1. Introduction
Patients with diabetes mellitus (DM) and acute myocardial infarction (AMI) are at high risk for recurrent cardiovascular events [
1,
2], in part due to a greater tendency towards thrombosis [
3,
4]. Diabetics are characterized by increased platelet reactivity [
5] including higher reactivity while on antiplatelet treatment [
6,
7]. ST-segment elevation myocardial infarction (STEMI) is characterized by a highly prothrombotic state [
8], the highest of which can be observed in diabetic STEMI patients [
9].
Primary percutaneous coronary intervention (pPCI) is the most effective and most recommended therapeutic approach in patients with STEMI and in those at very high-risk myocardial infarction without persistent ST-segment elevation (non-STEMI with ongoing ischemia) [
10].
Prasugrel and ticagrelor are the currently recommended treatment in patients with acute coronary syndromes, including STEMI, since they have been shown to reduce ischemic events compared to clopidogrel [
11].
Studies testing newer P2Y
12 inhibitors in acute coronary syndromes showed similar positive effects for ticagrelor and prasugrel on outcomes of patients with DM compared to non-diabetics [
1,
12]. However, the AMI populations in these studies were heterogeneous and included minor infarcts, in which the AMI diagnosis was based on highly sensitive tests for troponins. As such, the subgroup analyses of these studies provided only limited information on high-risk AMI populations treated with primary or immediate PCI.
The effect of prasugrel and ticagrelor, specifically on the STEMI population, has been even less well studied. To date, only the ATLANTIC trial selectively tested novel P2Y12 inhibitors in a STEMI population. The independent effect of diabetes on prognoses in the highest risk patients following an AMI, treated with improved, up-to-date techniques, is therefore still open to discussion.
The multicenter randomized PRAGUE-18 study was a comparison of prasugrel and ticagrelor in patients with AMI indicated to primary PCI [
13,
14]. The trial was unique since the AMI population was real life (with very few exclusion criteria) and homogenous with respect to the highest thrombotic risk.
This sub-study aims to evaluate the prognostic significance of DM in patients with AMI treated with pPCI in the era of potent antithrombotics and to investigate whether the most efficient treatment currently available, i.e., primary PCI and potent P2Y12 inhibitors can change the negative impact of DM on the prognoses of patients at the highest risk of major adverse cardiovascular events.
2. Methods
This analysis includes subjects randomized into the PRAGUE-18 trial stratified by DM status and by insulin treatment into prespecified subgroups. Subjects were classified according to the presence or absence of DM at baseline. The diagnosis of DM was based on patients’ history and on initial clinical examination. All diabetics on a diet, oral hypoglycemic medication, or insulin control were included. A subgroup of patients requiring insulin control was evaluated separately as these patients present mostly more advanced DM and are at higher risk. Nevertheless, the reasons for pre-randomization choice between insulin and other treatments were not analyzed. Potential risk factors in patient histories, pre-, peri-, and post-procedural pharmacotherapy, and characteristics related to the pPCI procedure were searched and analyzed for subgroup differences. The impact of DM and the impact of insulin treatment, relative to glucose control, on patient prognosis (study endpoints) were evaluated separately using multivariate analyses.
Enrollment criteria, the design, and the randomization process of the PRAGUE-18 study have been previously described [
13,
14]. Briefly, P2Y
12 inhibitor naive patients with AMI (STEMI and very high-risk non-STEMI) indicated to pPCI were randomized either to a prasugrel or ticagrelor loading dose and one-year therapy on top of aspirin treatment. Simple randomization using GraphPad scientific software was used in the study. The “sealed envelope” method was used for the distribution of the randomization codes. Since expenses for both drugs were not covered by insurance, patients were allowed to switch to clopidogrel during the study, under supervision of the treating physician. The study population consists of 1230 patients enrolled between May 2016 and November 2017. Hemodynamic instability was not an exclusion criterion for study participation. Nearly 4% of patients were in cardiogenic shock at baseline, and 5.2% were on mechanical ventilation. Almost all patients (99.2%) enrolled in the study underwent immediate PCI; primary PCI was performed in 94.6%. Radial access was used in two-thirds of patients and at least one intracoronary stent was implanted in 96% of patients.
The primary net-clinical endpoint was death, spontaneous MI, stroke, severe bleeding, or revascularization within 7 days. The secondary key efficacy endpoint was cardiovascular death, spontaneous MI, and stroke at 30 days and one year.
The occurrence of secondary endpoints, i.e., all-cause death, definite stent thrombosis (according to the Academic Research Consortium criteria), and bleeding (defined according to TIMI (Thrombolysis in Myocardial Infarction) and Bleeding Academic Research Consortium criteria) were also recorded. Data from the study were recorded using web-based case report forms and stored in a database system. An endpoint adjudication committee verified all study endpoints [
13].
The study design was approved by the multicenter ethics committee at the University Hospital Kralovske Vinohrady in Prague, Czech Republic (EK-VP/04/2013), and by the ethics committees of all participating sites. Study protocol is registered under PRAGUE-18 Clinicaltrials.gov NCT02808767.
Statistical Analysis
Continuous variables are presented as means or medians, and categorical variables are presented as numbers and percentages. The statistical significance of differences in categorical variables between patient groups was tested using the Fisher exact test; the Mann–Whitney U test was used for continuous variables. The occurrence of events over time was described and visualized using the Kaplan–Meier methodology; the statistical significance of differences between groups was tested using the log-rank test. The 1-dimensional and multidimensional Cox proportional hazards model were used for endpoint adjudications and described using hazard ratios (HR), 95% confidence intervals (CI), and statistical significance. In the multivariate analyses, the impact of DM on the prognosis was adjusted for age, sex, and presence of multivessel disease. All analyses were performed using SPSS version 24.0.0.1 (IBM Corporation, Armonk, NY, USA).
3. Results
Diabetic patients. DM was present in 250 (20%) patients. Prior to randomization, one-fourth (N = 64) of the diabetic patients were on long-term insulin treatment, 58.4% on oral hypoglycemic medication, and 16% were controlled by the diet only.
Baseline clinical characteristics of patients with and without DM, including initial ECG changes, chronic therapy before admission, initial laboratory results, and characteristics related to index angiography, are summarized in
Table 1.
Diabetic patients were more likely to be older, female, obese, with hypertension, hyperlipidemia, and a history of bleeding. DM was also associated with a more frequent presence of multivessel coronary disease and left main disease. In patients on insulin, the presence of multivessel coronary disease and left main disease was especially high (64% compared to 6.3% for non-diabetics).
The median time from symptom onset to hospital arrival was significantly longer in diabetic patients on insulin therapy compared to patients without DM (3.5 vs. 2.5 h; p = 0.030).
Before randomization, subjects with DM were more likely to receive chronic treatment with aspirin, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and statins.
Drug-eluting stents were used in two-thirds and thrombo-aspiration during pPCI was used in one-third of patients with and without DM. Glycoprotein IIb/IIIa inhibitors were used more often in patients with DM (
Table 1). The most frequent use of glycoprotein IIb/IIIa inhibitors was indeed observed in patients treated with insulin (28.1%). The result of pPCI in the DM group was more frequently evaluated by the treating interventional cardiologist as suboptimal or a procedural failure with the highest rate being patients on insulin (14.3%).
Discharge medications included aspirin in 97.3%, β-Blockers in 81.9%, ACE inhibitors/ARBs in 83.7%, and statins in 93.7% of the study population and did not differ between patients with and without DM (p = 0.999, p = 0.134, p = 0.626 and p = 0.270 respectively). The percentage of patients who switched their study treatment during the 12-month study course to clopidogrel (40.8%) did not differ between patients with or without DM (38.7%), p = 0.562.
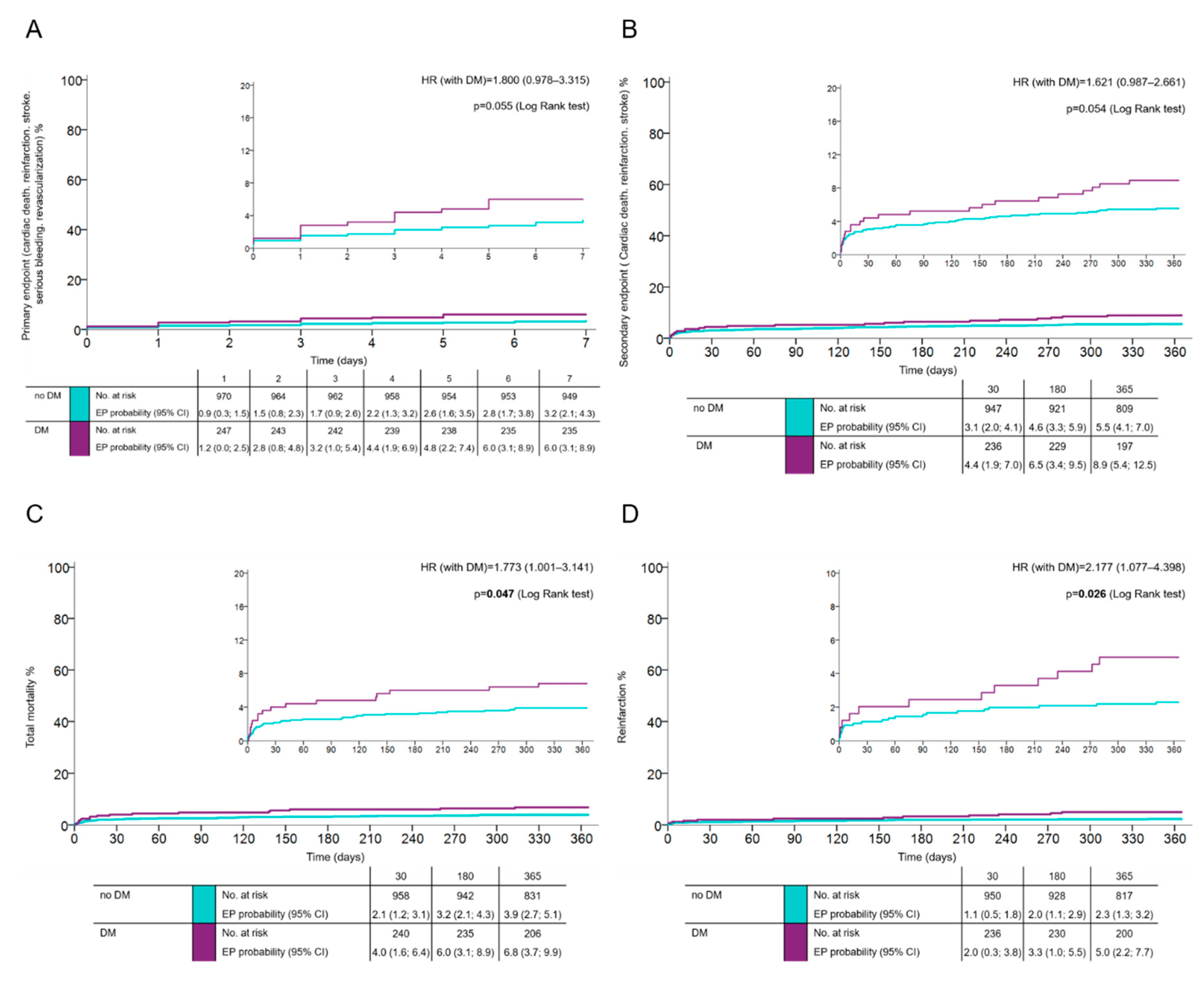
Outcomes: The primary net-clinical endpoint occurred in 6.1% of patients with, and in 3.5% of patients without DM (HR 1.8; 95% CI 0.978–3.315;
p = 0.055) (
Figure 1A). At one year, the key secondary endpoint (CV death, spontaneous MI, and stroke) occurred in 8.8% of diabetics and in 5.5% of patients without DM (HR 1.621; 95% CI 0.987–2.661;
p = 0.054) (
Figure 1B). The total one-year mortality and risk of nonfatal reinfarction in DM vs. non-DM were 6.8% vs. 3.9% (HR 1.773; 95% CI 1.001–3.141;
p = 0.047) and 4.8% vs. 2.2% (HR 2.177; 95% CI 1.077–4.398;
p = 0.026) (
Figure 1C,D), respectively; cardiac mortality at one year was 4% vs. 3% (HR 1.366; 95% CI (0.666–2.804;
p = 0.393), respectively. The risk of definite stent thrombosis was higher in diabetics (HR 2.37; 95% CI 0.864–6.541;
p = 0.08) (
Figure S1A in supplementary material). There was no risk of major bleeding related to the presence of DM (HR 0.861; 95% CI 0.554–1.339;
p = 0.506) (
Figure S1B).
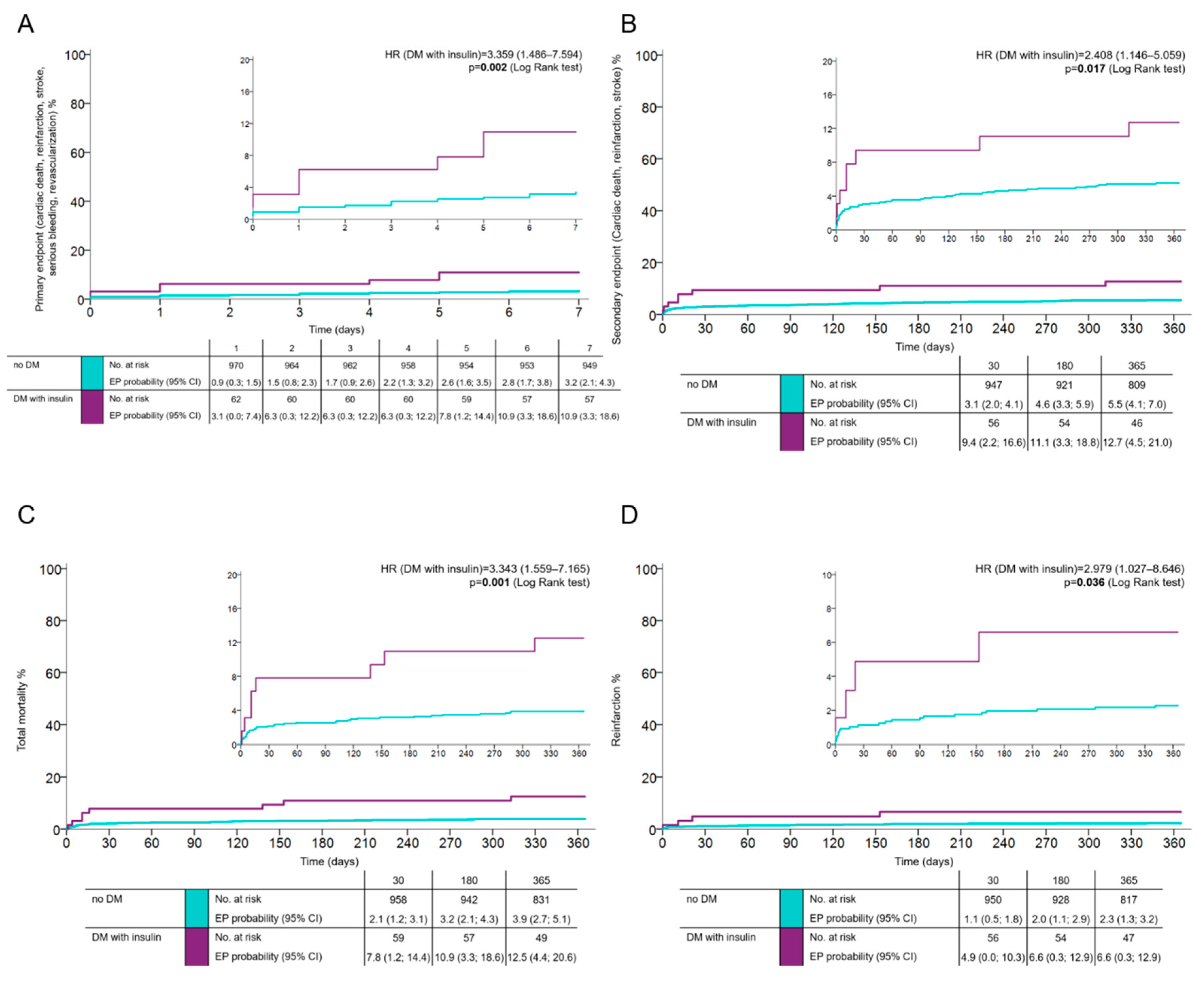
Patients with more advanced DM requiring insulin control exhibited all assessed endpoints more frequently compared with diabetic patients, not on insulin. The occurrence of the primary net clinical endpoint in this group of patients was 10.9% (HR 3.359; 95% CI 1.486–7.594;
p = 0.002 in comparison to the reference group of patients without DM) (
Figure 2A), the occurrence of the combined key ischemic endpoint at one year was 12.5% (HR 2.408; 95% CI 1.146–5.059;
p = 0.017) (
Figure 2B). The total mortality and risk of reinfarction at one year in DM patients on insulin was 12.5% (HR 3.343; 95% CI 1.559–7.165;
p = 0.001) (
Figure 2C) compared to 6.3% for patients without DM (HR 2.979; 95% CI 1.027–8.646;
p = 0.036) (
Figure 2D).
Using the Cox proportional hazards multivariate model adjusted for significant predictors of prognosis (see Methods section), DM remained an independent predictor of recurrent AMI at one year (HR 2.176; 95% CI 1.055–4.489; p = 0.035). Presence of DM on insulin was an independent predictor of total one-year mortality (HR 2.642; 95% CI, 1.223–5.709; p = 0.013).
4. Discussion
4.1. Study Population
The impact of DM on outcomes of patients with AMI has been explored in several previous studies. In contrast, this analysis examined a very precisely defined population of patients with the highest risk AMI. All enrolled patients received best practices treatment, i.e., pPCI using new-generation drug-eluting stents plus, new, potent oral antiplatelet therapy. Furthermore, the penetration of secondary preventive medication at discharge was very high in the PRAGUE-18 study population, including aspirin, statins, beta-blockers, and ACE inhibitors/ARB inhibitors.
4.2. Antiplatelet Treatment
All patients received potent antiplatelet therapy consisting of aspirin and ticagrelor or prasugrel. These newer (ticagrelor and prasugrel) antiplatelet agents are believed to overcome the problem with platelet resistance to clopidogrel, and they reduce thrombotic complications to a greater extent than clopidogrel in DM patients [
1,
12]. Data shows that antiplatelet therapy provided with prasugrel is of particular benefit to patients with DM. Subjects with DM had a greater reduction in ischemic events without an increase in major bleeding and therefore had a greater net treatment benefit with prasugrel compared to patients without DM [
12]. Ticagrelor exerts similar or greater inhibition of platelet reactivity compared to prasugrel in DM patients with coronary artery disease [
15]. Therefore, we hypothesized that we would observe a less negative impact of DM on the prognosis of patients in PRAGUE-18 compared to older studies.
4.3. Risk Factors
Similar to other published cohorts of AMI patients [
1,
16], DM patients were more likely to have several risk criteria, including multivessel disease, compared to non-diabetic patients. In some pPCI studies, longer time from symptom onset to hospital arrival was observed with DM patients [
16,
17]. In our study, this time difference was significant only with regard to DM patients treated with insulin, who, on average, arrived at the hospital one hour later than non-diabetics. This reflects frequent atypical or absent symptoms of ischemia in DM patients and can be one cause of worse outcomes.
4.4. PCI Procedures
Suboptimal results of pPCI with slower flow in the infarct-related artery was more often observed in the diabetic group. The finding is associated with a poor prognosis and it is generally believed to be the result of more prevalent distal embolization enhanced by lower coronary reserve and more diffuse coronary disease in diabetics. This highlights the importance of aggressive antithrombotic drug regimens to manage this population. Both prasugrel and ticagrelor have the potential to reduce distal embolization, but the time from randomization to infarct-related artery reperfusion in PRAGUE-18 was too short to expect full antiplatelet effects from either drug at the time of the procedure. This finding emphasizes the need for treatment of DM STEMI patients as soon as possible. If efficacious pretreatment is not possible, the use of fast-acting antiplatelet agents, such as cangrelor or a GP IIb/IIIa inhibitor, might be considered in DM patients. Patients with DM also have increased levels of procoagulation factors e.g., fibrinogen, tissue, or von Willebrand factors, and decreased levels of anticoagulation factors such as protein C and antithrombin III [
18]. Specific more aggressive anticoagulation treatment during pPCI in patients with DM might therefore be effective.
4.5. Outcomes
Our findings extend to prior observations on the adverse effect of diabetes on STEMI prognoses. Historically, it has been reported that patients with AMI and DM have a two to a fivefold higher risk for recurrent cardiovascular events, including death, compared to subjects without DM. Registries from the early 2000s report the cardiac mortality of patients with DM and acute coronary syndrome (ACS) are 2- to 3-fold higher compared to patients without DM [
19]. The hospital mortality rate of diabetics at that time was 13% [
20,
21]. Randomized studies from that period showed a 1.6–2-fold higher mortality of diabetic patients after STEMI, with hospital mortality of about 5% and one-year mortality over 13% [
16,
22]. In the control arm of a meta-analysis testing GP IIb/IIIa inhibitors in pPCI, the mortality rate was four-fold higher in diabetics compared with non-diabetics [
23]. A recent Atlantic study reported a 2-fold increase risk of clinically important ischemic events in diabetics [
24].
In the PRAGUE-18 study, despite a very high-risk AMI population, a one-year mortality rate of 6.8% among DM patients was low compared to older studies. However, DM remained associated with a 2-fold higher risk of reinfarction. DM patients on insulin were associated with 2.5-fold higher mortality at one year. These findings show an improving prognosis for DM patients, but the relative prognostic impact of DM remains. Diffuse coronary atherosclerosis, reduced coronary reserve, poorer collaterals, reduced compensatory capacity of the myocardium, and diabetic cardiomyopathy in DM patients likely play a role [
25].
DM patients use insulin mainly when other treatments are inadequate. The negative impact of insulin treatment on prognosis is likely related to more advanced DM among these patients. However, a direct negative impact of insulin on the prognosis cannot be excluded. Adverse effects of hyperinsulinemia on coagulation and smooth muscle cell proliferation and migration are well known.
4.6. Stent Thrombosis
In older studies, DM was associated with a higher risk of stent thrombosis with an up to a 5-fold increase [
26]. This was not surprising considering the higher platelet reactivity in DM patients whose platelet response to clopidogrel had attenuated [
27]. In the PRAGUE-18 study, the incidence of stent thrombosis in DM patients was 2% (HR 2.377; 95% CI, 0.864–6.541;
p = 0.084) (
Figure S1).
4.7. Bleeding
Unlike in older cohorts, we did not observe any increased risk of bleeding in DM patients compared to patients without DM (HR 0.861; 95% CI, 0.554–1.339;
p = 0.506) (
Figure S1). A similar result was seen in the TRITON TIMI 38 study, where DM was associated with higher bleeding risk in the clopidogrel but not in the prasugrel treatment groups. This was particularly interesting since there was a higher bleeding risk associated with prasugrel in patients without DM [
12]. Why prasugrel does not increase the bleeding risk in diabetics, while clopidogrel does, is not fully elucidated.
4.8. Causes of Higher Risk
One of the main causes of the negative prognostic impact of DM is believed to be increased platelet reactivity, a prothrombotic state, and a higher risk of thrombus embolization, which is poorly tolerated due to the more diffuse coronary disease, lower coronary reserve, and poorer collateralization in diabetics [
25]. Furthermore, DM patients were more likely to have a poor response to clopidogrel [
27]; the impact of platelet reactivity on cardiovascular events in patients with DM and coronary artery disease is documented [
6].
The extraordinary role of platelets in DM was observed in the studies, where the use of glycoprotein IIb/IIIa inhibitor abciximab with stent implantation during pPCI significantly decreased the risk of cardiovascular events in diabetics by one half [
23,
28].
5. Conclusions
Despite modern coronary interventions with new generation drug eluting stenting, intense antithrombotic therapy and high levels of guideline-based medical care, diabetes still has significant adverse effects on the prognosis of STEMI patients, which highlights the importance of further improvements in the management of this high-risk population.
The new ESC guidelines for diabetes management with the use of SGLT2 inhibitors and with the new DM specific targets might mitigate this significant risk factor in patients with STEMI.
6. Study Limitations
Although pre-specified, the present study is a subgroup post hoc analysis of the PRAGUE-18 trial with its limitations.
Supplementary Materials
The following are available online at
https://www.mdpi.com/2077-0383/9/8/2555/s1, Figure S1: The occurrence of stent thrombosis and serious bleeding in patients with and without diabetes mellitus (without diabetes mellitus, with diabetes mellitus on insulin therapy and not on insulin therapy) following acute myocardial infarction (AMI) treated with primary percutaneous coronary intervention and prasugrel or ticagrelor. (Time-to-event analysis was done using the Kaplan–Meier estimate of the survival function.), Supplementary list 1. List of Study Sites and Investigators.
Author Contributions
Conceptualization, Z.M.; data curation, S.S., O.H., P.K., M.H., J.K., I.V., J.D., F.T., J.M., and A.V.; formal analysis, S.S., Z.M., M.S., and J.J.; funding acquisition, Z.M.; investigation, S.S., O.H., P.K., M.H., J.K., I.V., J.D., F.T., J.M., and A.V.; methodology, Z.M.; project administration, J.J.; supervision, J.D., R.R., and F.T.; writing—original draft, S.S., Z.M., and J.J.; and writing—review and editing, Z.M., O.H., P.K., M.H., J.K., I.V., J.D., R.R., F.T., J.M., A.V., and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Charles University Cardiovascular Research Programs P-35 and PROGRES Q 38, Charles University, Prague, Czech Republic.
Acknowledgments
All authors have given their approval for the submission of the manuscript. All authors assume responsibility for the accuracy and completeness of the reported data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stefan, J.; Dominick, J.; Angiolillo, H.; David, E.; Steen, H.; Frederic, K.; Juan, M.; Josë, C.; Nicolau, J.S.; Robert, F.S.; et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: A substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur. Heart J. 2010, 31, 3006–3016. [Google Scholar]
- Norhammar, A.; Malmberg, K.; Diderholm, E.; Lagerqvist, B.; Lindahl, B.; Rydén, L.; Wallentin, L. Diabetes mellitus: The major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery dinase and benefits of revascularization. J. Am. Coll. Cardiol. 2004, 43, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Malmberg, K.; Ryden, L.; Tornvall, P.; Stenestrand, U.; Wallentin, L. Under utilisation of evidence-based treatment partially explains for the unfavourable prognosis in diabetic patients with acute myocardial infarction. Eur. Heart J. 2003, 24, 838–844. [Google Scholar] [CrossRef]
- Sobel, B.E. Optimizing cardiovascular outcomes in diabetes mellitus. Am. J. Med. 2007, 120 (Suppl. 2), S3–S11. [Google Scholar] [CrossRef]
- Colwell, J.A.; Halushka, P.V.; Sarji, K.; Levine, J.; Sagel, J.; Nair, R.M. Altered platelet function in diabetes mellitus. Diabetes 1976, 25 (2 Suppl.), 826–831. [Google Scholar]
- Angiolillo, D.J.; Bernardo, E.; Sabate, M.; Jiménez-Queved, P.; Costa, M.A.; Palazuelos, J.; Hernández-Antolín, R.; Moreno, R.; Escaned, J.; Alfonso, F.; et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Coll. Cardiol. 2007, 50, 1541–1547. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Shoemaker, S.B.; Desai, B.; Yuan, B.; Charlton, R.K.; Bernardo, E.; Zenni, M.M.; Guzman, L.A.; Bass, T.A.; Costa, M.A. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: Results of the optimizing antiplatelet therapy in diabetes mellitus (OPTIMUS) study. Circulation 2007, 115, 708–716. [Google Scholar] [CrossRef]
- Knudsen, E.C.; Seljeflot, I.; Abdelnoor, M.; Eritsland, J.; Mangschau, A.; Müller, C.; Arnesen, H.; Andersen, G. Elevated levels of PAI-1 activity and t-PA antigen are associated with newly diagnosed abnormal glucose regulation in patients with ST-elevation myocardial infarction. J. Thromb. Haemost. 2011, 9, 1468–1474. [Google Scholar] [CrossRef]
- Jakl, M.; Sevcik, R.; Fatorova, I.; Horacek, J.M.; Pudil, R. High on-treatment platelet reactivity: Risk factors and 5-year outcomes in patients with acute myocardial infarction. Anatol. J. Cardiol. 2017, 17, 113–118. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 1–96. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Wiviott, S.D.; Braunwald, E.; Angiolillo, D.J.; Meisel, S.; Dalby, A.J.; Verheugt, F.W.A.; Goodman, S.G.; Corbalan, R.; Purdy, D.A.; Murphy, S.A.; et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel–thrombolysis in myocardial infarction 38. Circulation 2008, 118, 1626–1636. [Google Scholar] [PubMed]
- Motovska, Z.; Hlinomaz, O.; Miklík, R.; Hromadka, M.; Varvarovsky, I.; Dusek, J.; Knot, J.; Jarkovsky, J.; Kala, P.; Rokyta, R.; et al. Prasugrel Versus Ticagrelor in Patients With Acute Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention: Multicenter Randomized PRAGUE-18 Study. Circulation 2016, 134, 1603–1612. [Google Scholar] [CrossRef]
- Motovska, Z.; Hlinomaz, O.; Kala, P.; Hromadka, M.; Knot, J.; Varvarovsky, I.; Dušek, J.; Jarkovsky, J.; Miklik, R.; Rokyta, R.; et al. 1-Year Outcomes of Patients Undergoing Primary Angioplasty for Myocardial Infarction Treated with Prasugrel Versus Ticagrelor. J. Am. Coll. Cardiol. 2018, 71, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Francesco, F.; Fabiana, R.; Niti, A.; Jenny, H.; Megha, K.; Ashwin, D.; Valeria, E.D.; Jung, R.C.; Latonya, B.; Martin, M.Z.; et al. Angiolillo Pharmacodynamic Comparison of Prasugrel Versus Ticagrelor in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease. Circulation 2016, 134, 780–792. [Google Scholar]
- Harjai, K.J.; Stone, G.W.; Boura, J.; Mattos, L.; Chandra, H.; Cox, D.; Grines, L.; O’Neill, W.; Grines, C. Primary Angioplasty in Myocardial Infarction Investigators. Comparison of outcomes of diabetic and nondiabetic patients undergoing primary angioplasty for acute myocardial infarction. Am. J. Cardiol. 2003, 91, 1041–1045. [Google Scholar] [CrossRef]
- Simek, S.; Aschermann, M.; Holm, F.; Humhal, J.; Linhart, A.; Vojácek, J.; Psenicka, M.; Hemzský, L.; Rohác, J.; Mrázek, V. Impact of primary angioplasty on prognosis of patients with diabetes mellitus and myocardial infarction. Vnitr. Lek. 2003, 49, 51–60. [Google Scholar]
- Grant, P.J. Diabetes mellitus as a prothrombotic condition. J. Intern. Med. 2007, 262, 157–172. [Google Scholar] [CrossRef]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef]
- Marso, S.P.; Giorgi, L.V.; Johnson, W.L.; Huber, K.C.; Laster, S.B.; Shelton, C.J.; McCallister, B.D.; Coen, M.M.; Rutherford, B.D. Diabetes mellitus is associated with a shift in the temporal risk profile of in hospital death after percutaneus coronary intervention: An analysis of 25,223 patients over 20 years. Am. Heart J. 2003, 145, 270–277. [Google Scholar] [CrossRef]
- Malmberg, K.; Yusuf, S.; Gerstein, H.C.; Brown, J.; Zhao, F.; Hunt, D.; Piegas, L.; Calvin, J.; Keltai, M.; Budaj, A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 2000, 102, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Donahoe, S.M.; Stewart, G.C.; McCabe, C.H.; Mohanavelu, S.; Murphy, S.A.; Cannon, C.P.; Antman, E.M. Diabetes and mortality following acute coronary syndromes. JAMA 2007, 298, 765–775. [Google Scholar] [CrossRef]
- Montalescot, G.; Antoniucci, D.; Kastrati, A.; Neumann, F.J.; Borentain, M.; Migliorini, A.; Boutron, C.; Collet, J.-P.; Vicaut, E. Abciximab in primary coronary stenting of ST-elevation myocardial infarction: A European meta-analysis on individual patients’ data with long-term follow-up. Eur. Heart J. 2007, 28, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Fabris, E.; Hof, A.V.; Hamm, C.W.; Lapostolle, F.; Lassen, J.F.; Goodman, S.G.; Berg, J.M.T.; Bolognese, L.; Cequier, A.; Chettibi, M.; et al. Pre-hospital administration of ticagrelor in diabetic patients with ST-elevation myocardial infarction undergoing primary angioplasty: A sub-analysis of the ATLANTIC trial. Catheter Cardiovasc Interv. 2018, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.; Kim, M.C.; Moreno, P.R.; Krishnan, P.; Ivan, O.C.; Sharma, S.K.; Sharma, S. Comparison of Coronary Flow Reserve and Fractional Flow Reserve in Patients with Versus Without Diabetes Mellitus and Having Elective Percutaneous Coronary Intervention and Abciximab Therapy. Am. J. Cardiol. 2008, 101, 796–800. [Google Scholar] [CrossRef]
- Silva, J.A.; Nunez, E.; White, C.J.; Collons, T.J.; Jenkins, J.S.; Zhang, S.Y.; Jain, S.P.; Ramee, S.R. Predictors of stent Thrombosis after primary stenting for acute myocardial infarction. Catheter Cardiovasc Interv. 1999, 47, 415–422. [Google Scholar] [CrossRef]
- Geisler, T.; Anders, N.; Paterok, M.; Langer, H.; Stellos, K.; Lindemann, S.; Herdeg, C.; May, A.E.; Gawaz, M. Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care 2007, 30, 372–374. [Google Scholar] [CrossRef][Green Version]
- Stone, G.W.; Grines, C.L.; Cox, D.A.; Garcia, E.; Tcheng, J.E.; Griffin, J.J.; Guagliumi, G.; Stuckey, T.; Turco, M.; Carroll, J.D.; et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N. Engl. J. Med. 2002, 346, 957–966. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).