Long-Term Evolution of Quality of Life and Symptoms Following Surgical Treatment for Endometriosis: Different Trajectories for Which Patients?
Abstract
:1. Introduction
2. Methods
2.1. Study Approval
2.2. Patients and Methods
2.3. Surgical Technique
2.4. Statistical Analyses
3. Results
3.1. Description of the Population
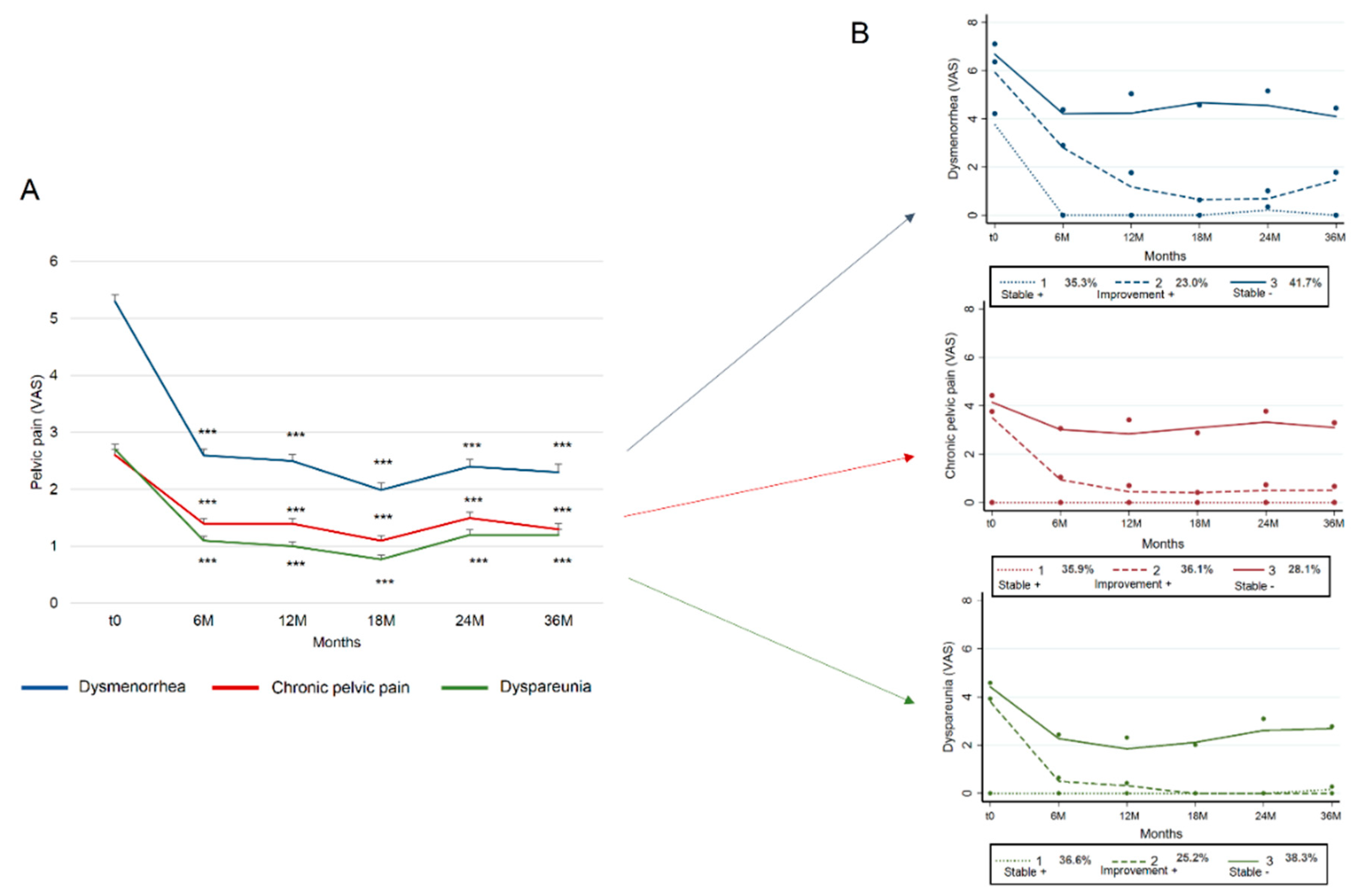
3.2. Trajectories
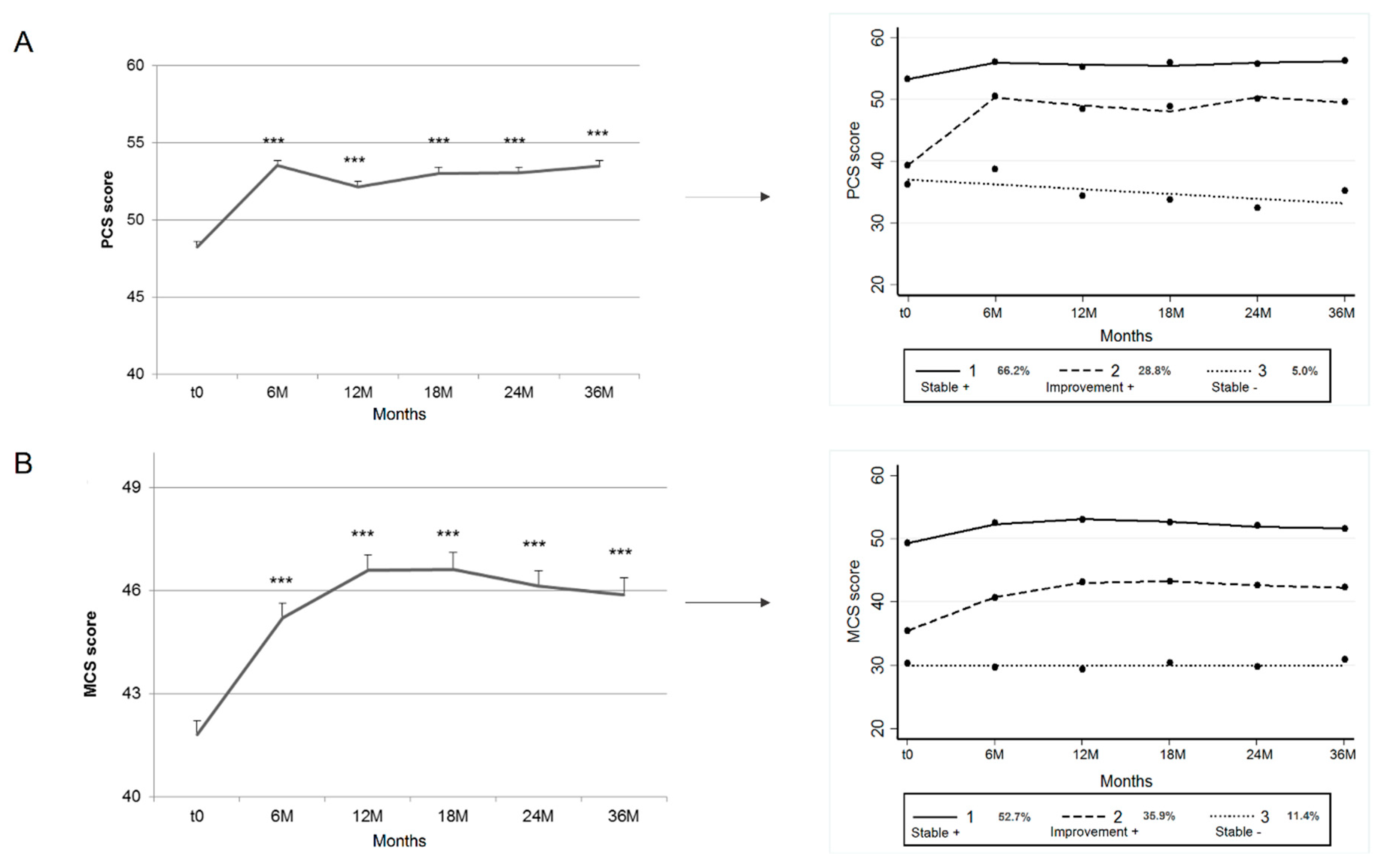
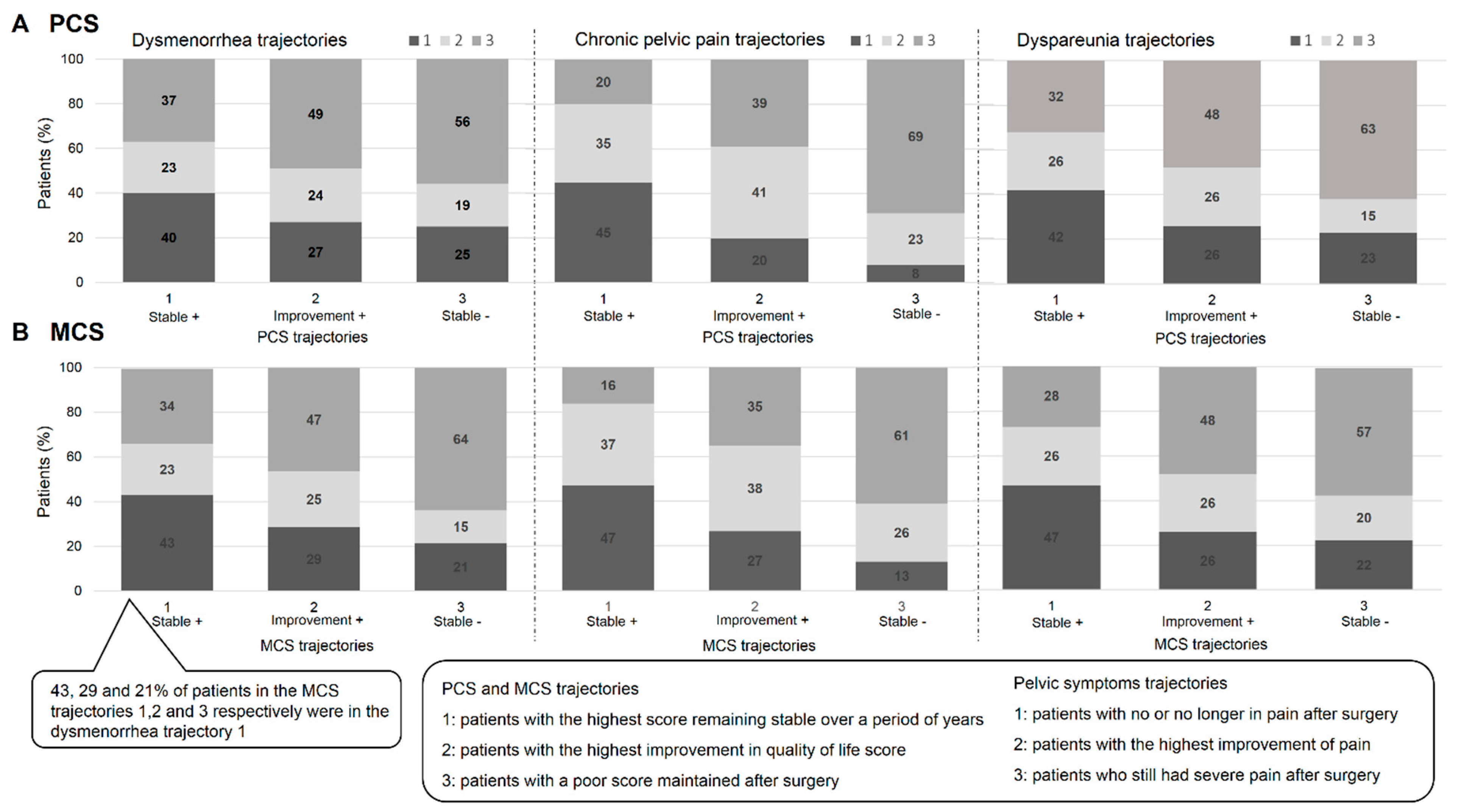
3.3. Comparison of Pelvic Pain and Quality-of-Life Trajectories
3.3.1. PCS and Pelvic Symptoms
3.3.2. MCS and Pelvic Symptoms
3.4. Multiple Correspondence Analysis (MCA) (at t0)
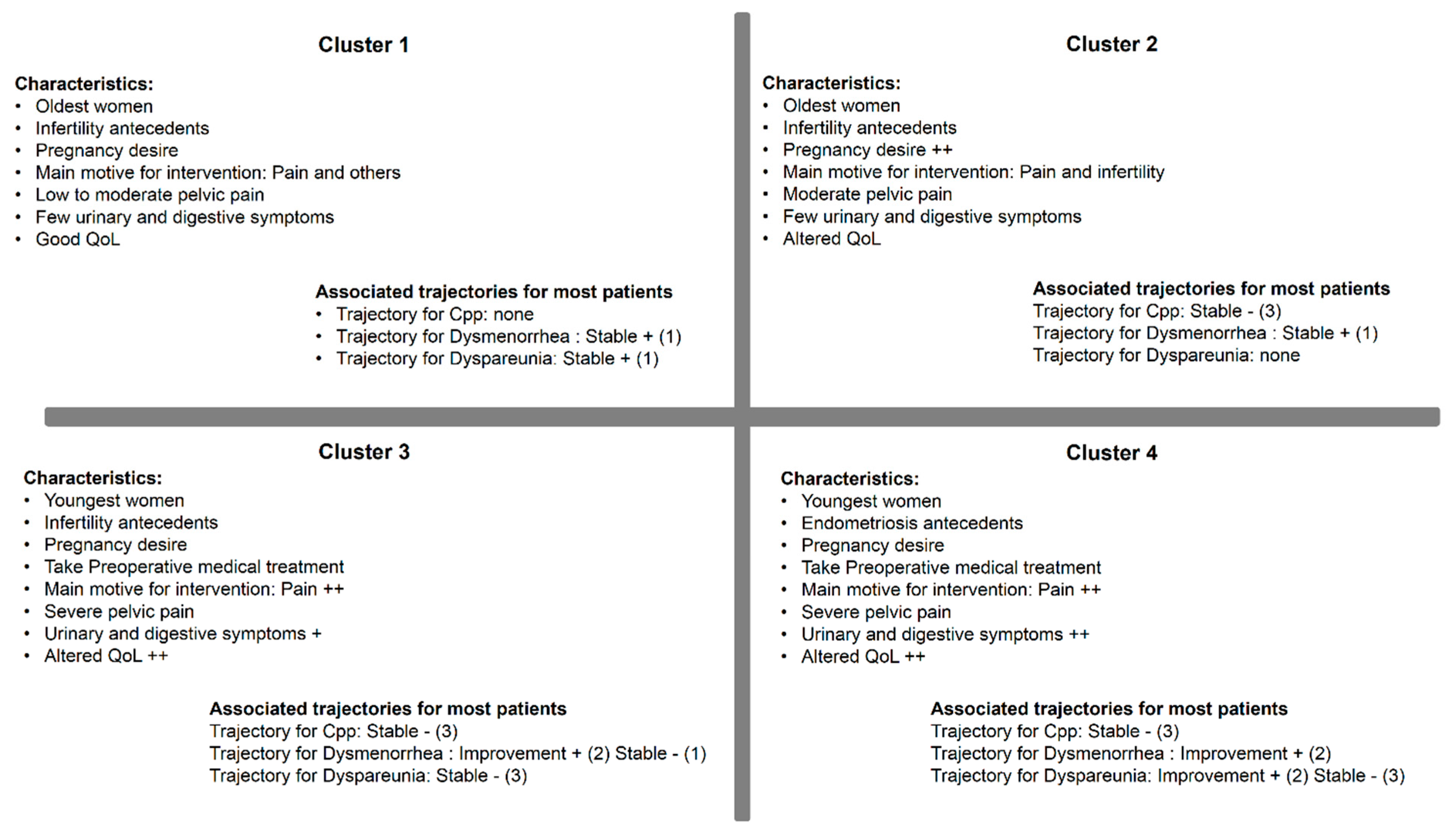
3.5. Comparison of Clusters and Trajectories
3.6. Profiles of Patients from Different Trajectories: Univariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mabrouk, M.; Montanari, G.; Guerrini, M.; Villa, G.; Solfrini, S.; Vicenzi, C.; Mignemi, G.; Zannoni, L.; Frasca, C.; Di Donato, N.; et al. Does laparoscopic management of deep infiltrating endometriosis improve quality of life? A prospective study. Heal. Qual. Life Outcomes 2011, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercellini, P.; Aimi, G.; Busacca, M.; Apolone, G.; Uglietti, A.; Crosignani, P.G. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis: Results of a randomized, controlled trial. Fertil. Steril. 2003, 80, 310–319. [Google Scholar] [CrossRef]
- Ribeiro, P.A.; Sekula, V.G.; Abdalla-Ribeiro, H.S.; Rodrigues, F.C.; Aoki, T.; Aldrighi, J.M. Impact of laparoscopic colorectal segment resection on quality of life in women with deep endometriosis: One year follow-up. Qual. Life Res. 2013, 23, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.A.; Podgaec, S.; Dias, J.A.; Filho, N.D.; Petta, C.A.; Abrão, M.S. Quality of Life after Segmental Resection of the Rectosigmoid by Laparoscopy in Patients with Deep Infiltrating Endometriosis with Bowel Involvement. J. Minim. Invasive Gynecol. 2011, 18, 730–733. [Google Scholar] [CrossRef]
- Parra, R.S.; Feitosa, M.R.; De Camargo, H.P.; Valério, F.P.; Zanardi, J.V.C.; Da Rocha, J.J.R.; Féres, O. The impact of laparoscopic surgery on the symptoms and wellbeing of patients with deep infiltrating endometriosis and bowel involvement. J. Psychosom. Obstet. Gynecol. 2020, 1–6. [Google Scholar] [CrossRef]
- Riiskjær, M.; Forman, A.; Kesmodel, U.S.; Andersen, L.M.; Ljungmann, K.; Seyer-Hansen, M. Pelvic Pain and Quality of Life Before and After Laparoscopic Bowel Resection for Rectosigmoid Endometriosis. Dis. Colon Rectum 2018, 61, 221–229. [Google Scholar] [CrossRef]
- Abbott, J.; Hawe, J.; Clayton, R.; Garry, R. The effects and effectiveness of laparoscopic excision of endometriosis: A prospective study with 2–5 year follow-up. Hum. Reprod. 2003, 18, 1922–1927. [Google Scholar] [CrossRef]
- Comptour, A.; Chauvet, P.; Canis, M.; Gremeau, A.-S.; Pouly, J.-L.; Rabischong, B.; Pereira, B.; Bourdel, N. Patient Quality of Life and Symptoms after Surgical Treatment for Endometriosis. J. Minim. Invasive Gynecol. 2019, 26, 717–726. [Google Scholar] [CrossRef]
- Byrne, D.; Curnow, T.; Smith, P.; Cutner, A.; Saridogan, E.; Clark, T.J.; Saraswat, L. Laparoscopic excision of deep rectovaginal endometriosis in BSGE endometriosis centres: A multicentre prospective cohort study. BMJ Open 2018, 8, e018924. [Google Scholar] [CrossRef] [PubMed]
- Darai, E.; Dubernard, G.; Coutant, C.; Frey, C.; Rouzier, R.; Ballester, M. Randomized Trial of Laparoscopically Assisted Versus Open Colorectal Resection for Endometriosis. Ann. Surg. 2010, 251, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Bourdel, N.; Comptour, A.; Bouchet, P.; Gremeau, A.-S.; Pouly, J.-L.; Slim, K.; Pereira, B.; Canis, M. Long-term evaluation of painful symptoms and fertility after surgery for large rectovaginal endometriosis nodule: A retrospective study. Acta Obstet. Gynecol. Scand. 2017, 97, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcoverde, F.V.L.; Andres, M.P.; Borrelli, G.M.; Barbosa, P.D.A.; Abrão, M.S.; Kho, R.M. Surgery for Endometriosis Improves Major Domains of Quality of Life: A Systematic Review and Meta-Analysis. J. Minim. Invasive Gynecol. 2019, 26, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Turco, L.C.; Scaldaferri, F.; Chiantera, V.; Cianci, S.; Ercoli, A.; Fagotti, A.; Fanfani, F.; Ferrandina, G.; Nicolotti, N.; Tamburrano, A.; et al. Long-term evaluation of quality of life and gastrointestinal well-being after segmental colo-rectal resection for deep infiltrating endometriosis (ENDO-RESECT QoL). Arch. Gynecol. Obstet. 2019, 301, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Roman, H.; Bubenheim, M.; Huet, E.; Bridoux, V.; Zacharopoulou, C.; Daraï, E.; Collinet, P.; Tuech, J.-J. Conservative surgery versus colorectal resection in deep endometriosis infiltrating the rectum: A randomized trial. Hum. Reprod. 2018, 33, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Rindos, N.B.; Fulcher, I.R.; Donnellan, N.M. Pain and Quality of Life after Laparoscopic Excision of Endometriosis. J. Minim. Invasive Gynecol. 2020. [Google Scholar] [CrossRef]
- Roman, H.; Tuech, J.-J.; Huet, E.; Bridoux, V.; Khalil, H.; Hennetier, C.; Bubenheim, M.; Branduse, L.A. Excision versus colorectal resection in deep endometriosis infiltrating the rectum: 5-year follow-up of patients enrolled in a randomized controlled trial. Hum. Reprod. 2019, 34, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Nagin, D.S. Group-Based Trajectory Modeling: An Overview. Ann. Nutr. Metab. 2014, 65, 205–210. [Google Scholar] [CrossRef]
- Comptour, A.; Pereira, B.; Lambert, C.; Chauvet, P.; Gremeau, A.-S.; Pouly, J.-L.; Canis, M.; Bourdel, N. Identification of Predictive Factors in Endometriosis for Improvement in Patient Quality of Life. J. Minim. Invasive Gynecol. 2020, 27, 712–720. [Google Scholar] [CrossRef]
- Leplège, A.; Ecosse, E.; Verdier, A.; Perneger, T.V. The French SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1013–1023. [Google Scholar] [CrossRef]
- Stull, D.E.; Wasiak, R.; Kreif, N.; Raluy, M.; Colligs, A.; Seitz, C.; Gerlinger, C. Validation of the SF-36 in patients with endometriosis. Qual. Life Res. 2013, 23, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Bourdel, N.; Alves, J.; Pickering, G.; Ramilo, I.; Roman, H.; Canis, M. Systematic review of endometriosis pain assessment: How to choose a scale? Hum. Reprod. Updat. 2014, 21, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Bourdel, N.; Roman, H.; Mage, G.; Canis, M. Chirurgie des endométriomes ovariens: De la physiopathologie à la prise en charge pratique pré-, per- et postopératoire. Gynécol. Obstét. Fertil. 2011, 39, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Kondo, W.; Bourdel, N.; Tamburro, S.; Cavoli, D.; Jardon, K.; Rabischong, B.; Botchorishvili, R.; Pouly, J.; Mage, G.; Canis, M. Complications after surgery for deeply infiltrating pelvic endometriosis. BJOG: Int. J. Obstet. Gynaecol. 2010, 118, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Bourdel, N.; Cognet, S.; Canis, M.; Berdugo, O.; Botchorishvili, R.; Rabischong, B.; Jardon, K.; Botchorishvilli, R. Laparoscopic Ureteroneocystostomy: Be Prepared! J. Minim. Invasive Gynecol. 2015, 22, 827–833. [Google Scholar] [CrossRef]
- Shah, N.H.; Hipwell, A.E.; Stepp, S.D.; Chang, C.-C.H. Measures of discrimination for latent group-based trajectory models. J. Appl. Stat. 2014, 42, 1–11. [Google Scholar] [CrossRef]
- Nagin, D.S.; Jones, B.L.; Passos, V.L.; Tremblay, R.E. Group-based multi-trajectory modeling. Stat. Methods Med. Res. 2016, 27, 2015–2023. [Google Scholar] [CrossRef]
- Nagin, D. Group-Based Modeling of Development; Harvard University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Feise, R.J. Do multiple outcome measures require p-value adjustment? BMC Med Res. Methodol. 2002, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Nagin, D.S.; Odgers, C.L. Group-Based Trajectory Modeling in Clinical Research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar] [CrossRef] [Green Version]
- Kho, R.M.; Andres, M.P.; Borrelli, G.M.; Neto, J.S.; Zanluchi, A.; Abrão, M.S. Surgical treatment of different types of endometriosis: Comparison of major society guidelines and preferred clinical algorithms. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 51, 102–110. [Google Scholar] [CrossRef]
- Valentin, L.; Canis, M.; Pouly, J.L.; Lasnier, C.; Jaffeux, P.; Aublet-Cuvelier, B.; Bourdel, N. SF-36 preoperative interest of predicting improvement of quality of life after laparoscopic management of minimal endometriosis. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 137–142. [Google Scholar] [CrossRef]
- Stratton, P.; Berkley, K.J. Chronic pelvic pain and endometriosis: Translational evidence of the relationship and implications. Hum. Reprod. Updat. 2010, 17, 327–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercellini, P.; Trespidi, L.; De Giorgi, O.; Cortesi, I.; Parazzini, F.; Crosignani, P.G. Endometriosis and pelvic pain: Relation to disease stage and localization. Fertil. Steril. 1996, 65, 299–304. [Google Scholar] [CrossRef]
- Bourdel, N.; Chauvet, P.; Billone, V.; Douridas, G.; Fauconnier, A.; Gerbaud, L.; Canis, M. Systematic review of quality of life measures in patients with endometriosis. PLoS ONE 2019, 14, e0208464. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.S.K.; Gross, E.; Geraedts, K.; Rauchfuss, M.; Wölfler, M.M.; Häberlin, F.; Von Orelli, S.; Eberhard, M.; Imesch, P.; Imthurn, B.; et al. The use of home remedies and complementary health approaches in endometriosis. Reprod. Biomed. Online 2019, 38, 260–271. [Google Scholar] [CrossRef]
- Mira, T.; Buen, M.M.; Borges, M.G.; Yela, D.A.; Benetti-Pinto, C.L. Systematic review and meta-analysis of complementary treatments for women with symptomatic endometriosis. Int. J. Gynecol. Obstet. 2018, 143, 2–9. [Google Scholar] [CrossRef]
- Expectations of Women with Endometriosis: What Information to Deliver? CNGOF-HAS Endometriosis Guidelines. Available online: https://pubmed.ncbi.nlm.nih.gov/29530554/ (accessed on 10 March 2020).
- Jones, G.; Jenkinson, C.; Kennedy, S. Evaluating the responsiveness of the Endometriosis Health Profile Questionnaire: The EHP-30. Qual. Life Res. 2004, 13, 705–713. [Google Scholar] [CrossRef]
- Chauvet, P.; Auclair, C.; Mourgues, C.; Canis, M.; Gerbaud, L.; Bourdel, N. Psychometric properties of the French version of the Endometriosis Health Profile-30, a health-related quality of life instrument. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 235–242. [Google Scholar] [CrossRef]
| n = 962 | n = 540 | n = 422 | |
|---|---|---|---|
| Demographic Data | |||
| Age (years) | 33.0 ± 7.1 | 34.0 ± 7.5 | 31.8 ± 6.3 |
| Tobacco (%) | 36.7 | 36.9 | 36.5 |
| BMI (kg/m2) | 22.1 ± 3.7 | 22.2 ± 4.0 | 22.0 ± 3.4 |
| In couple (%) | 78.0 | 76.0 | 80.6 |
| Antecedent | |||
| Endometriosis antecedents (%) | 10.3 | 12.0 | 8.3 |
| Preoperative medical treatment (%) | 29.2 | 27.5 | 31.3 |
| Infertility (%) | 41.7 | 36.8 | 47.9 |
| Symptoms | |||
| Dysmenorrhea (VAS) | 6.0 ± 3.4 | 5.0 ± 3.9 | 7.2 ± 2.1 |
| Chronic pelvic pain (VAS) | 2.9 ± 3.4 | 2.8 ± 3.5 | 3.1 ± 3.4 |
| Dyspareunia (VAS) | 3.0 ± 3.2 | 2.7 ± 3.2 | 3.4 ± 3.2 |
| Preoperative Characteristics | |||
| Pregnancy desire (%) | 53.1 | 49.0 | 57.8 |
| Period between first consultation and diagnosis (years) | 6 (2; 15) | 5 (2; 15) | 6 (2; 15) |
| Pelvic ultrasound (%) | 94.5 | 93.9 | 95.3 |
| MRI (%) | 23.9 | 23.4 | 24.5 |
| Motive for intervention (%) | |||
| Pain | 51.4 | 49.0 | 54.5 |
| Sterility | 20.6 | 18.0 | 23.9 |
| Endometriosis | 13.7 | 16.1 | 10.7 |
| Others | 14.3 | 16.9 | 10.9 |
| Operative Characteristics | |||
| rAFS stage (%) | |||
| Stage I: minimal | 27.4 | 26.0 | 29.1 |
| Stage II: mild | 28.3 | 29.6 | 26.8 |
| Stage III: moderate | 20.7 | 20.3 | 21.1 |
| Stage IV: severe | 23.6 | 24.1 | 23.0 |
| Duration of surgery (minutes) | 75 (60; 120) | 75 (60; 120) | 80 (55; 120) |
| Procedure | |||
| Surgical procedure (%) | 96.5 | 96.2 | 96.9 |
| Adhesiolysis (%) | 61.0 | 61.5 | 60.5 |
| Peritoneal superficial lesions (%) | 73.9 | 69.6 | 79.3 |
| Ovarian endometrioma (%) | 47.8 | 50.0 | 45.0 |
| Endometriosis nodule (%) | 58.0 | 58.0 | 57.9 |
| Digestive intervention (%) | 4.9 | 5.6 | 4.1 |
| Urological intervention (%) | 7.1 | 7.4 | 6.7 |
| Postoperative medical treatment (%) | 44.2 | 40.4 | 49.2 |
| Dysmenorrhea | |||
|---|---|---|---|
| Trajectory 1 (Stable +) | Trajectory 2 (improvement +) | Trajectory 3 (Stable −) | |
| Observed probabilities | 35.3 | 23.0 | 41.7 |
| Posterior probabilities | 29.9 | 32.2 | 38.0 |
| Average posterior probabilities | 79.0 | 83.4 | 78.9 |
| Odds of correct classification | 6.9 | 16.9 | 5.2 |
| Cpp | |||
| Trajectory 1 (Stable +) | Trajectory 2 (improvement +) | Trajectory 3 (Stable −) | |
| Observed probabilities | 35.9 | 36.1 | 28.1 |
| Posterior probabilities | 28.1 | 40.4 | 31.5 |
| Average posterior probabilities | 78.4 | 82.2 | 84.5 |
| Odds of correct classification | 6.5 | 8.2 | 14 |
| Dyspareunia | |||
| Trajectory 1 (Stable +) | Trajectory 2 (improvement +) | Trajectory 3 (Stable −) | |
| Observed probabilities | 36.6 | 25.2 | 38.3 |
| Posterior probabilities | 29.9 | 30.4 | 39.7 |
| Average posterior probabilities | 81.7 | 84.2 | 90.0 |
| Odds of correct classification | 7.7 | 15.9 | 14.5 |
| PCS | |||
| Trajectory 1 (Stable +) | Trajectory 2 (improvement +) | Trajectory 3 (Stable −) | |
| Observed probabilities | 66.2 | 28.8 | 5.0 |
| Posterior probabilities | 65.2 | 28.9 | 6.0 |
| Average posterior probabilities | 92 | 80.6 | 88.1 |
| Odds of correct classification | 5.9 | 10.3 | 140.8 |
| MCS | |||
| Trajectory 1 (Stable +) | Trajectory 2 (improvement +) | Trajectory 3 (Stable −) | |
| Observed probabilities | 52.7 | 35.9 | 11.4 |
| Posterior probabilities | 51.3 | 35.0 | 13.7 |
| Average posterior probabilities | 89.1 | 76.6 | 82.4 |
| Odds of correct classification | 7.3 | 5.9 | 36.4 |
| Cluster 1 (n = 76) | Cluster 2 (n = 182) | Cluster 3 (n = 102) | Cluster 4 (n = 62) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 0.05 | ||||
| <29 | 19.7 | 35.7 | 35.3 | 41.9 | |
| 29 to 34 | 47.4 | 40.1 | 33.3 | 27.4 | |
| ≥35 | 32.9 | 24.2 | 31.4 | 30.7 | |
| Body mass index < 25 kg/m2 | 86.8 | 86.8 | 82.4 | 83.9 | 0.73 |
| Tobacco | 30.3 | 33 | 44.1 | 41.9 | 0.13 |
| Single | 26.3 | 16.5 | 20.6 | 17.7 | 0.32 |
| Menarche ≤ 12 years | 40.8 | 48.9 | 49 | 43.5 | 0.6 |
| Infertility antecedents | 44.7 | 57.1 | 45.1 | 29 | 0.001 |
| Endometriosis antecedents | 6.6 | 8.2 | 3.9 | 17.7 | 0.02 |
| Pregnancy desire | 53.9 | 64.8 | 52 | 51.6 | 0.09 |
| Preoperative medical treatment | 23.7 | 23.6 | 42.2 | 45.2 | <0.001 |
| Main motive for intervention | <0.001 | ||||
| Pain | 36.8 | 51.6 | 59.8 | 75.8 | |
| Sterility | 23.7 | 31.9 | 16.7 | 12.9 | |
| Endometriosis | 11.8 | 8.8 | 16.7 | 4.8 | |
| Other | 27.6 | 7.7 | 6.9 | 6.5 | |
| VAS dysmenorrhea | <0.001 | ||||
| <3 | 17.1 | 0 | 2 | 0 | |
| 3 to 6 | 60.5 | 14.3 | 20.6 | 12.9 | |
| ≥7 | 22.4 | 85.7 | 77.4 | 87.1 | |
| VAS chronic pelvic pain | <0.001 | ||||
| <3 | 82.9 | 80.8 | 8.8 | 1.6 | |
| 3 to 6 | 15.8 | 15.4 | 58.8 | 25.8 | |
| ≥7 | 1.3 | 3.8 | 32.4 | 72.6 | |
| VAS dyspareunia | <0.001 | ||||
| <3 | 65.8 | 44.5 | 33.3 | 30.6 | |
| 3 to 6 | 27.6 | 40.1 | 33.3 | 37.1 | |
| ≥7 | 6.6 | 15.4 | 33.3 | 32.3 | |
| Dyschezia | 14.5 | 39.6 | 39.2 | 48.4 | <0.001 |
| Nausea | 5.3 | 28.6 | 39.2 | 40.3 | <0.001 |
| Rectal bleeding | 2.6 | 3.8 | 2.9 | 11.3 | 0.08 |
| Constipation | 15.8 | 30.2 | 39.2 | 37.1 | 0.006 |
| Diarrhea | 17.1 | 28.6 | 26.5 | 33.9 | 0.14 |
| Painful urination | 3.9 | 10.4 | 17.6 | 14.5 | 0.03 |
| Fatigue | 40.8 | 59.3 | 76.5 | 83.9 | <0.001 |
| Headache | 26.3 | 42.9 | 52.9 | 56.5 | 0.001 |
| PCS ≤ 50 | 17.1 | 42.3 | 66.7 | 83.9 | <0.001 |
| MCS ≤ 40 | 23.7 | 36.8 | 52.9 | 69.4 | <0.001 |
| Analgesic treatment during menstruations | 59.2 | 92.3 | 86.3 | 91.9 | <0.001 |
| Analgesic treatment outside menstruations | 6.6 | 6 | 50 | 77.4 | <0.001 |
| rAFS stage | 0.48 | ||||
| Stage I: minimal | 38.2 | 25.8 | 32.4 | 22.6 | |
| Stage II: mild | 21.1 | 28.6 | 28.4 | 25.8 | |
| Stage III: moderate | 21.1 | 19.8 | 21.6 | 24.2 | |
| Stage IV: severe | 19.7 | 25.8 | 17.6 | 27.4 |
| Clusters | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | p-Value | |
| (n = 76) | (n = 182) | (n = 102) | (n= 62) | ||
| Trajectories of Cpp | 0.16 | ||||
| 1 | 32.9 | 28.0 | 26.5 | 24.2 | |
| 2 | 35.5 | 23.1 | 22.5 | 27.4 | |
| 3 | 31.6 | 48.9 | 51.0 | 48.4 | |
| Trajectories of dysmenorrhea | <0.001 | ||||
| 1 | 52.6 | 51.7 | 0.0 | 0.0 | |
| 2 | 35.5 | 28.0 | 53.9 | 59.7 | |
| 3 | 11.8 | 20.3 | 46.1 | 40.3 | |
| Trajectories of dyspareunia | 0.001 | ||||
| 1 | 47.4 | 32.4 | 18.6 | 19.4 | |
| 2 | 25.0 | 29.7 | 29.4 | 37.1 | |
| 3 | 27.6 | 37.9 | 52.0 | 43.5 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comptour, A.; Lambert, C.; Chauvet, P.; Figuier, C.; Gremeau, A.-S.; Canis, M.; Pereira, B.; Bourdel, N. Long-Term Evolution of Quality of Life and Symptoms Following Surgical Treatment for Endometriosis: Different Trajectories for Which Patients? J. Clin. Med. 2020, 9, 2461. https://doi.org/10.3390/jcm9082461
Comptour A, Lambert C, Chauvet P, Figuier C, Gremeau A-S, Canis M, Pereira B, Bourdel N. Long-Term Evolution of Quality of Life and Symptoms Following Surgical Treatment for Endometriosis: Different Trajectories for Which Patients? Journal of Clinical Medicine. 2020; 9(8):2461. https://doi.org/10.3390/jcm9082461
Chicago/Turabian StyleComptour, Aurélie, Céline Lambert, Pauline Chauvet, Claire Figuier, Anne-Sophie Gremeau, Michel Canis, Bruno Pereira, and Nicolas Bourdel. 2020. "Long-Term Evolution of Quality of Life and Symptoms Following Surgical Treatment for Endometriosis: Different Trajectories for Which Patients?" Journal of Clinical Medicine 9, no. 8: 2461. https://doi.org/10.3390/jcm9082461
APA StyleComptour, A., Lambert, C., Chauvet, P., Figuier, C., Gremeau, A.-S., Canis, M., Pereira, B., & Bourdel, N. (2020). Long-Term Evolution of Quality of Life and Symptoms Following Surgical Treatment for Endometriosis: Different Trajectories for Which Patients? Journal of Clinical Medicine, 9(8), 2461. https://doi.org/10.3390/jcm9082461






