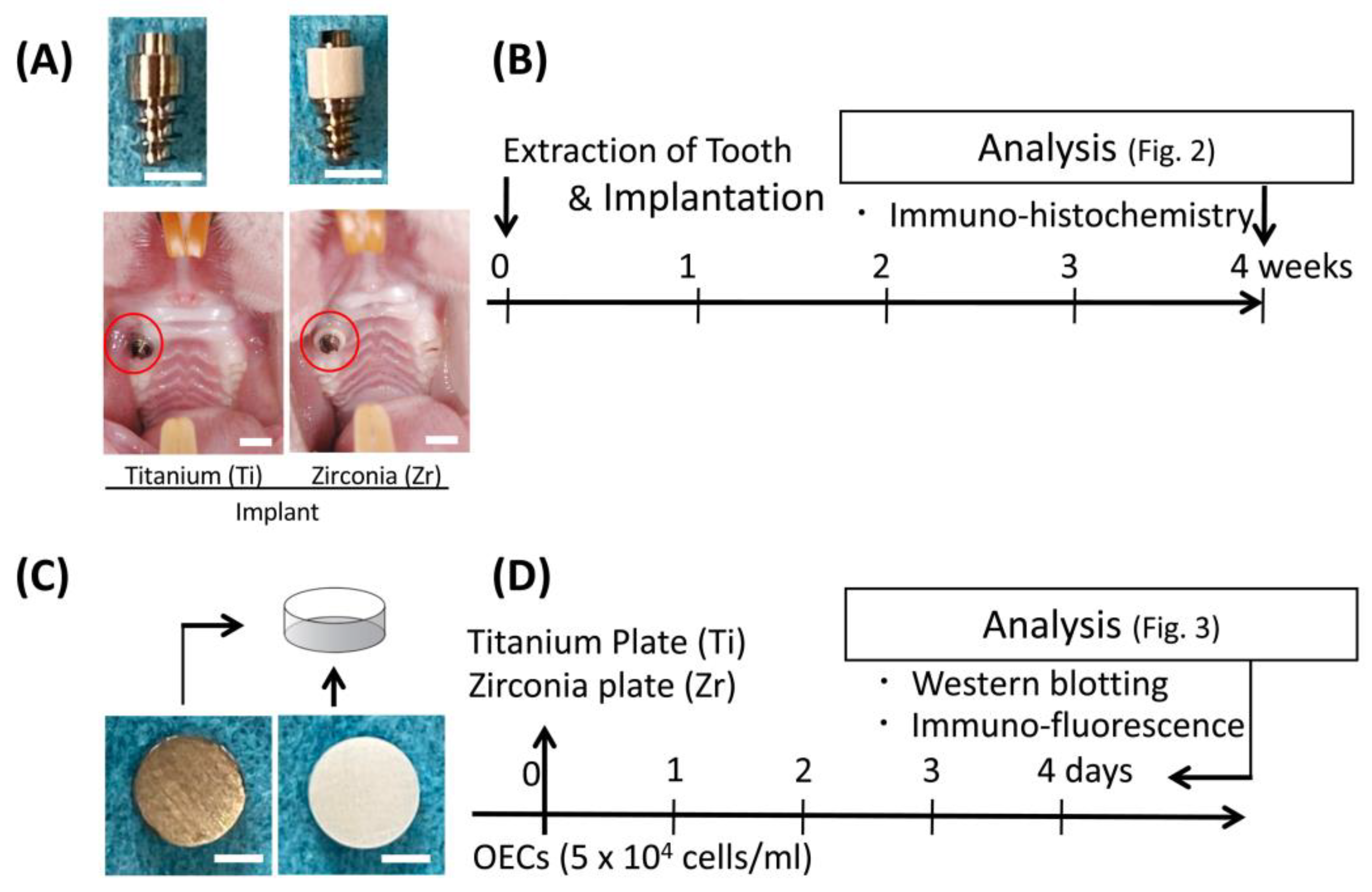
2.2. Experimental Implants
2.3. Implantation
2.4. Immunohistochemistry for Animal Experiments
2.5. Statistical Analysis
2.6. Culture Experiments
2.7. Immunofluorescence Staining for Culture Experiment
2.8. Western Blotting
3. Results
3.1. In Vivo Localization of Inβ4 around Experimental Implant
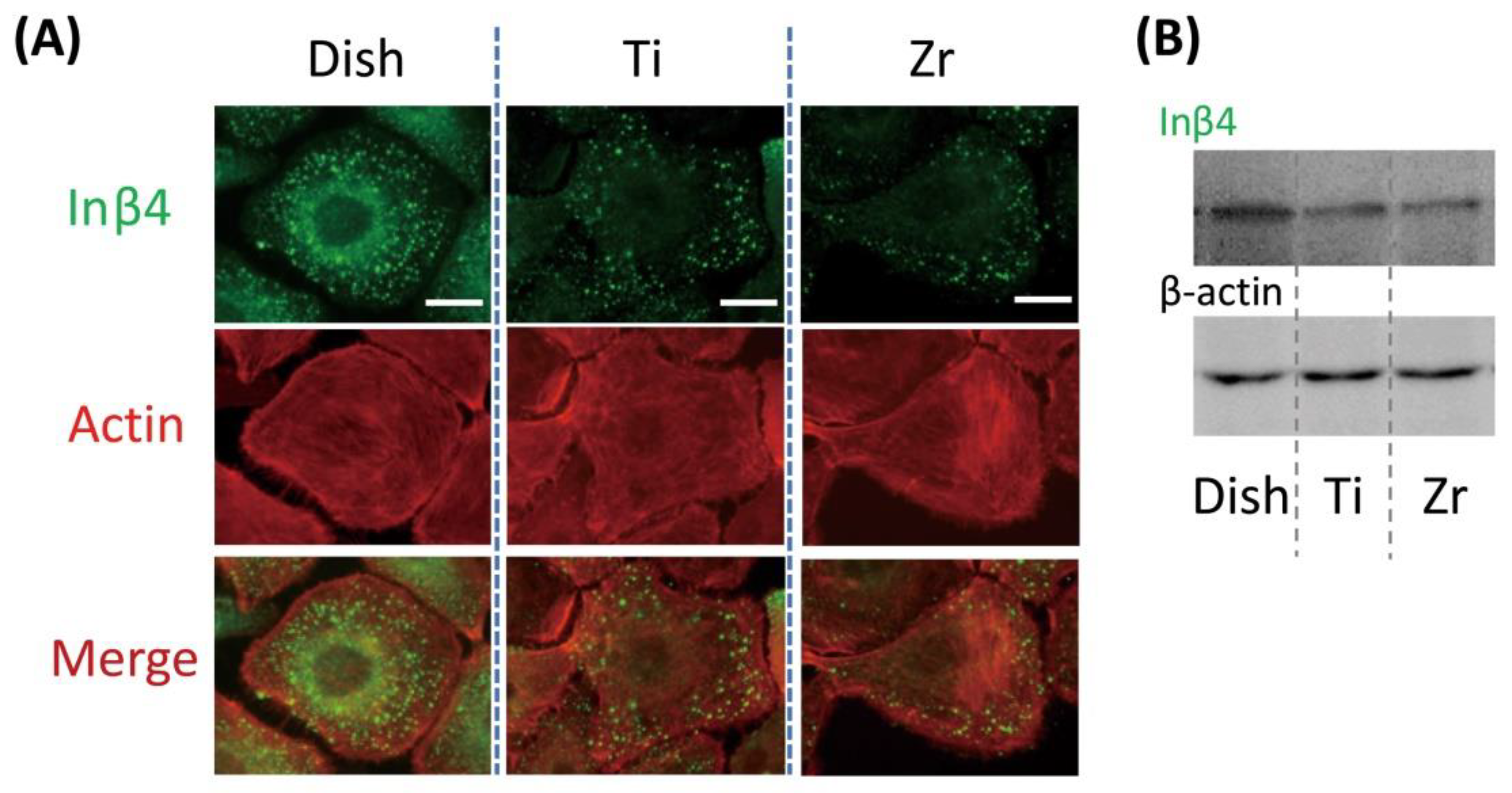
3.2. In Vitro Localization of Inβ4 in OECs on Titanium or Zirconia Plate
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Linkevicius, T.; Vaitelis, J. The effect of zirconia or titanium as abutment material on soft peri-implant tissues: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2015, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Ioannidis, A.; Cathomen, E.; Hammerle, C.H.; Husler, J.; Jung, R.E. Discoloration of the Peri-implant Mucosa Caused by Zirconia and Titanium Implants. Int. J. Periodontics Restor. Dent 2016, 36, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kinumatsu, T.; Hashimoto, S.; Muramatsu, T.; Sasaki, H.; Jung, H.S.; Yamada, S.; Shimono, M. Involvement of laminin and integrins in adhesion and migration of junctional epithelium cells. J. Periodontal Res. 2009, 44, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Spurr-Michaud, S.; Tisdale, A.; Elwell, J.; Gipson, I.K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc. Natl. Acad. Sci. USA 1990, 87, 8970–8974. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, T.; Hashimoto, S.; Kinumatsu, T.; Muramatsu, T.; Jung, H.S.; Yamada, S.; Shimono, M. Immunolocalization of laminin and integrin in regenerating junctional epithelium of mice after gingivectomy. J. Periodontal Res. 2009, 44, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Yamaza, T.; Yoshinari, M.; Ohsaki, Y.; Ayukawa, Y.; Kido, M.A.; Inoue, T.; Shimono, M.; Koyano, K.; Tanaka, T. Ultrastructural and immunoelectron microscopic studies of the peri-implant epithelium-implant (Ti-6Al-4V) interface of rat maxilla. J. Periodontol. 2000, 71, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Ultrastructural localization of laminin-5 (gamma2 chain) in the rat peri-implant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; Group, N.C.R.R.G.W. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. J. Gene Med. 2010, 12, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Atsuta, I.; Ayukawa, Y.; Zhou, T.; Narimatsu, I.; Koyano, K. Effect of titanium or zirconia implant abutments on epithelial attachments after ultrasonic cleaning. J. Oral Sci. 2020, 62, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Atsuta, I.; Ayukawa, Y.; Zhou, X.; Dwi Rakhmatia, Y.; Koyano, K. The impact of surface alteration on epithelial tissue attachment after the mechanical cleaning of titanium or zirconia surface. J. Oral Rehabil. 2019, 47, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, I.; Atsuta, I.; Ayukawa, Y.; Oshiro, W.; Yasunami, N.; Furuhashi, A.; Koyano, K. Epithelial and Connective Tissue Sealing around Titanium Implants with Various Typical Surface Finishes. ACS Biomater. Sci. Eng. 2019, 5, 4976–4984. [Google Scholar] [CrossRef]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Atsuta, I.; Ayukawa, Y.; Narimatsu, I.; Zhou, T.; Hu, J.; Koyano, K. Effects of Different Divalent Cation Hydrothermal Treatments of Titanium Implant Surfaces for Epithelial Tissue Sealing. Materials 2020, 13, 2038. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, C.J.; Bernal, G.; Rungcharassaeng, K.; Kan, J.Y.K. Clinical complications with implants and implant prostheses. J. Prosthet. Dent. 2003, 90, 121–132. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Mino, S.; Goto, T.; Kido, M.A.; Terada, Y.; Tanaka, T. Changes in the distribution of laminin-5 during peri-implant epithelium formation after immediate titanium implantation in rats. Biomaterials 2005, 26, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Pulkkinen, L. Molecular complexity of the cutaneous basement membrane zone. Mol. Biol. Rep. 1996, 23, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Rausch-Fan, X.; Wieland, M.; Matejka, M.; Schedle, A. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J. Biomed. Mater. Res. A 2007, 82, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Taylor, A.F.; Li, Z.; Vogler, E.A.; Donahue, H.J. Integrin expression and osteopontin regulation in human fetal osteoblastic cells mediated by substratum surface characteristics. Tissue Eng. 2005, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, T.; Ayukawa, Y.; Shibata, Y.; Takeshita, T.; Atsuta, I.; Ogino, Y.; Yasunami, N.; Yamashita, Y.; Koyano, K. Effect of Calcium Chloride Hydrothermal Treatment of Titanium on Protein, Cellular, and Bacterial Adhesion Properties. J. Clin. Med. 2020, 9, 2627. [Google Scholar] [CrossRef] [PubMed]
- Takamori, Y.; Atsuta, I.; Nakamura, H.; Sawase, T.; Koyano, K.; Hara, Y. Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin. Oral Implant. Res. 2017, 28, 163–170. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayukawa, Y.; Atsuta, I.; Moriyama, Y.; Jinno, Y.; Koyano, K. Localization of Integrin Beta-4 Subunit at Soft Tissue–Titanium or Zirconia Interface. J. Clin. Med. 2020, 9, 3331. https://doi.org/10.3390/jcm9103331
Ayukawa Y, Atsuta I, Moriyama Y, Jinno Y, Koyano K. Localization of Integrin Beta-4 Subunit at Soft Tissue–Titanium or Zirconia Interface. Journal of Clinical Medicine. 2020; 9(10):3331. https://doi.org/10.3390/jcm9103331
Chicago/Turabian StyleAyukawa, Yasunori, Ikiru Atsuta, Yasuko Moriyama, Yohei Jinno, and Kiyoshi Koyano. 2020. "Localization of Integrin Beta-4 Subunit at Soft Tissue–Titanium or Zirconia Interface" Journal of Clinical Medicine 9, no. 10: 3331. https://doi.org/10.3390/jcm9103331
APA StyleAyukawa, Y., Atsuta, I., Moriyama, Y., Jinno, Y., & Koyano, K. (2020). Localization of Integrin Beta-4 Subunit at Soft Tissue–Titanium or Zirconia Interface. Journal of Clinical Medicine, 9(10), 3331. https://doi.org/10.3390/jcm9103331




