Differential Diagnosis and Management of Diarrhea in Patients with Neuroendocrine Tumors
Abstract
1. Introduction
2. Diarrhea: Basic Notions
2.1. Etiology Criteria
2.1.1. Secretory Diarrhea
2.1.2. Osmotic Diarrhea
2.1.3. Diarrhea Secondary to Altered Bowel Motility
2.1.4. Inflammatory Diarrhea
3. Diarrhea Associated with Peculiar Neuroendocrine Tumor Syndromes
3.1. Carcinoid Syndrome
3.2. Zollinger–Ellison Syndrome
3.3. Verner–Morrison Syndrome
3.4. Becker Syndrome (Glucagonoma)
3.5. Syndrome Associated with Somatostatin Hypersecretion (Somatostatinoma)
4. Diarrhea Not Associated with Hormone Secretion (Non-Functioning Tumors)
4.1. Diarrhea Caused by PEI
4.2. Diarrhea Caused by Bile Acid Malabsorption
4.3. Diarrhea Secondary to Short Bowel Syndrome after Extensive Small Bowel Resections
4.4. Diarrhea Associated with Antineoplastic Treatments in NETs
4.5. Other Causes of Diarrhea
5. Diagnostic Workup
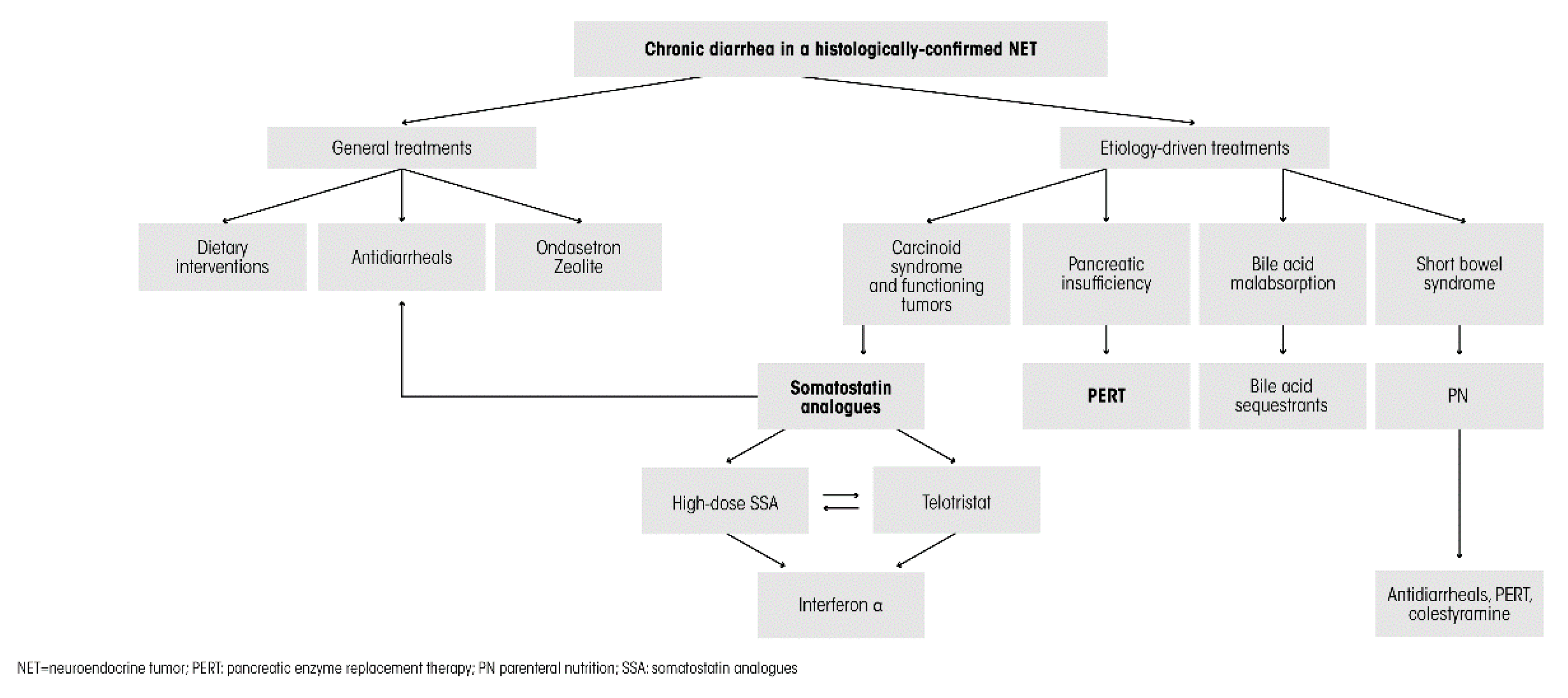
6. Management Options and Treatment Strategies
6.1. General Treatments
6.1.1. Dietary and Nutritional Intervention
6.1.2. Antidiarrheals
6.1.3. 5-HT3 Receptor Antagonists, Antihistamine–Antiserotonin Compound
6.2. Etiology-Driven Specific Treatments
6.2.1. Diarrhea Associated with Carcinoid Syndrome or other Hormonal Hypersecretion Syndromes
6.2.2. Diarrhea Caused by Bile Acid Malabsorption
6.2.3. Pancreatic Enzyme Replacement Therapy in Patient with Pancreatic Insufficiency
6.2.4. Diarrhea Secondary to Short Bowel Syndrome after Extensive Small-Bowel Resections
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naraev, B.G.; Halland, M.; Halperin, D.M.; Purvis, A.J.; O’dorisio, T.M.; Halfdanarson, T.R. Management of Diarrhea in Patients with Carcinoid Syndrome. Pancreas 2019, 48, 961–972. [Google Scholar] [CrossRef]
- Darbà, J.; Marsà, A. Exploring the current status of neuroendocrine tumours: A population-based analysis of epidemiology, management and use of resources. BMC Cancer 2019, 19, 1226. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Joish, V.N.; Perez-Olle, R.; Dharba, S.; Balaji, K.; Halperin, D.M. Nutritional complications and the management of patients with gastroenteropancreatic neuroendocrine tumors. World J. Gastroenterol. 2019, 25, 6857–6865. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.; Sundin, A.; Perren, A.; Berruti, A. ESMO Guidelines Committee Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Laing, E.; Kiss, N.; Michael, M.; Gough, K.; Krishnasamy, M.; Whyand, T.; Auer, B. Investigating Nutrition-Related Complications and Quality of Life in Patients With Gastroenteropancreatic Neuroendocrine Tumors: Protocol for a Mixed-Methods Prospective Study. JMIR Res. Protoc. 2018, 7, e11228. [Google Scholar] [CrossRef] [PubMed]
- Zandee, W.; Kamp, K.; Van Adrichem, R.C.; Feelders, R.A.; De Herder, W.W. Effect of hormone secretory syndromes on neuroendocrine tumor prognosis. Endocrine-Related Cancer 2017, 24, R261–R274. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.; Feliberti, E.; Perry, R.R. Pancreatic Polypeptide (PPoma). In Endotext; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Arasaradnam, R.P.; Brown, S.; Forbes, A.; Fox, M.R.; Hungin, P.; Kelman, L.; Major, G.; O’Connor, M.; Sanders, D.S.; Sinha, R.; et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut 2018, 67, 1380–1399. [Google Scholar] [CrossRef]
- Nemeth, V.; Zulfiqar, H.; Pfleghaar, N. Diarrhea. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Sandhu, D.K.; Surawicz, C. Update on Chronic Diarrhea: A Run-Through for the Clinician. Curr. Gastroenterol. Rep. 2012, 14, 421–427. [Google Scholar] [CrossRef]
- Gallo, M.; Muscogiuri, G.; Pizza, G.; Ruggeri, R.M.; Barrea, L.; Faggiano, A.; Colao, A.; on behalf of NIKE Group. The management of neuroendocrine tumours: A nutritional viewpoint. Crit. Rev. Food Sci. Nutr. 2017, 59, 1046–1057. [Google Scholar] [CrossRef]
- Raman, M. Testing for Chronic Diarrhea. Adv. Clin. Chem. 2017, 79, 199–244. [Google Scholar] [CrossRef]
- Schiller, L.R.; Pardi, D.S.; Sellin, J.H. Chronic Diarrhea: Diagnosis and Management. Clin. Gastroenterol. Hepatol. 2017, 15, 182–193.e3. [Google Scholar] [CrossRef] [PubMed]
- Juckett, G.; Trivedi, R. Evaluation of chronic diarrhea. Am. Fam. Physician 2011, 84, 1119–1126. [Google Scholar] [PubMed]
- Burgers, K.; Lindberg, B.; Bevis, Z.J. Chronic Diarrhea in Adults: Evaluation and Differential Diagnosis. Am. Fam. Physician 2020, 101, 472–480. [Google Scholar] [PubMed]
- Woods, T.A. Diarrhea. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Sweetser, S. Evaluating the Patient with Diarrhea: A Case-Based Approach. Mayo Clin. Proc. 2012, 87, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Koumarianou, A.; Alexandraki, K.I.; Wallin, G.; Kaltsas, G.; Daskalakis, K. Pathogenesis and Clinical Management of Mesenteric Fibrosis in Small Intestinal Neuroendocine Neoplasms: A Systematic Review. J. Clin. Med. 2020, 9, 1777. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Salazar-Lindo, E.; León-Barúa, R. Differentiation of osmotic and secretory diarrhoea by stool carbohydrate and osmolar gap measurements. Arch. Dis. Child. 1997, 77, 201–205. [Google Scholar] [CrossRef]
- Wang, R.; Zheng-Pywell, R.; Chen, H.A.; Bibb, J.A.; Chen, H.; Rose, J.B. Management of Gastrointestinal Neuroendocrine Tumors. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419884058. [Google Scholar] [CrossRef]
- Clement, D.S.V.M.; Tesselaar, M.E.T.; Van Leerdam, M.E.; Srirajaskanthan, R.; Ramage, J. Nutritional and vitamin status in patients with neuroendocrine neoplasms. World J. Gastroenterol. 2019, 25, 1171–1184. [Google Scholar] [CrossRef]
- Boudreaux, J.P.; Klimstra, D.S.; Hassan, M.M.; Woltering, E.A.; Jensen, R.T.; Goldsmith, S.J.; Nutting, C.; Bushnell, D.L.; Caplin, M.E.; Yao, J.C. The NANETS Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors. Pancreas 2010, 39, 753–766. [Google Scholar] [CrossRef]
- Norton, J.A. Gastrinomas: Medical or surgical treatment. Endocrinol. Metab. Clin. North 2018, 47, 577–601. [Google Scholar] [CrossRef]
- Linee Guida AIOM 2019. Neoplasie Neuroendocrine. Available online: www.aiom.it>uploads>2019_LG_AIOM_Neuroendocrini (accessed on 25 October 2019).
- Ito, T.; Igarashi, H.; Jensen, R.T. Pancreatic neuroendocrine tumors: Clinical features, diagnosis and medical treatment: Advances. Best Pr. Res. Clin. Gastroenterol. 2012, 26, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, G.; Besser, G.M.; Grossman, A.B. The Diagnosis and Medical Management of Advanced Neuroendocrine Tumors. Endocr. Rev. 2004, 25, 458–511. [Google Scholar] [CrossRef]
- Guarnotta, V.; Martini, C.; Davì, M.V.; Pizza, G.; Colao, A.; Faggiano, A. The Zollinger-Ellison syndrome: Is there a role for somatostatin analogues in the treatment of the gastrinoma? Endocrine 2017, 60, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Lee, L.; Jensen, R.T. Treatment of symptomatic neuroendocrine tumor syndromes: Recent advances and controversies. Expert Opin. Pharmacother. 2016, 17, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Venzon, D.J.; Shojamanesh, H.; Abou-Saif, A.; Peghini, P.; Doppman, J.L.; Gibril, F.; Jensen, R.T. Zollinger-Ellison Syndrome: Clinical Presentation in 261 Patients. Medicine 2000, 79, 379–411. [Google Scholar] [CrossRef]
- Sandhu, S.; Jialal, I. ViPoma; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Capurso, G.; Traini, M.; Piciucchi, M.; Signoretti, M.; Arcidiacono, P.G. Exocrine pancreatic insufficiency: Prevalence, diagnosis, and management. Clin. Exp. Gastroenterol. 2019, 12, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Haupt, M.E.; Geller, D.E.; Hall, J.A.; Diez, P.M.Q. Less common etiologies of exocrine pancreatic insufficiency. World J. Gastroenterol. 2017, 23, 7059–7076. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Domínguez-Muñoz, J.E.; Layer, P.; Lerch, M.M. Pancreatic Exocrine Insufficiency as a Complication of Gastrointestinal Surgery and the Impact of Pancreatic Enzyme Replacement Therapy. Dig. Dis. 2019, 38, 53–68. [Google Scholar] [CrossRef]
- Brennan, G.T.; Saif, M.W. Pancreatic enzyme replacement therapy: A concise review. JOP 2019, 20, 121–125. [Google Scholar]
- Domínguez-Muñoz, J.E.; Nieto-García, L.; Díaz, J.L.; Lariño-Noia, J.; Abdulkader, I.; Iglesias-Garcia, J. Impact of the treatment of pancreatic exocrine insufficiency on survival of patients with unresectable pancreatic cancer: A retrospective analysis. BMC Cancer 2018, 18, 534. [Google Scholar] [CrossRef]
- Ii, J.G.L.; Draganov, P.V. Pancreatic function testing: Here to stay for the 21st century. World J. Gastroenterol. 2008, 14, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; Grace, F.; Kilgore, L.; Smith, D.J.; Norris, S.R.; Gardner, A.W.; Ringseis, R.; Eder, K.; Shephard, R.J.; Kokkinos, P.; et al. Pancreatic Insufficiency; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; p. 690. [Google Scholar]
- Andreasi, V.; Partelli, S.; Capurso, G.; Muffatti, F.; Balzano, G.; Crippa, S.; Falconi, M. Long-Term Pancreatic Functional Impairment after Surgery for Neuroendocrine Neoplasms. J. Clin. Med. 2019, 8, 1611. [Google Scholar] [CrossRef] [PubMed]
- Rinzivillo, M.; De Felice, I.; Magi, L.; Annibale, B.; Panzuto, F. Occurrence of exocrine pancreatic insufficiency in patients with advanced neuroendocrine tumors treated with somatostatin analogs. Pancreatology 2020, S1424–S3903. [Google Scholar] [CrossRef] [PubMed]
- Sagar, V.M.; Cooper, S.C.; Johnson, J.; Shetty, S.; Shah, T. Gastrointestinal manifestations of neuroendocrine tumours: Their investigation and management. Postgrad. Med. J. 2017, 93, 494–497. [Google Scholar] [CrossRef]
- Valentin, N.; Camilleri, M.; Altayar, O.; Vijayvargiya, P.; Acosta, A.; Nelson, A.D.; Murad, M.H. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: A systematic review and meta-analysis. Gut 2015, 65, 1951–1959. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Hörsch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; De Vries, E.G.E.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.W.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Phan, A.T.; Halperin, D.M.; Chan, J.A.; Fogelman, D.R.; Hess, K.R.; Malinowski, P.; Regan, E.; Ng, C.S.; Yao, J.C.; Kulke, M.H. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: A multicentre, single-group, phase 2 study. Lancet Oncol. 2015, 16, 695–703. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Caccaro, R.; D’Incà, R.; Pathak, S.; Sturniolo, G.C. Clinical utility of calprotectin and lactoferrin in patients with inflammatory bowel disease: Is there something new from the literature? Expert Rev. Clin. Immunol. 2012, 8, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Fine, K.D.; Seidel, R.H.; Do, K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest. Endosc. 2000, 51, 318–326. [Google Scholar] [CrossRef]
- Shah, R.J.; Fenoglio-Preiser, C.; Bleau, B.L.; Giannella, R.A. Usefulness of colonoscopy with biopsy in the evaluation of patients with chronic diarrhea. Am. J. Gastroenterol. 2001, 96, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Schiller, L.R. Chronic diarrhea: To biopsy or not to biopsy. Gastrointest. Endosc. 2005, 61, 376–377. [Google Scholar] [CrossRef]
- Daher, R.; Yazbeck, T.; Jaoude, J.B.; Abboud, B. Consequences of dysthyroidism on the digestive tract and viscera. World J. Gastroenterol. 2009, 15, 2834–2838. [Google Scholar] [CrossRef]
- Abraham, B.P.; Sellin, J.H. Drug-induced, factitious, and idiopathic diarrhoea. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 633–648. [Google Scholar] [CrossRef]
- Chassany, O.; Michaux, A.; Bergmann, J.-F. Drug-Induced Diarrhoea. Drug Saf. 2000, 22, 53–72. [Google Scholar] [CrossRef]
- Langbein, T.; Dathe, W.; Deuerling, A.; Baum, R.P. Efficacy of Detoxsan® powder on diarrhea caused by gastrointestinal neuroendocrine tumors. World J. Gastroenterol. 2019, 25, 2133–2143. [Google Scholar] [CrossRef]
- Pusceddu, S.; Prinzi, N.; Raimondi, A.; Corti, F.; Buzzoni, R.; De Braud, F.G.M.; Seregni, E.; Maccauro, M.; Coppa, J.; Milione, M.; et al. Entering the third decade of experience with octreotide LAR in neuroendocrine tumors: A review of current knowledge. Tumori J. 2018, 105, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Faggiano, A.; Brighi, N.; Tafuto, S.; Ibrahim, T.; Brizzi, M.P.; Pusceddu, S.; Albertelli, M.; Massironi, S.; Panzuto, F.; et al. Nonconventional Doses of Somatostatin Analogs in Patients With Progressing Well-Differentiated Neuroendocrine Tumor. J. Clin. Endocrinol. Metab. 2019, 105, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.A.; Wolin, E.M.; Liyanage, N.; Lowenthal, S.P.; Mirakhur, B.; Pommier, R.F.; Shaheen, M.; Vinik, A.; ELECT Study Group. Patient-Reported Symptom Control of Diarrhea and Flushing in Patients with Neuroendocrine Tumors Treated with Lanreotide Depot/Autogel: Results from a Randomized, Placebo-Controlled, Double-Blind and 32-Week Open-Label Study. Oncologist 2017, 23, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kvols, L.K.; Moertel, C.G.; O’Connell, M.J.; Schutt, A.J.; Rubin, J.; Hahn, R.G. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N. Engl. J. Med. 1986, 315, 663–666. [Google Scholar] [CrossRef]
- Rubin, J.; Ajani, J.; Schirmer, W.; Venook, A.P.; Bukowski, R.; Pommier, R.; Saltz, L.B.; Dandona, P.; Anthony, L. Octreotide Acetate Long-Acting Formulation Versus Open-Label Subcutaneous Octreotide Acetate in Malignant Carcinoid Syndrome. J. Clin. Oncol. 1999, 17, 600. [Google Scholar] [CrossRef]
- Anthony, L.; Vinik, A.I. Evaluating the Characteristics and the Management of Patients with Neuroendocrine Tumors Receiving Octreotide LAR During a 6-Year Period. Pancreas 2011, 40, 987–994. [Google Scholar] [CrossRef]
- Weber, J.M.; Feldman, M.; Kvols, L.; Strosberg, J.R. Above-label doses of octreotide-LAR in patients with metastatic small-intestinal carcinoid tumors. J. Clin. Oncol. 2012, 30, e14579. [Google Scholar] [CrossRef]
- Fisher, G.A.; Wolin, E.M.; Liyanage, N.; Lowenthal, S.P.; Mirakhur, B.; Pommier, R.F.; Shaheen, M.; Vinik, A.I. ELECT Study Investigators lanreotide therapy in carcinoid syndrome: Prospective analysis of patient-reported symptoms in patients responsive to prior octreotide therapy and patients naïve to somatostatin analogue therapy in the elect phase 3 study. Endocr. Pr. 2018, 24, 243–255. [Google Scholar] [CrossRef]
- O’Toole, D.; Ducreux, M.; Bommelaer, G.; Wemeau, J.L.; Bouche, O.; Catus, F.; Blumberg, J.; Ruszniewski, P. Treatment of carcinoid syndrome: A prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer 2000, 88, 770–776. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Benson, A.B.; Huynh, L.; Duh, M.S.; Goldman, J.; Sahai, V.; Rademaker, A.W.; Kulke, M.H. Clinical Benefits of Above-Standard Dose of Octreotide LAR in Patients with Neuroendocrine Tumors for Control of Carcinoid Syndrome Symptoms: A Multicenter Retrospective Chart Review Study. Oncologist 2014, 19, 930–936. [Google Scholar] [CrossRef]
- Al-Efraij, K.; Aljama, M.A.; Kennecke, H. Association of dose escalation of octreotide long-acting release on clinical symptoms and tumor markers and response among patients with neuroendocrine tumors. Cancer Med. 2015, 4, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Wolin, E.M.; Jarzab, B.; Eriksson, B.; Walter, T.; Toumpanakis, C.; Morse, M.A.; Tomassetti, P.; Weber, M.M.; Fogelman, D.R.; Ramage, J.; et al. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des. Dev. Ther. 2015, 9, 5075–5086. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and Safety Study in Pancreatic or Midgut Neuroendocrine Tumours Having Progressed Radiologically While Previously Treated With Lanreotide Autogel® 120 mg (CLARINET FORTE). Available online: https://clinicaltrials.gov/ct2/show/study/NCT02651987?term=NCT02651987&draw=2&rank=1 (accessed on 28 July 2020).
- Kulke, M.H.; Hörsch, D.; Caplin, M.E.; Anthony, L.B.; Bergsland, E.; Öberg, K.; Welin, S.; Warner, R.R.; Lombard-Bohas, C.; Kunz, P.L.; et al. Telotristat Ethyl, a Tryptophan Hydroxylase Inhibitor for the Treatment of Carcinoid Syndrome. J. Clin. Oncol. 2017, 35, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Gross, D.J.; Benavent, M.; Perros, P.; Srirajaskanthan, R.; Warner, R.R.P.; Kulke, M.H.; Anthony, L.B.; Kunz, P.L.; Horsch, D.; et al. Telotristat ethyl in carcinoid syndrome: Safety and efficacy in the TELECAST phase 3 trial. Endocrine-Related Cancer 2018, 25, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.S.; Kulke, M.H.; Hörsch, D.; Anthony, L.B.; Warner, R.R.P.; Bergsland, E.; Welin, S.; O’Dorisio, T.M.; Kunz, P.L.; McKee, C.; et al. Time to Sustained Improvement in Bowel Movement Frequency with Telotristat Ethyl: Analyses of Phase III Studies in Carcinoid Syndrome. J. Gastrointest. Cancer 2020, 1–10. [Google Scholar] [CrossRef]
- Molina-Cerrillo, J.; Alonso-Gordoa, T.; Martínez-Sáez, O.; Grande, E. Inhibition of Peripheral Synthesis of Serotonin as a New Target in Neuroendocrine Tumors. Oncologist 2016, 21, 701–707. [Google Scholar] [CrossRef]
- Strosberg, J. Gastroenteropancreatic Neuroendocrine Tumors. Am. Cancer Soc. Oncol. Pract. 2018, 68, 552–570. [Google Scholar] [CrossRef]
- Roberts, K.; Bannister, C.; Schrem, H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology 2019, 19, 114–121. [Google Scholar] [CrossRef]
- Roberts, K.J.; Schrem, H.; Hodson, J.; Angelico, R.; Dasari, B.V.M.; Coldham, C.A.; Marudanayagam, R.; Sutcliffe, R.P.; Muiesan, P.; Isaac, J.; et al. Pancreas exocrine replacement therapy is associated with increased survival following pancreatoduodenectomy for periampullary malignancy. HPB 2017, 19, 859–867. [Google Scholar] [CrossRef]
- Landers, A.; Brown, H.; Strother, M. The effectiveness of pancreatic enzyme replacement therapy for malabsorption in advanced pancreatic cancer, a pilot study. Palliat. Care Res. Treat. 2019, 12, 1178224218825270. [Google Scholar] [CrossRef]
- Pezzilli, R.; Caccialanza, R.; Capurso, G.; Brunetti, O.; Milella, M.; Falconi, M. Pancreatic Enzyme Replacement Therapy in Pancreatic Cancer. Cancers 2020, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.M.; Izbicki, J.; Varga-Szabó, L.; Czako, L.; Fiók, J.; Sperti, C.; Lerch, M.M.; Pezzilli, R.; Vasileva, G.; Pap, A.; et al. Randomised clinical trial: A 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment. Pharmacol. Ther. 2013, 37, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Thorat, V.; Reddy, N.; Bhatia, S.; Bapaye, A.; Rajkumar, J.S.; Kini, D.D.; Kalla, M.M.; Ramesh, H. Randomized clinical trial: The efficacy and safety of pancreatin enteric-coated minimicrospheres (Creon 40000 MMS) in patients with PEI due to chronic pancreatitis—A double-blind, placebo-controlled study. Aliment Pharmacol. Ther. 2012, 36, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Rausa, E.; Invernizzi, P.; Braga, M.; Vecchi, M. Understanding short bowel syndrome: Current status and future perspectives. Dig. Liver Dis. 2020, 52, 253–261. [Google Scholar] [CrossRef]
- Pironi, L.; Steiger, E.; Brandt, C.; Joly, F.; Wanten, G.; Chambrier, C.; Aimasso, U.; Sasdelli, A.S.; Zeraschi, S.; Kelly, D.; et al. Home parenteral nutrition provision modalities for chronic intestinal failure in adult patients: An international survey. Clin. Nutr. 2020, 39, 585–591. [Google Scholar] [CrossRef]
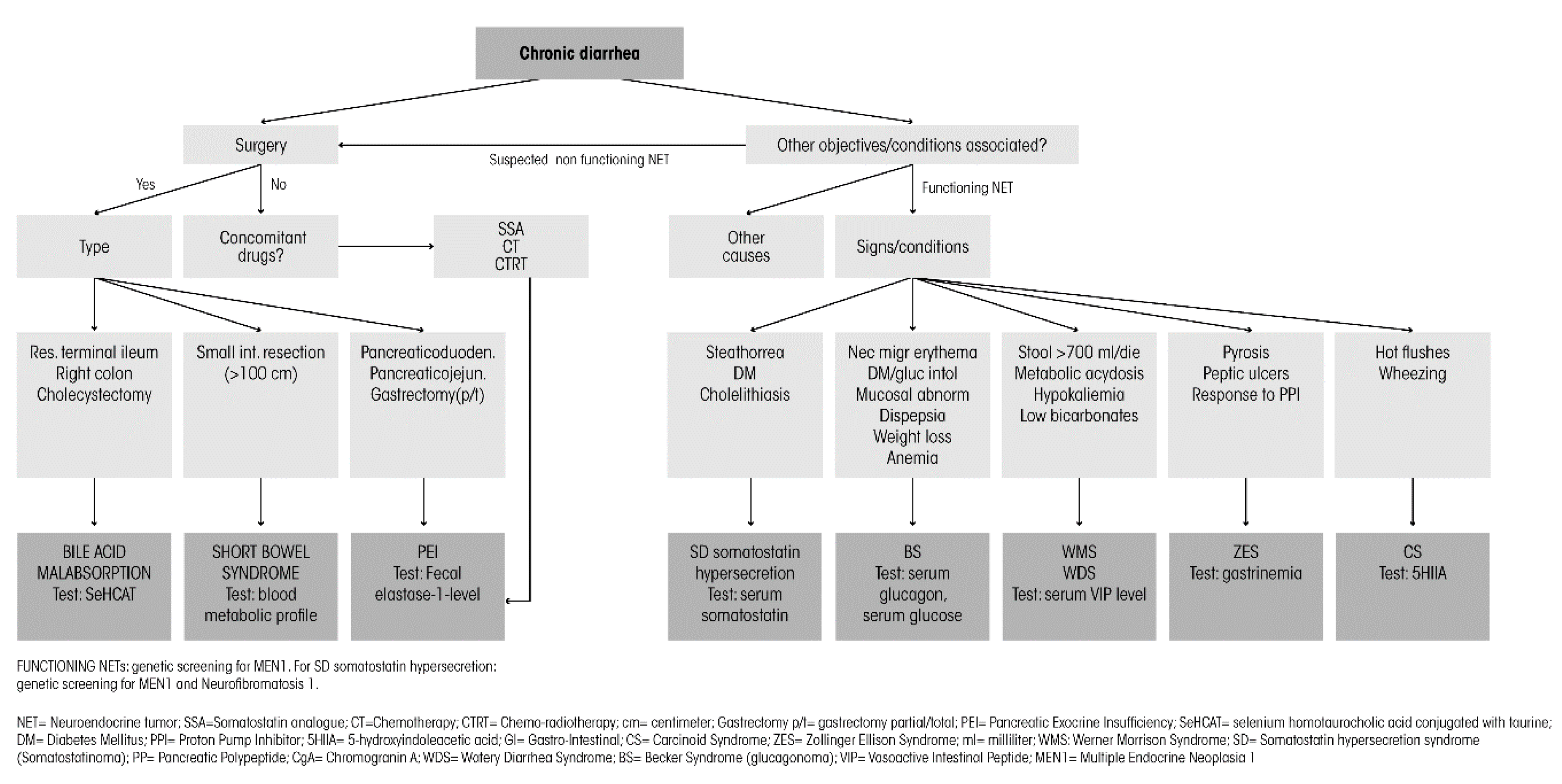
| Syndrome | Signs and Symptoms |
|---|---|
| Carcinoid syndrome | Diarrhea (mild or severe, usually after meals), hot flushes and wheezing, less frequently, heart failure, vomiting and bronchoconstriction [21] |
| Zollinger–Ellison syndrome | Severe peptic ulcer disease, pyrosis and recurrent diarrhea with watery or oily stools, responsive to PPI [23,24] |
| Verner–Morrison syndrome | Persistent watery diarrhea (after a 48 h fast) associated with depletion of liquids and electrolytes, stool amount overcomes 700 mL per day, metabolic acidosis through bicarbonate depletion, hypokalemia [6] |
| Becker syndrome (glucagonoma) | Weight loss [25], anemia [6], typical skin lesions named necrolytic migratory erythema, diabetes mellitus or glucose intolerance, secretory diarrhea, cheilitis, glossitis, stomatitis, hypoaminoacidemia [25] and dyspepsia [24] |
| Syndrome associated with somatostatin hypersecretion (somatostatinoma) | Diabetes mellitus, diarrhea, steatorrhea, and cholelithiasis [26] |
| Disease | PEI Prevalence (%) | Factors Associated with PEI Occurrence |
|---|---|---|
| PEI Caused by Pancreatic Disorders | ||
| Chronic pancreatitis | 30–90 | Long disease duration Alcoholic etiology Extensive calcification Ductal obstruction |
| Acute pancreatitis | Mild: 15–20 Severe: 30–40 | Necrosis extent (>30) Alcoholic etiology |
| Autoimmune pancreatitis | 30–60 | |
| Unresectable pancreatic cancer | 20–60 | Head localization Large site Ductal obstruction Coexistent chronic pancreatitis |
| Pancreatic neoplasm after surgery | Pancreaticoduodenectomy: 80–90 Distal pancreatectomy: 20–50 | Whipple intervention Gastropancreatic anastomosis |
| Benign pancreatic tumor (before surgery) | 30–60 | Head localization Large size Ductal obstruction Coexistent chronic pancreatitis |
| Cystic fibrosis | 80–90 | Class I, II, III, IV CTFR mutations |
| Scwachman–Diamond syndrome | 80–90 | |
| PEI Caused by Extrahepatic Disorders | ||
| Type I diabetes | 30–50 | High insulin requirement Poor glycemic control Early diabetes onset |
| Type II diabetes | 20–30 | Insulin requirement Poor glycemic control Long diabetes duration |
| Inflammatory bowel disease | Ulcerative colitis: 10 Crohn’s disease: 4 | Disease reactivation (only for temporary PEI) Long disease duration Surgical patients |
| Celiac disease | 5–90 | Untreated disease (no gluten-free diet) |
| Pediatric intestinal transplantation | 10 | |
| HIV syndrome | 10–50 | Retroviral therapy |
| Gastrointestinal surgery | Total/subtotal gastrectomy: 40–90 | Extensive intestinal resection |
| Esophagectomy | 16 | Vagal denervation |
| Sjogren’s syndrome | 10–30 | |
| Aging | 15–30 | Age > 80 years |
| Tobacco usage | 10–20 | Alcohol usage |
| Somatostatin analogs therapy | 20 | |
| Surgery | Effect of Surgery | |||
|---|---|---|---|---|
| Decreased Neural Stimulation of Pancreatic Secretion | Decreased Hormonal Stimulation of Pancreatic Secretion | Postcibal Asynchrony | Loss of Pancreatic Tissue | |
| Pancreaticoduodenectomy | Present | Present | Present | Present |
| Pancreaticojejunostomy | Present | Present | Present | Absent |
| Gastrectomy | Present | Present | Present | Absent |
| Partial gastrectomy | Present | Present | Present | Absent |
| Therapeutic Drug | Percentage of Patients with Diarrhea as AE in the Different Arms | RCT | Patient Number | Reference |
|---|---|---|---|---|
| Everolimus (10 mg/day) + octreotide depot vs. placebo plus octreotide depot | All grades 27% vs. 16% | Randomized, Double-blind Placebo-controlled, Multicenter Phase 3 Study in Patients With Advanced Carcinoid Tumor (RADIANT-2) (NCT00412061) | 429 | [42] |
| Everolimus (RAD001 10 mg/day) + best supportive care vs. placebo + best supportive care | All grades 34% vs. 10% | Randomized Double-blind Phase 3 Study in Patients With Advanced NET (RADIANT-3) (NCT00510068) | 410 | [43] |
| Everolimus (RAD001) + best supportive care vs. placebo + best supportive care | All grades 31% vs. 16% | Randomized, Double-blind, Multicenter, Phase 3 Study of in Patients With Advanced NET (Gastrointestinal (GI) or Lung Origin) (RADIANT-4) (NCT01524783) | 302 | [44] |
| Sunitinib (SU011248, SUTENT) vs. placebo | All grades 59% vs. 39% | Phase 3, Randomized, Double-Blind Study In Patients With Progressive Advanced/Metastatic Well-Differentiated Pancreatic Islet Cell Tumors (NCT00428597) | 171 | [45] |
| Octreotide LAR (long-acting release) 30 mg vs. placebo | All grades 22% vs. 28% | Placebo-controlled, double-blind, prospective, randomized Phase 3 Study on Antiproliferative Effect of Octreotide in Patients With Metastasized Neuroendocrine Tumors of the Midgut (NCT00171873) | 85 | [46] |
| Pazopanib (GW786034) | All grades 63% | Open Label Phase 2 Study in Advanced Low-Grade or Intermediate-Grade Neuroendocrine Carcinoma | 52 | [47] |
| Treatment | Diarrhea Outcome | Other Outcomes | Type of Study | References |
|---|---|---|---|---|
| Octreotide (150 μg three times daily) | Rapid palliation in 88% of patients | Reduction in 5-HIAA levels in 72% of patients | Single arm trial | [60] |
| Intramuscular octreotide LAR (10, 20, or 30 mg every 4 weeks) + subcutaneous octreotide (every 8 h) | Best control of flushing with 20 mg and subcutaneous treatments | Randomized phase 3 trial | [61] | |
| Octreotide LAR depot 30 mg, 20 m, and 40 mg for at least 4 months | Diarrhea improvement in 48% of patients | Flushing improvement in 60% of patients | Retrospective study | [62] |
| Octreotide LAR (doses equal or higher than 30 mg every 4 weeks) | Diarrhea improvement in 62% of patients | Flushing improvement in 56% of patients | Retrospective chart-review | [63] |
| Lanreotide depot vs. placebo | Reductions of days patients reported symptoms: 16 weeks and sustained over 32 weeks | Reductions of days patients reported symptoms of flushing: 16 weeks and sustained over 32 weeks | Double blind, randomized placebo controlled phase 3 clinical trial | [64] |
| Octreotide 200µg 2/3 times daily for 1 month followed by lanreotide 30 mg every 10 days for 1 month and vice versa | Disappearance or improvement of diarrhea in 45.4% of patients on lanreotide and 50% of patients on octreotide | Disappearance or improvement in flushes in 53.8% of patients on lanreotide and in 68% on octreotide; reduced urinary 5-HIAA levels with both treatments | Prospective, open, multicenter, crossover study | [65] |
| Octreotide LAR above 30, 40 or 60 mg every 4 weeks | Improvement or resolution of diarrhea in 79% of patients after dose escalation | Improvement or resolution of flushing in 81% of patients after dose escalation | Multicenter retrospective chart review study | [66] |
| Octreotide LAR > 30 mg | Decrease in diarrhea in 62% of patients | Decrease in flushing in 91% of patients; Reduction in 5-HIAA levels in 23% of the patients | Retrospective review | [67] |
| Pasireotide LAR (60 mg) vs. octreotide LAR (40 mg) | Diarrhea (all grades) 6% with pasireotide LAR Diarrhea (all grades) 2% with octreotide LAR | 42% of flushing events after 6 months with pasireotide LAR 49% of flushing episodes with octreotide LAR | Randomized, double-blind, phase 3 study | [68] |
| Lanreotide 120 mg vs. Placebo every 4 weeks, followed by 32 weeks’ initial open label lanreotide | Days with moderate/severe diarrhea and/or flushing with lanreotide 23.4% vs. placebo 35.8%; diarrhea scores improved between double-blind and initial open label treatment | Randomized, placebo-controlled, double-blind and 32 week open-label study | [59] | |
| Lanreotide Autogel® (120 mg every 14 days) | − | − | Open label, phase 2 | NCT02651987 [69] |
| High doses of SSAs | Adverse events 15% | Retrospective analysis | [58] | |
| High doses of octreotide LAR | Uncommon treatment discontinuations due to adverse events; cholelithiasis, may increase with longer duration of treatment | Review | [57] | |
| Telotristat etiprate 250 mg or 500 mg three times/day +SSAs vs. placebo + SSAs | ≥30% bowel movement frequency reduction in ≥50% of patients (20%, 44%, and 42% for placebo, telotristat 250 mg and 500 mg, respectively); reduced mean urinary 5HIAA (both treatments) vs. placebo at week 12 (p < 0.001) | Randomized, placebo-controlled, parallel group, multicenter, double-blind, phase 3 Study (TELESTAR) | [70] | |
| Telotristat etiprate 250 mg or 500 mg three times/daily + SSAs vs. placebo + SSAs | 19%, 16% and 8% diarrhea for placebo, telotristat 250 mg and 500 mg, respectively | Reductions in 5-HIAA (−54.0% and −89.7% median difference from placebo for telotristat 250 mg and 500 mg, respectively) | Randomized, placebo-controlled, multicenter, double-blind, phase 3 Study (TELECAST) | [71] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusceddu, S.; Rossi, R.E.; Torchio, M.; Prinzi, N.; Niger, M.; Coppa, J.; Giacomelli, L.; Sacco, R.; Facciorusso, A.; Corti, F.; et al. Differential Diagnosis and Management of Diarrhea in Patients with Neuroendocrine Tumors. J. Clin. Med. 2020, 9, 2468. https://doi.org/10.3390/jcm9082468
Pusceddu S, Rossi RE, Torchio M, Prinzi N, Niger M, Coppa J, Giacomelli L, Sacco R, Facciorusso A, Corti F, et al. Differential Diagnosis and Management of Diarrhea in Patients with Neuroendocrine Tumors. Journal of Clinical Medicine. 2020; 9(8):2468. https://doi.org/10.3390/jcm9082468
Chicago/Turabian StylePusceddu, Sara, Roberta Elisa Rossi, Martina Torchio, Natalie Prinzi, Monica Niger, Jorgelina Coppa, Luca Giacomelli, Rodolfo Sacco, Antonio Facciorusso, Francesca Corti, and et al. 2020. "Differential Diagnosis and Management of Diarrhea in Patients with Neuroendocrine Tumors" Journal of Clinical Medicine 9, no. 8: 2468. https://doi.org/10.3390/jcm9082468
APA StylePusceddu, S., Rossi, R. E., Torchio, M., Prinzi, N., Niger, M., Coppa, J., Giacomelli, L., Sacco, R., Facciorusso, A., Corti, F., Raimondi, A., Prisciandaro, M., Colombo, E., Beninato, T., Del Vecchio, M., Milione, M., Di Bartolomeo, M., & de Braud, F. (2020). Differential Diagnosis and Management of Diarrhea in Patients with Neuroendocrine Tumors. Journal of Clinical Medicine, 9(8), 2468. https://doi.org/10.3390/jcm9082468











