Hypofractionated Radiotherapy in Locally Advanced Myxoid Liposarcomas of Extremities or Trunk Wall: Results of a Single-Arm Prospective Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
- A necessity for a secondary operation under general or regional anesthesia for wound repair (debridement, operative drainage, and secondary wound closure including rotationplasty, free flaps, or skin grafts)—Grade 3 according to CTCAE v.4
- Wound infection requiring systemic antibiotics—Grade 3 according to CTC AE v 4.
- Persistent deep packing for 90 days or longer (due to wound dehiscence)—Grade 2 according to CTCAE v4.
- Hospital readmission for wound care.
2.1. Assessment
2.2. Treatment
2.3. Patient Samples
2.4. Statistical Analysis
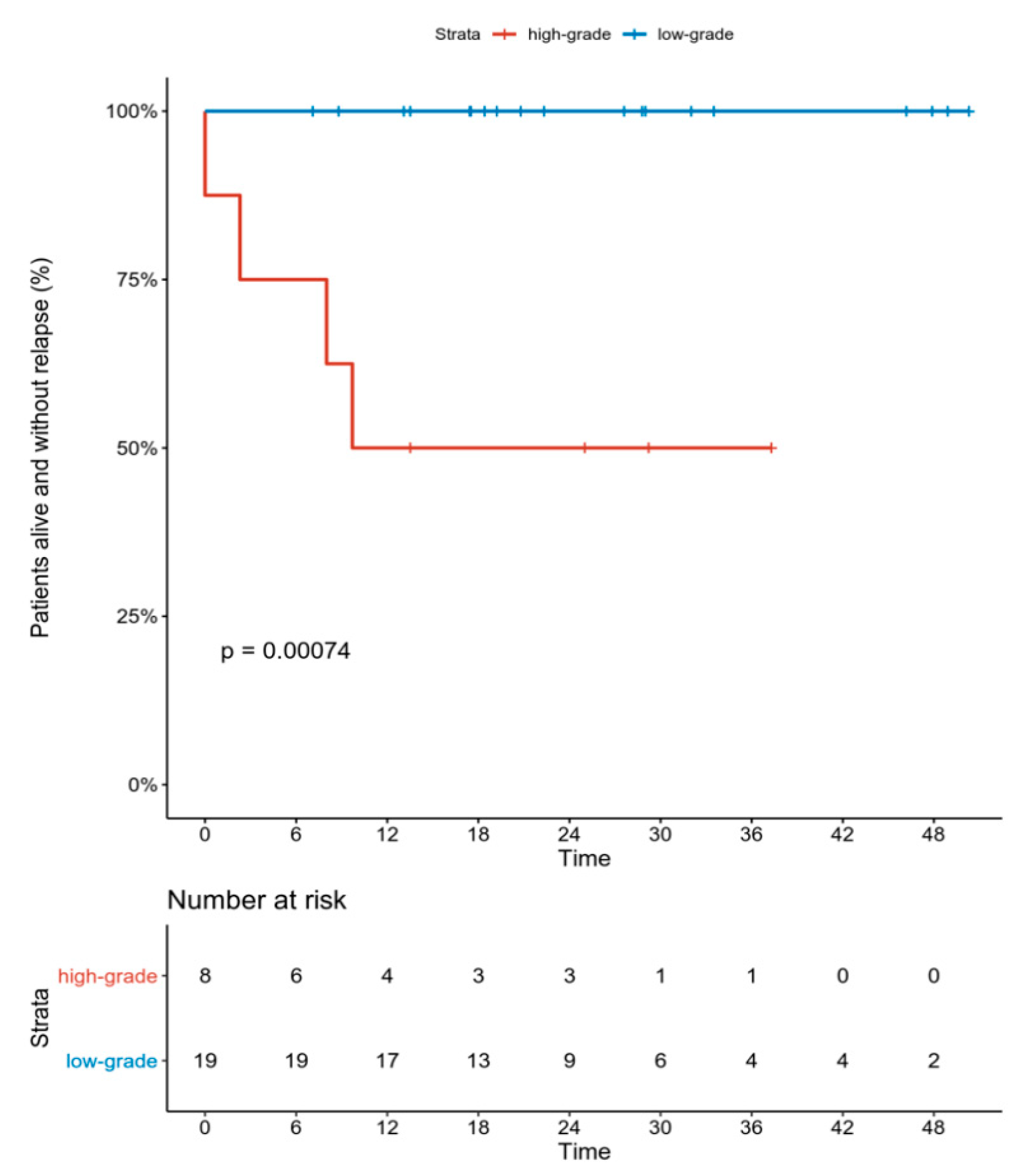
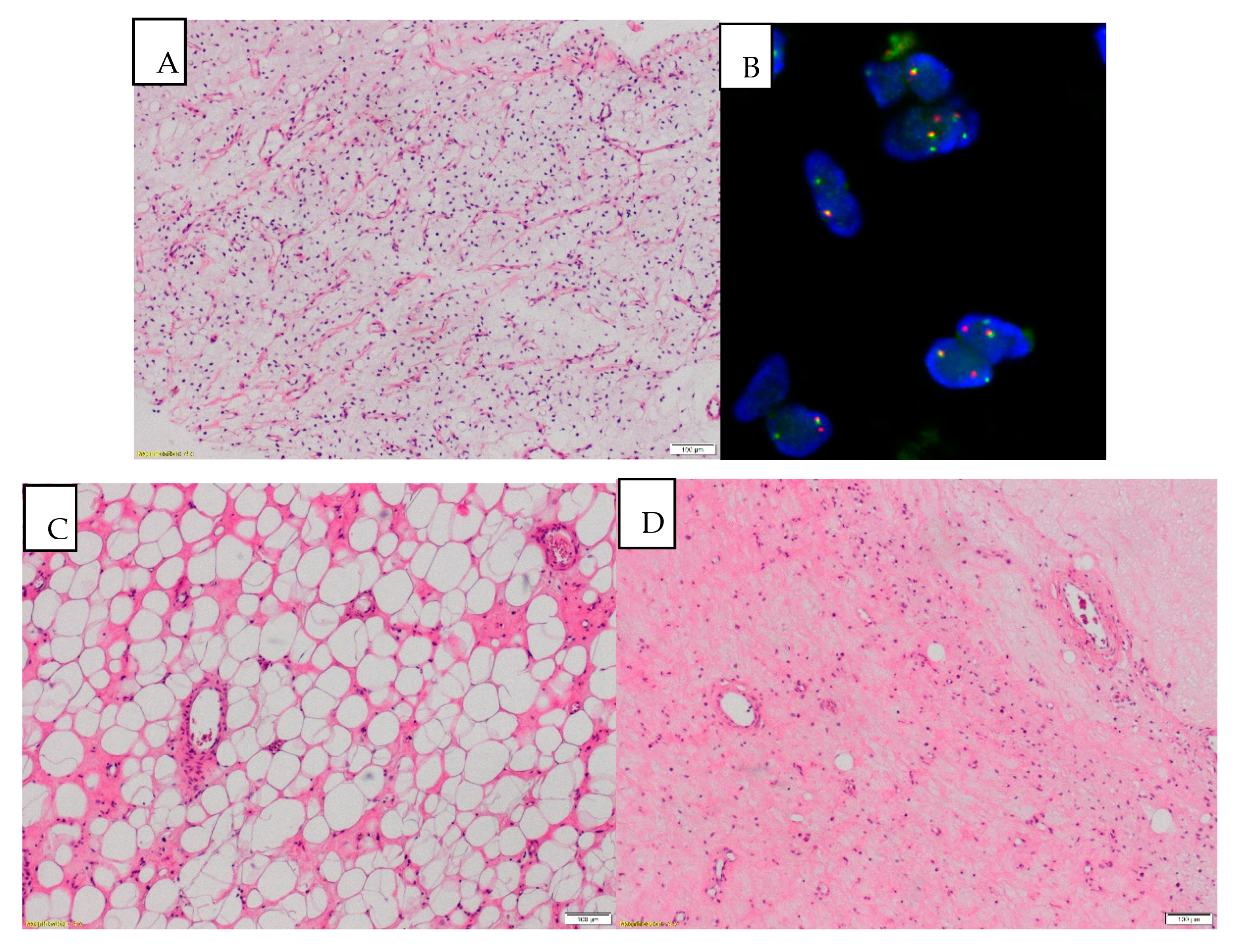
3. Results
Toxicity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sex | Age | Max Tumor Size (cm) | Tumor Grade | Surgical Margin | Radiological Response to Treatment (by RECIST) | Response to Treatment According to EORTC-STBSG Response Score | Metastases | Alive | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | M | 62 | 12 | High | R0 | SD | D | Yes | No |
| 2. | M | 38 | 20 | Low | R0 | NA | D | No | Yes |
| 3. | F | 67 | 15 | High | R0 | PR | C | No | Yes |
| 4. | F | 28 | 14 | High | R0 | PR | C | No | Yes |
| 5. | F | 60 | 13 | Low | R1 | NA | E | No | Yes |
| 6. | F | 48 | 11 | Low | R0 | PR | D | No | Yes |
| 7. | M | 67 | 13 | High | R0 | NA | C | Yes | Yes |
| 8. | F | 24 | 18 | Low | R0 | NA | C | No | Yes |
| 9. | F | 28 | 15 | Low | R0 | NA | E | No | Yes |
| 10. | F | 32 | 15 | Low | R0 | SD | E | No | Yes |
| 11. | F | 43 | 5 | Low | R0 | PR | A | No | Yes |
| 12. | M | 44 | 16 | Low | R0 | NA | D | No | Yes |
| 13. | M | 32 | 13 | Low | R1 | NA | D | No | Yes |
| 14. | F | 82 | 12 | High | R0 | NA | D | No | Yes |
| 15. | M | 31 | 20 | High | R0 | PR | C | Yes | No |
| 16. | M | 28 | 9 | Low | R0 | PR | E | No | Yes |
| 17. | F | 40 | 14 | High | R0 | NA | E | No | Yes |
| 18. | M | 50 | 5 | Low | R0 | NA | D | No | Yes |
| 19. | F | 33 | 10 | Low | R0 | NA | C | No | Yes |
| 20. | F | 64 | 9 | Low | R0 | NA | D | No | Yes |
| 21. | M | 58 | 14 | High | R0 | NA | D | No | Yes |
| 22. | F | 43 | 7 | Low | R0 | NA | C | No | Yes |
| 23. | M | 55 | 5 | Low | R0 | SD | E | No | Yes |
| 24. | M | 30 | 10 | Low | R0 | NA | E | No | Yes |
| 25. | M | 36 | 15 | Low | R0 | SD | E | No | Yes |
| 26. | M | 59 | 15 | High | R0 | SD | C | Yes | Yes |
| 27. | M | 35 | 20 | Low | R0 | PR | D | No | Yes |
References
- Kilpatrick, S.E.; Doyon, J.; Choong, P.F.; Sim, F.H.; Nascimento, A.G. The clinicopathologic spectrum of myxoid and round cell liposarcoma. A study of 95 cases. Cancer 1996, 77, 1450–1458. [Google Scholar] [CrossRef]
- Durr, H.R.; Rauh, J.; Baur-Melnyk, A.; Knosel, T.; Lindner, L.; Roeder, F.; Jansson, V.; Klein, A. Myxoid liposarcoma: Local relapse and metastatic pattern in 43 patients. BMC Cancer 2018, 18, 304. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Grosso, F.; Lo Vullo, S.; Pennacchioli, E.; Stacchiotti, S.; Ferrari, A.; Collini, P.; Lozza, L.; Mariani, L.; Casali, P.G.; et al. Myxoid/round cell and pleomorphic liposarcomas: Prognostic factors and survival in a series of patients treated at a single institution. Cancer 2007, 109, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Dei Tos, A.P. Liposarcomas: Diagnostic pitfalls and new insights. Histopathology 2014, 64, 38–52. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Tschernyavsky, S.J.; Decuseara, R.; Leung, D.H.; Woodruff, J.M.; Brennan, M.F.; Bridge, J.A.; Neff, J.R.; Goldblum, J.R.; Ladanyi, M. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: A molecular and clinicopathologic study of 82 cases. Clin. Cancer Res. 2001, 7, 3977–3987. [Google Scholar]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv268–iv269. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Haas, R.L.; Delaney, T.F.; O’Sullivan, B.; Keus, R.B.; Le Pechoux, C.; Olmi, P.; Poulsen, J.P.; Seddon, B.; Wang, D. Radiotherapy for management of extremity soft tissue sarcomas: Why, when, and where? Int. J. Radiat Oncol. Biol. Phys. 2012, 84, 572–580. [Google Scholar] [CrossRef]
- Kosela-Paterczyk, H.; Szacht, M.; Morysinski, T.; Lugowska, I.; Dziewirski, W.; Falkowski, S.; Zdzienicki, M.; Pienkowski, A.; Szamotulska, K.; Switaj, T.; et al. Preoperative hypofractionated radiotherapy in the treatment of localized soft tissue sarcomas. Eur. J. Surg. Oncol. 2014, 40, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, K.; Bergh, P.; Cederlund, C.G.; Hultborn, R.; Willen, H.; Aman, P.; Kindblom, L.G.; Meis-Kindblom, J.M. Irradiation of myxoid/round cell liposarcoma induces volume reduction and lipoma-like morphology. Acta Oncol. 2007, 46, 838–845. [Google Scholar] [CrossRef]
- Betgen, A.; Haas, R.L.; Sonke, J.J. Volume changes in soft tissue sarcomas during preoperative radiotherapy of extremities evaluated using cone-beam CT. J. Radiat. Oncol. 2013, 2, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kosela-Paterczyk, H.; Szumera-Cieckiewicz, A.; Szacht, M.; Haas, R.; Morysinski, T.; Dziewirski, W.; Prochorec-Sobieszek, M.; Rutkowski, P. Efficacy of neoadjuvant hypofractionated radiotherapy in patients with locally advanced myxoid liposarcoma. Eur. J. Surg. Oncol. 2016, 42, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Easley, K.A.; Goldblum, J.R. Myxoid/round cell liposarcoma of the extremities. A clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am. J. Surg. Pathol. 1996, 20, 171–180. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial (Ed.) Soft Tissue and Bone Tumours; IARC: Lyon, France, 2020. [Google Scholar]
- Wardelmann, E.; Haas, R.L.; Bovee, J.V.; Terrier, P.; Lazar, A.; Messiou, C.; LePechoux, C.; Hartmann, W.; Collin, F.; Fisher, C.; et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur. J. Cancer 2016, 53, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.; Harrison, L.B.; Leung, D.H.; Woodruff, J.M.; Casper, E.S.; Brennan, M.F. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J. Clin. Oncol. 1996, 14, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Chang, A.E.; Baker, A.R.; Sindelar, W.F.; Danforth, D.N.; Topalian, S.L.; DeLaney, T.; Glatstein, E.; Steinberg, S.M.; Merino, M.J.; et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998, 16, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef]
- Haas, R.L.; Gronchi, A.; van de Sande, M.A.J.; Baldini, E.H.; Gelderblom, H.; Messiou, C.; Wardelmann, E.; Le Cesne, A. Perioperative Management of Extremity Soft Tissue Sarcomas. J. Clin. Oncol. 2018, 36, 118–124. [Google Scholar] [CrossRef]
- Pitson, G.; Robinson, P.; Wilke, D.; Kandel, R.A.; White, L.; Griffin, A.M.; Bell, R.S.; Catton, C.N.; Wunder, J.S.; O’Sullivan, B. Radiation response: An additional unique signature of myxoid liposarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 522–526. [Google Scholar] [CrossRef]
- Choi, H. Role of Imaging in Response Assessment and Individualised Treatment for Sarcomas. Clin. Oncol. (R Coll. Radiol.) 2017, 29, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Colombo, C.; Pizzamiglio, S.; Verderio, P.; Callegaro, D.; Stacchiotti, S.; Martin Broto, J.; Lopez-Pousa, A.; Ferrari, S.; Poveda, A.; et al. High-risk soft tissue sarcomas treated with perioperative chemotherapy: Improving prognostic classification in a randomised clinical trial. Eur. J. Cancer 2018, 93, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Assi, T.; Kattan, J.; El Rassy, E.; Honore, C.; Dumont, S.; Mir, O.; Le Cesne, A. A comprehensive review of the current evidence for trabectedin in advanced myxoid liposarcoma. Cancer Treat. Rev. 2019, 72, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Bui, B.N.; Bonvalot, S.; Pilotti, S.; Ferrari, S.; Hohenberger, P.; Hohl, R.J.; Demetri, G.D.; Le Cesne, A.; Lardelli, P.; et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann. Oncol. 2012, 23, 771–776. [Google Scholar] [CrossRef]
- Gronchi, A.; Hindi, N.; Cruz, J.; Blay, J.Y.; Lopez-Pousa, A.; Italiano, A.; Alvarez, R.; Gutierrez, A.; Rincon, I.; Sangalli, C.; et al. Trabectedin and RAdiotherapy in Soft Tissue Sarcoma (TRASTS): Results of a Phase I Study in Myxoid Liposarcoma from Spanish (GEIS), Italian (ISG), French (FSG) Sarcoma Groups. EClinicalMedicine 2019, 9, 35–43. [Google Scholar] [CrossRef]
- Chung, P.W.; Deheshi, B.M.; Ferguson, P.C.; Wunder, J.S.; Griffin, A.M.; Catton, C.N.; Bell, R.S.; White, L.M.; Kandel, R.A.; O’Sullivan, B. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: A comparison with other soft tissue sarcomas. Cancer 2009, 115, 3254–3261. [Google Scholar] [CrossRef]
- Haas, R.L.; Miah, A.B.; LePechoux, C.; DeLaney, T.F.; Baldini, E.H.; Alektiar, K.; O’Sullivan, B. Preoperative radiotherapy for extremity soft tissue sarcoma; past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiother. Oncol. 2016, 119, 14–21. [Google Scholar] [CrossRef]
- Dei Tos, A.P.; Bonvalot, S.; Haas, R. Evolution in the management of soft tissue sarcoma: Classification, surgery and use of radiotherapy. Expert Rev. Anticancer Ther. 2020, 20 (Suppl. 1), 3–13. [Google Scholar] [CrossRef]
- Vos, M.; Blaauwgeers, H.G.T.; Ho, V.K.Y.; van Houdt, W.J.; van der Hage, J.A.; Been, L.B.; Bonenkamp, J.J.; Bemelmans, M.H.A.; van Dalen, T.; Haas, R.L.; et al. Increased survival of non low-grade and deep-seated soft tissue sarcoma after surgical management in high-volume hospitals: A nationwide study from the Netherlands. Eur. J. Cancer 2019, 110, 98–106. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Bjornlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallbook, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Kalbasi, A.; Kamrava, M.; Chu, F.I.; Telesca, D.; Van Dams, R.; Yang, Y.; Ruan, D.; Nelson, S.D.; Dry, S.M.; Hernandez, J.; et al. A Phase II Trial of 5-Day Neoadjuvant Radiotherapy for Patients with High-Risk Primary Soft Tissue Sarcoma. Clin. Cancer Res. 2020, 26, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Moreau, L.C.; Turcotte, R.; Ferguson, P.; Wunder, J.; Clarkson, P.; Masri, B.; Isler, M.; Dion, N.; Werier, J.; Ghert, M.; et al. Myxoid\round cell liposarcoma (MRCLS) revisited: An analysis of 418 primarily managed cases. Ann. Surg. Oncol. 2012, 19, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Spalek, M.; Kosela-Paterczyk, H.; Borkowska, A.W.M.; Cieszanowski, A.; Castaneda-Wysocka, P.; Switaj, T.; Dudzisz-Sledz, M.; Dabrowska-Szewczyk, E.; Rutkowski, P. Preoperative hypofractionated radiotherapy (rt) combined with chemotherapy in primary marginally resectable high grade soft tissue sarcomas (sts) of extremities or trunk wall: Interim analysis of prospective phase ii clinical trial. Ann. Oncol. 2018, 29 (Suppl. 8), viii576–viii595. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Eisenberg, B.L.; Kane, J.M.; Li, X.A.; Lucas, D.; Petersen, I.A.; DeLaney, T.F.; Freeman, C.R.; Finkelstein, S.E.; et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J. Clin. Oncol. 2015, 33, 2231–2238. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Griffin, A.M.; Dickie, C.I.; Sharpe, M.B.; Chung, P.W.; Catton, C.N.; Ferguson, P.C.; Wunder, J.S.; Deheshi, B.M.; White, L.M.; et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer 2013, 119, 1878–1884. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Brennan, M.F.; Healey, J.H.; Singer, S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J. Clin. Oncol. 2008, 26, 3440–3444. [Google Scholar] [CrossRef]

| N = 27 (%) | ||
|---|---|---|
| Sex (%) | female | 13 (48) |
| male | 14 (52) | |
| Age (median [IQR]) | 43 [32,58.5] | |
| Grade (%) | high-grade | 9 (33.3) |
| low-grade | 18 (66.6) | |
| Biopsy in NRI (%) | no | 7 (26) |
| yes | 20 (74) | |
| Size (median [IQR]) | 13 [10,15] | |
| Size (%) | T1 | 3 (11) |
| T2 | 5 (18.5) | |
| T3 | 14 (52) | |
| T4 | 5 (18.5) | |
| Necrosis in biopsy (%) | absent | 20 (74) |
| present | 5 (18.5) | |
| unknown | 2 (7.5) |
| n = 29(%) | All | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Radiation dermatitis | 12 (41.4) | 10 (34.4) | 1 (3.4) | 1 (3.4) |
| Acute toxicity (≤3 months from surgery) | 11 (37.9) * | |||
| Wound complication | 8 (29.6) | 3 (11.1) | - | |
| Wound infection requiring systemic antibiotic | 5 (18.5) | |||
| Late toxicity (>3 months from surgery) | 4 (13.8) | |||
| Lymphedema | 1 (3.4) | 1 (3.4) | - | |
| Fibrosis | 1 (3.4) | 1 (3.4) | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koseła-Paterczyk, H.; Spałek, M.; Borkowska, A.; Teterycz, P.; Wągrodzki, M.; Szumera-Ciećkiewicz, A.; Morysiński, T.; Castaneda-Wysocka, P.; Cieszanowski, A.; Zdzienicki, M.; et al. Hypofractionated Radiotherapy in Locally Advanced Myxoid Liposarcomas of Extremities or Trunk Wall: Results of a Single-Arm Prospective Clinical Trial. J. Clin. Med. 2020, 9, 2471. https://doi.org/10.3390/jcm9082471
Koseła-Paterczyk H, Spałek M, Borkowska A, Teterycz P, Wągrodzki M, Szumera-Ciećkiewicz A, Morysiński T, Castaneda-Wysocka P, Cieszanowski A, Zdzienicki M, et al. Hypofractionated Radiotherapy in Locally Advanced Myxoid Liposarcomas of Extremities or Trunk Wall: Results of a Single-Arm Prospective Clinical Trial. Journal of Clinical Medicine. 2020; 9(8):2471. https://doi.org/10.3390/jcm9082471
Chicago/Turabian StyleKoseła-Paterczyk, Hanna, Mateusz Spałek, Aneta Borkowska, Paweł Teterycz, Michał Wągrodzki, Anna Szumera-Ciećkiewicz, Tadeusz Morysiński, Patrycja Castaneda-Wysocka, Andrzej Cieszanowski, Marcin Zdzienicki, and et al. 2020. "Hypofractionated Radiotherapy in Locally Advanced Myxoid Liposarcomas of Extremities or Trunk Wall: Results of a Single-Arm Prospective Clinical Trial" Journal of Clinical Medicine 9, no. 8: 2471. https://doi.org/10.3390/jcm9082471
APA StyleKoseła-Paterczyk, H., Spałek, M., Borkowska, A., Teterycz, P., Wągrodzki, M., Szumera-Ciećkiewicz, A., Morysiński, T., Castaneda-Wysocka, P., Cieszanowski, A., Zdzienicki, M., Goryń, T., & Rutkowski, P. (2020). Hypofractionated Radiotherapy in Locally Advanced Myxoid Liposarcomas of Extremities or Trunk Wall: Results of a Single-Arm Prospective Clinical Trial. Journal of Clinical Medicine, 9(8), 2471. https://doi.org/10.3390/jcm9082471







